Abstract
We previously determined the effects of dietary selenium (Se) deficiency or excess on mRNA abundance of 12 selenoprotein genes in pig tissues. In this study, we determined the effect of dietary Se on mRNA levels of the remaining porcine selenoprotein genes along with protein production of 4 selenoproteins (Gpx1, Sepp1, Selh, and Sels) and body glucose homeostasis. Weanling male pigs (n = 24) were fed a Se-deficient (<0.02 mg Se/kg), basal diet supplemented with 0, 0.3, or 3.0 mg Se/kg as Se-enriched yeast (Angel Yeast) for 16 wk. Although mRNA abundance of the 13 selenoproteins in 10 tissues responded to dietary Se in 3 patterns, there was no common regulation for any given gene across all tissues or for any given tissue across all genes. Dietary Se affected (P < 0.05) 2, 3, 3, 5, 6, 7, 7, and 8 selenoprotein genes in muscle, hypothalamus, liver, kidney, heart, spleen, thyroid, and pituitary, respectively. Protein abundance of Gpx1, Sepp1, Selh, and Sels in 6 tissues was regulated (P < 0.05) by dietary Se concentrations in 3 ways. Compared with those fed 0.3 mg Se/kg, pigs fed 3.0 mg Se/kg became hyperinsulinemic (P < 0.05) and had lower (P < 0.05) tissue levels of serine/threonine protein kinase. In conclusion, dietary Se exerted no global regulation of gene transcripts or protein levels of individual selenoproteins across porcine tissues. Pigs may be a good model for studying mechanisms related to the potential prodiabetic risk of high-Se intake in humans.
Introduction
Selenium (Se) is an essential nutrient for humans and many other species. Classical symptoms of dietary Se deficiency in farm and laboratory animals were characterized in the 1950s to 1960s (1–8), and supranutrition of Se was found to decrease cancer mortality in the 1990s (9–13). However, the underlying molecular mechanism for these multiple-phased functions of Se in the body has remained unclear. Although 24–25 selenoprotein genes were identified in mammals (14), only a few studies have determined collective responses of these genes to dietary Se concentrations ranging from deficiency to moderately high levels (15–20). Thus, systematic data are needed to link selenoprotein gene expression profiles to metabolic impacts of dietary Se deficiency or excess in tissues. Because pigs are not only an important food-producing species worldwide but are also an excellent model for human nutrition (21, 22) and medicine (23, 24), we have determined the expression profiles of 12 selenoprotein genes (Gpx1, Gpx2, Gpx4, Dio1, Dio3, Selk, Sep15, Sephs2, Sepn1, Sepp1, Sepw1, and Txnrd1) in liver, testis, thyroid, and pituitary of pigs fed Se-deficient (<0.02 mg/kg), -adequate (0.3 mg/kg), and -excess (3 mg/kg) diets (15). Although dietary Se resulted in dose-dependent increases (P < 0.05) in Se concentrations and Gpx activities in all of these 4 tissues, it did not alter mRNA levels of any selenoprotein gene in thyroid or pituitary. More intriguingly, the expression of 7 selenoprotein genes was not affected at all by dietary Se concentrations, even in the liver. This observed resistance of the assayed selenoprotein genes to dietary Se change has raised the following questions: 1) how dietary Se affects expressions of the remaining selenoprotein genes in various tissues in addition to the 4 mentioned above; 2) if feeding longer than 8 wk is needed to show full effects of dietary Se on the selenoprotein gene expression in tissues, in particular endocrine organs such as thyroid; and 3) what the impact is of dietary Se concentrations on protein production of selected selenoproteins in various tissues.
Recently, a number of human studies have suggested a surprising link between high body Se status and adverse blood profiles of glucose and lipid or diabetic susceptibility (25–28). Because supranutrition of Se was considered to be anticarcinogenesis (12), it is imperative to clarify if prolonged intake of high dietary Se directly impairs glucose homeostasis or insulin function. Although we and others have attempted to reveal this link in rodents (29–33), pigs are better models for humans because of their great digestive and metabolic similarities to humans (21–24). Two abundant selenoproteins in tissues and frequently used biomarkers of body Se status (34, 35), glutatathione peroxidase-1 (Gpx1) and selenoprotein P (Sepp1) are associated with type-2 diabetes-like phenotypes or insulin abnormality (36–38). Meanwhile, selenoprotein S (Sels) is also regulated by glucose (39). Despite no such link reported, selenoprotein H (Selh), a nucleolar thioredoxin-like protein, exhibits Gpx-like activity and distinct expression patterns in highly proliferative tissues (40). Because serine/threonine protein kinase (Akt) plays a central role in insulin signaling (41), dysregulation of this protein often leads to insulin resistance or malfunction (36). Therefore, we used growing pigs and conducted a 16-wk feeding experiment to: 1) compare the effects of 0, 0.3, and 3.0 mg Se/kg on mRNA expression profiles of 13 selenoproteins in 10 tissues; 2) determine if the prolonged high Se intake induced dysregulation of plasma glucose, lipid, and insulin; and 3) explore how tissue protein levels of Gpx1, Sepp1, Selh, and Sels, along with Akt, were related to dietary Se deficiency or excess and the resultant metabolic disorders.
Materials and Methods
Expt. 1
Cloning of novel selenoprotein genes in porcine tissues.
Because of the lack of information in the database of National Center for Biotechnology Information (NCBI)8 or other sources, we cloned Selh, Selm, Selv, and Selx to design respective primers for the intended qPCR analysis. We used a strategy of in silico cloning followed by PCR and data mining to obtain these genes (42). Primers (Supplemental Table 1) were designed based on homology analysis of the expressed sequence tag fragment of the target selenoprotein gene with the same gene from other species or based on the highly conserved region of the gene aligned from human, mouse, rat, and cattle. As previously described (15), the target genes were amplified by RT-PCR, sequenced 3 times, and aligned using the BLAST program at NCBI. Total RNA of a Se-adequate, adult male Landrace pig was isolated from liver to clone Selh, Selm, and Selx and from testis to clone Selv.
Expt. 2
Animal, diet, and sample collection.
Our protocols were approved by Sichuan Agriculture University. A total of 24 weanling male pigs (3 wk old, Duroc-Landrace-Yorkshire crossbred, Swine Research Farm) were selected and fed a Se-deficient, corn-soybean meal (produced in the Se-deficient area in Sichuan, China) basal diet (BD) for 3 wk to adjust their Se status (Table 1). The pigs were divided into 3 groups (n = 8) and fed the BD supplemented with 0, 0.3, or 3.0 mg Se/kg as Se-enriched yeast (Angel Yeast) for 16 wk. Two BD were formulated for better meeting nutrient requirements of pigs at body weights ≤20 kg and >20 kg. The analyzed Se concentration (mg/kg) was 0.005 for corn, 0.023 for soybean, 1000 for the Se-rich yeast, 0.02 for pigs at body weights ≤20 kg, and 0.01 for pigs at body weights >20 kg, respectively. Body weights of individual pigs were recorded at baseline and then monthly. Blood samples were collected to prepare plasma samples (15) from individual pigs (feed deprived overnight for 8 h from 1200 to 0800 of sampling time) at baseline (wk 0), mid-point (wk 8), and final (wk 16). At the end of the study (wk 16), 6 pigs (feed deprived as described above) from each treatment group were killed to collect blood, liver, kidney, muscle (longissimus), testis, thyroid, pituitary, spleen, heart, and hypothalamus (15, 16).
TABLE 1.
Composition of BD as fed1
| ≤20 kg2 | >20 kg | |
| g/kg | ||
| Corn | 564.9 | 730.5 |
| Soybean | 275.0 | 230.0 |
| Whey powder | 120.0 | 0.0 |
| Choline | 1.0 | 1.0 |
| l-Lysine | 3.0 | 3.2 |
| DL-Methionine | 0.2 | 0.1 |
| L-Threonine | 0.6 | 0.7 |
| Salt | 2.0 | 3.0 |
| CaHPO4 | 14.0 | 12.0 |
| CaCO3 | 7.0 | 9.0 |
| Trace mineral premix3 | 10.0 | 10 |
| Vitamin premix4 | 0.3 | 0.5 |
The analyzed Se concentrations of corn, soybean, and Se-rich yeast (Angel Yeast) were 0.005, 0.023, and 1000 mg/kg, respectively. The analyzed Se concentrations in the BD were 0.02 and 0.01 mg/kg for ≤20 and >20 kg, respectively. BD, basal diet.
Two BD were prepared to better meet the nutrient requirements of pigs at different ages (weights).
Trace mineral premix provided/kg diet: FeSO4·7H2O, 993 mg; CuSO4·5H2O, 786 mg; ZnSO4·7H2O, 440 mg; MnSO4, 27.5 mg; KI, 0.4 mg; and colistin sulfate, 40 mg.
Vitamin premix provided/kg diet: retinyl acetate, 3027 g; cholecalciferol, 22 μg; dl-α-tocopheryl acetate, 32 mg; menadione, 2 mg; thiamin, 4 mg; riboflavin, 14 mg; calcium pantothenate, 40 mg; niacin, 60 mg; pyridoxol, 6 mg; d-biotin, 0.2 mg; folacin, 1.2 mg; and cobalamin, 72 μg.
Plasma and liver biochemical measures.
The hydride generation-atomic fluorescence spectrometer (AFS-830, Titan Instruments) (43) was used to measure feed, tissue, and plasma Se concentrations against a standard Se reference (15, 16). The activities of Gpx were measured by the coupled method (44) (0.12 mmol/L of H2O2 as substrate) (15, 16). Concentrations of protein were determined using the Bradford method (45). An automated clinical chemistry analyzer (Roche Model 800) was applied, along with respective kit, to determine plasma glucose (Glu-OX kit, ReeBio), TG (TG-test kit, ReeBio), and insulin (Iodine [125I]Insulin Radioimmunoassay kit, Groundwork Biotechnology Diagnosticate).
qPCR analyses of the selenoprotein mRNA abundance.
Total RNA was isolated from liver, kidney, muscle, testis, thyroid, pituitary, spleen, heart, hypothalamus, and blood of 4 pigs from each treatment group (n = 4). The relative mRNA abundances of 16 selenoprotein genes were assayed. These genes included 12 novel genes of pigs (Diol2, Gpx3, Selh, Seli, Selm, Selo, Sels, Selt, Selv, Selx, Txnrd2, and Txnrd3) and 4 genes (Gpx1, Sep15, Sephs2, and Sepp1) tested in our previous study (15) as inter-experimental controls. However, the results of Txnrd2, Txnrd3, and Diol2 were of poor quality and were not reported herein. Primers (Supplemental Table 2) for the rest of 13 selenoprotein genes and 2 reference genes, β-actin (Actb) and Gapdh, were designed using Primer Express 3.0 (Applied Biosystems). The RNA sample preparation, real time qPCR procedure, and relative mRNA abundance quantification were the same as previously described by our group (15, 16) except for that in the present study, the expression of selenoprotein gene was set as 1 assuming its application cycle threshold-Ct at 25 and that of the reference gene Actb at 15.02 (ΔCt = Cttarget – Ctreference = 25 – 15.02), and this ΔCt was then used as the control to normalize the relative expression level of target sample genes (2–(ΔCt sample – ΔCt control), or 2–ΔΔCt). To confirm the specific amplification, melting curve and cycle threshold analyses were performed (Supplemental Figs. 1 and 2).
Western-blot analyses.
Tissues were homogenized with the T-per Tissue Protein Extraction Reagent (Catalog no. 78510, Thermo Fisher Scientific) and centrifuged at 14,000 × g for 10 min at 4°C. The resulting supernatants of the homogenates (10~40 μg protein/lane) were loaded onto a SDS-PAGE (12%), transferred to polyvinylidene difluoride membranes, and incubated with appropriate antibodies (Supplemental Table 3) as previously described (36).
Statistical analyses.
One-way ANOVA followed by Duncan’s multiple range test (SPSS for Windows 13.0) was used to test effects of the 3 dietary Se concentrations on mRNA or protein levels of a given selenoprotein or Akt within each tissue. Plasma biochemical measures and growth performance of pigs were analyzed using 1-way ANOVA with time-repeated measurement. Data are presented as mean ± SE and the significance level was set at P < 0.05.
Results
Expt. 1
Porcine Selh, Selm, Selv, and Selx were cloned and their sequences were submitted to NCBI (Table 2). The number of basepairs in the gene coding sequence and the number of amino acids in the deduced peptide sequence were 369 and 123 for Selh, 429 and 143 for Selm, 1026 and 342 for Selv, and 348 and 116 for Selx, respectively. There was only one TGA codon for selenocysteine each in all these 4 genes. The homology of the coding sequences and the deduced peptide sequences in the cloned porcine genes to their human homologs ranged from 82 to 91% for Selh, Selm, and Selx. However, the cloned porcine Selv exhibited a rather low homology of gene coding sequence (70%) and peptide sequence (55%) compared with that of humans.
TABLE 2.
Characteristics of the 4 cloned novel pig selenoprotein genes
| Gene | Accession no. | Length1 | Homologous to human,2 % | Selenocysteine position3 | ||
| Gene | Peptide | Gene | Peptide | |||
| Selh | HM018602 | 369 | 123 | 89 | 89 | 43 |
| Selm | FJ968780 | 429 | 143 | 82 | 84 | 45 |
| Selv | GQ478346 | 1026 | 342 | 70 | 55 | 269 |
| Selx | EF113597 | 348 | 116 | 88 | 91 | 95 |
The numbers are the base pairs in the coding sequence of the gene and the amino acids in the deduced peptides.
As percentage to that of the human gene or peptide compared using BLASTN (57).
The number indicates its position from the N terminus in the N terminus.
Expt. 2
Growth performance and biochemical measures in plasma and liver.
Pigs fed BD, 0.3 mg Se/kg, and 3.0 mg Se/kg had similar body weights at wk 0 (9.2 ± 0.5 kg) and 16 (66.4 ± 7.4 kg). The 3 groups of pigs also had similar daily feed intakes and gain/feed efficiencies (data not shown). The final plasma and liver Se concentrations were 90 and 86% lower (P < 0.05) in pigs fed BD and 1.4- and 2.7-fold higher (P < 0.05), respectively, in pigs fed 3.0 mg Se/kg than those in pigs fed 0.3 mg Se/kg (Table 3). Compared with the initial values, plasma Gpx activities at wk 8 decreased (P < 0.05) by ~50% in pigs fed BD but were more than doubled (P < 0.05) in those fed 0.3 or 3.0 mg Se/kg. These opposite changes led to >5-fold differences in the activities (P < 0.05) between the BD and 2 Se-supplemented groups. At wk 16, pigs fed BD and 3.0 mg Se/kg maintained their respective plasma Gpx activities similar to those at wk 8, but pigs fed 0.3 mg Se/kg group had a slight decrease. Thus, the final plasma Gpx activity was 66% lower (P < 0.05) in pigs fed BD and 33% higher (P < 0.05) in pigs fed 3.0 mg Se/kg than that of pigs fed 0.3 mg Se/kg. Likewise, dietary Se deficiency decreased (P < 0.05) liver Gpx activity by 90% compared with the 0.3 mg Se/kg group. However, increasing dietary Se from 0.3 to 3.0 mg/kg did not produce any further change in the activity. Although final plasma glucose concentrations were similar among the 3 groups of pigs, the high-Se diet (3.0 mg/kg) resulted in a 50% higher (P < 0.05) plasma insulin concentration compared with that of 0.3 mg Se/kg or BD. The final plasma TG concentration in the BD group was 50% lower (P < 0.05) than that of pigs fed 0.3 mg Se/kg.
TABLE 3.
Effects of dietary Se concentrations on plasma and liver biochemical indicators of pigs fed the experimental diets for 16 wk1
| Measures | BD | +0.3 mg Se/kg | +3.0 mg Se/kg |
| Plasma Se, mmol/L | |||
| wk 0 | 0.03 ± 0.003 | 0.03 ± 0.01 | 0.03 ± 0.003 |
| wk16 | 0.02 ± 0.01a | 0.21 ± 0.01b | 0.51 ± 0.22c |
| Liver Se, nmol/g tissue | |||
| wk 16 | 0.1 ± 0.05a | 0.7 ± 0.06b | 2.6 ± 0.91c |
| Plasma Gpx, U/mg protein2 | |||
| wk 0 | 11.6 ± 1.2 | 11.2 ± 1.3 | 9.4 ± 1.6 |
| wk 8 | 5.0 ± 2.8a | 25.4 ± 2.6b | 28.5 ± 4.0b |
| wk16 | 6.3 ± 2.3a | 19.3 ± 2.7b | 25.7 ± 2.5c |
| Liver Gpx, U/mg protein | |||
| wk 16 | 28.6 ± 7.1a | 252 ± 67.3b | 220 ± 31.3b |
| Plasma glucose, mmol/L | |||
| wk 16 | 5.1 ± 0.1 | 5.6 ± 0.7 | 5.0 ± 0.2 |
| Plasma TG, mmol/L | |||
| wk 16 | 0.4 ± 0.05a | 0.8 ± 0.07b | 0.6 ± 0.09ab |
| Plasma insulin, pmol/L | |||
| wk 16 | 130 ± 3.5a | 132 ± 13.8a | 198 ± 31.2b |
Values are mean ± SE, = 4–6. Means in a row with superscripts without a common letter differ, P < 0.05.
The unit for the enzyme activity is defined as 1 μmol glutathione oxidized per min at 37°C.
Abundances of selenoprotein mRNA.
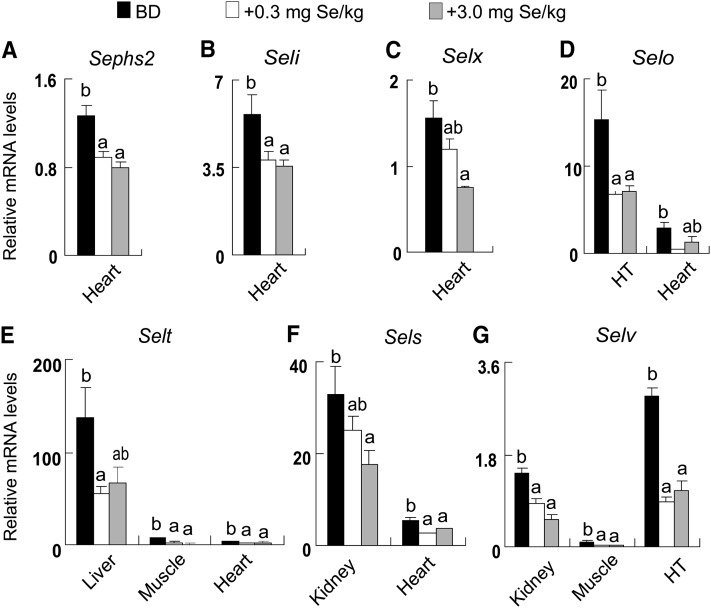
Expression of selenoprotein genes in various tissues responded to dietary Se concentrations in 3 patterns. The first was that dietary Se deficiency resulted in lower (P < 0.05) tissue mRNA levels compared with those of supplementing Se at 0.3 and 3.0 mg/kg (Fig. 1). These decreases included Gpx1 in liver, kidney, thyroid, and blood (Fig. 1A); Gpx3 in pituitary and testis (Fig. 1B); Sels in thyroid, pituitary, blood, and spleen (Fig. 1C); and Sepp1 in liver and kidney (Fig. 1D). The second pattern was manifested as higher (P < 0.05) mRNA levels in the BD group than those in the Se-supplemented groups (0.3 and 3.0 mg Se/kg) (Fig. 2). These upregulations included Sephs2 (Fig. 2A), Seli (Fig. 2B), and Selx (Fig. 2C) in heart; Selo in hypothalamus and heart (Fig. 2D); Selt in liver, muscle, and heart (Fig. 2E); Sels in kidney and heart (Fig. 2F); and Selv in kidney, muscle, and hypothalamus (Fig. 2G). The third way had similar downregulations of mRNA levels by both BD and 3.0 mg Se/kg compared with 0.3mg Se/kg (Fig. 3). These included Gpx1 in pituitary and spleen (Fig. 3A); Sephs2 in pituitary, thyroid, and hypothalamus (Fig. 3B); Selv in thyroid (Fig. 3C); Selm in thyroid and spleen (Fig. 3D), Seli in thyroid, pituitary, and spleen (Fig. 3E); Selx in spleen (Fig. 3F); Sep15 in thyroid, pituitary, spleen, and kidney (Fig. 3G); and Selh in kidney, thyroid, pituitary, and spleen (Fig. 3H). However, dietary Se deprivation or excess had a nonsignificant effect on the expression of many selenoprotein genes in various tissues (Supplemental Table 4).
FIGURE 1.
Effects of dietary Se concentrations on mRNA abundance of Gpx1(A), Gpx3 (B), Sels (C), and Sepp1 (D) in various tissues of pigs at wk 16. Data are mean ± SE, n = 5. Means for a gene within a tissue without a common letter differ, P < 0.05.
FIGURE 2.
Effects of dietary Se concentrations on mRNA abundance of Sephs2 (A), Seli (B), Selx (C), Selo (D), Selt (E), Sels (F), and Selv (G) in various tissues of pigs at wk 16. Data are mean ± SE, n = 5. Means for a gene within a tissue without a common letter differ, P < 0.05. HT, hypothalamus.
FIGURE 3.
Effects of dietary Se concentrations on mRNA abundance of Gpx1 (A), Sephs2 (B), Selv (C), Selm (D), Seli (E), Selx (F), Sep15 (G), and Selh (H) in various tissues of pigs at wk 16. Data are mean ± SE, n = 5. Means for a gene within a tissue without a common letter differ, P < 0.05. HT, hypothalamus.
Tissue abundances of selenoproteins.
Effects of dietary Se on tissue protein levels of Gpx1 (Fig. 4A), Sepp1 (Fig. 4B), Selh (Fig. 4C), and Sels (Fig. 4D) were also shown in 3 patterns. The first pattern was manifested with lower (P < 0.05) abundances in the BD group compared with those of pigs fed 0.3 and 3.0 mg Se/kg. These downregulations included Gpx1 in liver and muscle, Sepp1 in liver, kidney, and muscle, Selh in thyroid and kidney, and Sels in liver, kidney, and muscle. The second pattern was that tissue selenoproteins further increased (P < 0.05) by 3.0 over 0.3 mg Se/kg. These changes included Sepp1 in thyroid and testis, Selh in liver, and Sels in thyroid. The third pattern was no response of tissue selenoprotein level to dietary Se concentrations. These included Sepp1 and Sels in heart. Although Gpx1 in thyroid, kidney, and testis showed as pattern 1, Sels in testis fell into pattern 3, and Selh in testis increased by 0.3 mg Se/kg, but not 3.0 mg Se/kg, compared with the BD (Supplemental Fig. 3A–C), statistical analysis of these changes was impossible due to the lack of replications. Likewise, the relative distribution of Selh and Sels in 5–6 tissues was determined in only single assays. Liver Akt protein levels were decreased (P < 0.05) as dietary Se concentration rose (Fig. 4E). Similar decreases of Akt occurred in thyroid and heart, whereas the opposite change of the protein occurred in testis (Supplemental Fig. 3D). However, these changes of Akt required further statistical verification.
FIGURE 4.
Effects of dietary Se concentrations on protein levels of Gpx1 (A), Sepp1 (B), Selh (C), Sels (D), and Akt (E) in various tissues of pigs at wk 16. The band was a representative image of 3–5 independent analyses. Values below the protein band were relative density and are mean ± SE, n = 3–5. Means without a common letter differ, P < 0.05.
Discussion
Expression of 13 selenoprotein genes was determined in 10 tissues of growing pigs in the present study and illustrated 3 types of responses to dietary Se deficiency (BD), adequacy (0.3 mg/kg), and excess (3.0 mg/kg). The 3 dietary Se concentrations exerted the intended impacts on the body Se status of pigs, as shown by plasma and liver Se concentrations and Gpx activities. The successful cloning of Selh, Selm, Selv, and Selx and the available information on other novel porcine selenoprotein genes enabled us to design primers for qPCR to complete the systematic analysis of effects of dietary Se on porcine selenoprotein gene expression profiles initiated in our previous study (15). Specifically, 4 genes (Gpx1, Gpx3, Sels, and Sepp1) were downregulated in 7 tissues by dietary Se deficiency compared with the Se-supplemented groups. These genes seemed to code for relatively high abundant selenoproteins in the body and their responses were consistent with previous reports on pigs (15), chicks (16), mice (17), turkey poults (18), and rats (19). Meanwhile, dietary Se deficiency upregulated mRNA levels of 7 genes (Seli, Selo, Sels, Selt, Selv, Selx, and Sephs2) in 5 tissues compared with 0.3 and 3.0 mg Se/kg. Similar upregulation of Sephs2 expression by Se deficiency was also seen in rat kidney and muscle (19). It remains to be revealed if this type of upregulation implicates a unique mechanism for various tissues to prioritize selenoprotein gene expression and/or protein synthesis under alimited Se supply. Lastly, dietary Se deficiency and excess, compared with 0.3 mg Se/kg, resulted in symmetric (an inverted U shape) downregulations of 8 genes (Gpx1, Sep15, Sephs2, Selh, Seli, Selm, Selv, and Selx) in 6 tissues. In our previous pig study, testis Txnrd1 and Sep15 mRNA levels were decreased by 3.0 mg Se/kg compared with those of 0.3 mg Se/kg. The mirrored impacts by the 2 extremes of dietary Se intake on these 8 genes implied a homeostatic regulation of these genes in response to dietary Se fluctuations. This view and its potential metabolic importance were further supported by the fact that tissues involved in this pattern were mainly endocrine organs.
Despite the above-described 3 types of responses, no single selenoprotein gene exhibited any common response to dietary Se across various tissues. Meanwhile, no single tissue showed any common response to dietary Se across various selenoprotein genes. Therefore, our findings support the view (17) that no simple or universal molecular mechanism such as the position and local context of the UGA codon in the DNA sequence or the secondary structure of selenocysteine insertion element sequence and its affinity to binding proteins for a given selenoprotein gene can accurately predict its response to dietary Se regulation. Reciprocally, there is no tissue-specific regulator to control expression of different selenoprotein genes in a global way. In our previous 8-wk feeding experiment (15), mRNA levels of 13 genes in thyroid or pituitary were not altered by dietary Se deficiency or excess. In contrast, more selenoprotein genes (7–8 vs. 3–5) were affected by dietary Se in these tissues than in liver or kidney after the 16-wk feeding in the present study. Seemingly, expression of selenoprotein genes in endocrine tissues may be resistant to short-term changes of dietary Se intake but highly responsive to prolonged depletion or overdose of the nutrient. This scenario should be considered in searching for biomarkers to assess body Se status or in choosing the experimental duration to determine the effects of dietary Se on endocrine function. While our results illustrated an overrepresentation of endocrine tissues in the unique regulatory patterns of selenoprotein gene expression (an up-regulation or increase by Se deficiency compared with the Se adequacy; and similar down-regulations or decreases by both dietary Se deficiency and excess compared with the Se adequacy to form an inverted “U” shape curve) and protein production (up with high Se, see below), an earlier observation (46) suggested that endocrine tissues were high on the hierarchy of selenoprotein synthesis. The historical aspects of this hierarchy concept were well elaborated by a recent review along with microarray expression data in rats (20). Mechanistically, the high hierarchy of endocrine tissue selenogenome transcripts and proteins, along with their unique responses to dietary Se supply, may be associated with their high Se demands (46), special Se transport or uptake (46), and metabolic interactions between relevant hormones and Se (24).
The present study revealed novel regulations of protein production of Gpx1, Sepp1, Selh, and Sels in 6 tissues of pigs by dietary Se. The lack of reagents (antibodies), along with other technical difficulties, disallowed much previous research in laboratory animals (17, 19, 32), let alone in large animals like pigs (15), to study the regulation of dietary Se beyond gene expression. Thus, the results from those studies have limited value for functional analysis. In the present study, we identified appropriate antibodies from multiple sources to assay 4 major selenoproteins that may be related to body glucose metabolism (see below). Compared with their respective mRNA changes, the tissue protein levels of these 4 selenoproteins responded to dietary Se concentrations in 3 patterns. The first was that both mRNA and protein showed similar downregulations by dietary Se deficiency. This type included Gpx1 in liver and muscle, Sepp1 in liver, kidney, and muscle, Selh in thyroid and kidney, and Sels in liver, kidney, and muscle. The second was that the protein was elevated by 3.0 over 0.3 mg Se/kg despite a plateau of mRNA at 0.3 mg Se/kg. This type included Sepp1 in thyroid and testis, Selh in liver, and Sels in thyroid. The third was no change of protein regardless of mRNA responses. This type included Sepp1 in heart and Sels in heart and testis. Altogether, the 3 distinct types of responses underscore a disparity or the lack of simple regulatory mechanism for selenoproteins by dietary Se in pig tissues.
It was fascinating for us to find that feeding pigs with 3.0 mg Se/kg for 16 wk induced hyperinsulinemia compared with those fed 0.3 mg Se/kg. More specifically, the Se-overdosed pigs had >50% plasma insulin than the Se-adequate pigs to maintain similar plasma glucose concentrations, indicating an early sign of insulin resistance. Hyperinsulinemia and insulin resistance were induced by overexpressing the most abundant selenoprotein, Gpx1, in mice (36) and feeding gestating rats with high dietary Se (3.0 mg Se/kg) (31). Strikingly, the high-Se induced hyperinsulinemia was concurrent with a downregulation of Akt protein in liver and other tissues. Because Akt is a key protein in the insulin signaling cascade (41), its decrease or dysregulation could cause insulin resistance (47, 48) and thus may serve as one of the primary mechanisms for the high Se-induced hyperinsulinemia (31). Unlike rats (33, 49), pigs fed the high-Se diet (3 mg/kg) did not develop hyperlipidemia compared with those fed 0.3 mg Se/kg. However, the higher Se diet did result in greater protein levels of Gpx1 in heart and testis, Sepp1 in thyroid and testis, Selh in liver and kidney, and Sels in thyroid. Earlier studies indicate that overexpressing Gpx1 impaired insulin synthesis or sensitivity (36, 47), whereas knockout of Gpx1 (48, 50) or Sepp1 (37) genes actually improves body insulin sensitivity. In addition, Selh and Sels might also have the biochemical potential to be involved in glucose metabolism (39, 40, 51–53). Although it is unclear if the upregulations of tissue Gpx1, Sepp1, Selh, and Sels detected in the present study contributed to the induced hyperinsulinemia and/or the downregulation of tissue Akt protein, our findings indicate that pigs can be used to model the possible association of high Se intake with increased risk of diabetes in humans (54). Furthermore, the high sequence homology between pigs and humans in selenoprotein genes reinforces the notion that pigs are a better model than rodents to study Se metabolism in human health (55, 56).
Supplementary Material
Acknowledgments
X.G.L. and H.Z. designed the research; Y.L., H.Z., Q.Z., J.T., Ke Li., X-J.X., K-N.W., Kui Li, and X.G.L. conducted the experiments and analyzed the data; Y.L, H.Z, and X.G.L. wrote the paper; and X.G.L. and H.Z. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported in part by the Chang Jiang Scholars Program of the Chinese Ministry of Education (X-G.L.), the Chinese Natural Science Foundation (30628019, 30700585, and 30871844), and National Institutes of Health DK 53018 (X-G.L.).
Supplemental Tables 1–4 and Figures 1–3 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org
Abbreviations used: BD, basal diet; Ct, cycle threshold; NCBI, National Center for Biotechnology Information.
Literature Cited
- 1.Nesheim MC, Scott ML. Studies of the nutritive effects of selenium for chicks. J Nutr. 1958;65:601–18 [DOI] [PubMed] [Google Scholar]
- 2.Irving JT. Curative effect of selenium upon the incisor teeth of rats deficient in vitamin E. Nature. 1959;184:645–6 [DOI] [PubMed] [Google Scholar]
- 3.Hartley WJ. Selenium and ewe fertility. Proc New Zealand Soc Anim Prod. 1963;23:20–7 [Google Scholar]
- 4.Andrews ED, Hartley WJ, Grant AB. Selenium responsive diseases of animals in New Zealand. N Z Vet J. 1968;16:3–17 [DOI] [PubMed] [Google Scholar]
- 5.Jensen LS. Selenium deficiency and impaired reproduction in Japanese quail. Proc Soc Exp Biol Med. 1968;128:970–2 [Google Scholar]
- 6.McCoy KEM, Weswig PH. Some selenium response in the rat not related to vitamin E. J Nutr. 1969;98:383–9 [DOI] [PubMed] [Google Scholar]
- 7.Thompson JN, Scott ML. Role of selenium in the nutrition of the chick. J Nutr. 1969;97:335–42 [DOI] [PubMed] [Google Scholar]
- 8.Wu SH, Oldfield JE, Muth OH, Whanger PD, Weswig PH. Effect of selenium in reproduction. Proc West Sec Am Soc An Sci. 1969;20:85–9 [Google Scholar]
- 9.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–92 [DOI] [PubMed] [Google Scholar]
- 10.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. JAMA. 1996;276:1957–63 [PubMed] [Google Scholar]
- 11.Clark LC, Dalkin B, Krongrad A, Combs GF, Jr, Turnbul BW, Slate EH, Witherington R, Herlong JH, Janosko E, Carpenter D, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–4 [DOI] [PubMed] [Google Scholar]
- 12.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–54 [DOI] [PubMed] [Google Scholar]
- 13.Jiang C, Jiang W, Ip C, Ganther H, Lu J. Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Mol Carcinog. 1999;26:213–25 [DOI] [PubMed] [Google Scholar]
- 14.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–43 [DOI] [PubMed] [Google Scholar]
- 15.Zhou JC, Zhao H, Li JG, Xia XJ, Wang KN, Zhang YJ, Liu Y, Zhao Y, Lei XG. Selenoprotein gene expression in thyroid and pituitary of young pigs is not affected by dietary selenium deficiency or excess. J Nutr. 2009;139:1061–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang JQ, Li DL, Zhao H, Sun LH, Xia XJ, Wang KN, Luo X, Lei XG. The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr. 2011;141:1605–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunde RA, Raines AM, Barnes KM, Evenson JK. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep. 2009;29:329–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunde RA, Hadley KB. Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med (Maywood). 2010;235:23–31 [DOI] [PubMed] [Google Scholar]
- 19.Barnes KM, Evenson JK, Raines AM, Sunde RA. Transcript analysis of the selenoproteome indicates that dietary selenium requirements of rats based on selenium-regulated selenoprotein mRNA levels are uniformly less than those based on glutathione peroxidase activity. J Nutr. 2009;139:199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunde RA, Raines AM. Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents. Adv Nutr. 2011;2:138–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller ER, Ullrey DE. The pig as a model for human nutrition. Annu Rev Nutr. 1987;7:361–82 [DOI] [PubMed] [Google Scholar]
- 22.Rowan AM, Moughan PJ, Wilson MN, Maher K, Tasman-Jones C. Comparison of the ileal and faecal digestibility of dietary amino acids in adult humans and evaluation of the pig as a model animal for digestion studies in man. Br J Nutr. 1994;71:29–42 [DOI] [PubMed] [Google Scholar]
- 23.Tory PS, William HE, Stephen CD, Patricia M. Wound repair and regeneration: the pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. [DOI] [PubMed]
- 24.Wassen FW, Klootwijk W, Kaptein E, Duncker DJ, Visser TJ, Kuiper GG. Characteristics and thyroid state-dependent regulation of iodothyronine deiodinases in pigs. Endocrinology. 2004;145:4251–63 [DOI] [PubMed] [Google Scholar]
- 25.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–23 [DOI] [PubMed] [Google Scholar]
- 26.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT). JAMA. 2009;301:39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stranges S, Laclaustra M, Ji C, Cappuccio FP, Navas-Acien A, Ordovas JM, Rayman M, Guallar E. Higher selenium status is associated with adverse blood lipid profile in British adults. J Nutr. 2010;140:81–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30:829–34 [DOI] [PubMed] [Google Scholar]
- 29.Mueller AS, Pallauf J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J Nutr Biochem. 2006;17:548–60 [DOI] [PubMed] [Google Scholar]
- 30.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14:2327–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng MS, Li X, Liu Y, Zhao H, Zhou JC, Li K, Huang JQ, Sun LH, Tang JY, Xia XJ, et al. A high-selenium diet induced insulin resistance in gestating rats and their offspring. Free Radic Biol Med. 2012;52:1335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosse AC, Pallauf J, Hommel B, Sturm M, Fischer S, Wolf NM, Mueller AS. Impact of selenite and selenate on differentially expressed genes in rat liver examined by microarray analysis. Biosci Rep. 2010;30:293–306 [DOI] [PubMed] [Google Scholar]
- 33.Mueller AS, Bosse AC, Most E, Klomann SD, Schneider S, Pallauf J. Regulation of the insulin antagonistic protein tyrosine phosphatase 1B by dietary Se studied in growing rats. J Nutr Biochem. 2009;20:235–47 [DOI] [PubMed] [Google Scholar]
- 34.Lei XG, Cheng WH. New roles for an old selenoenzyme: evidence from glutathione peroxidase-1 null and overexpressing mice. J Nutr. 2005;135:2295–8 [DOI] [PubMed] [Google Scholar]
- 35.Burk RF, Hill KE. Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu Rev Nutr. 2005;25:215–35 [DOI] [PubMed] [Google Scholar]
- 36.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101:8852–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misu H, Takamura T, Takayama H, Hayashi H, Matsuzawa-Nagata N, Kurita S, Ishikura K, Ando H, Takeshita Y, Ota T, et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010;12:483–95 [DOI] [PubMed] [Google Scholar]
- 38.Walter PL, Steinbrenner H, Barthel A, Klotz LO. Stimulation of selenoprotein P promoter activity in hepatoma cells by FoxO1a transcription factor. Biochem Biophys Res Commun. 2008;365:316–21 [DOI] [PubMed] [Google Scholar]
- 39.Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, Quick M. Kantham L, Collier GR. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress: SelS is a novel glucose-regulated protein. FEBS Lett. 2004;563:185–90 [DOI] [PubMed] [Google Scholar]
- 40.Novoselov SV, Kryukov GV, Xu XM, Carlson BA, Hatfield DL, Gladyshev VN. Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. J Biol Chem. 2007;282:11960–8 [DOI] [PubMed] [Google Scholar]
- 41.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA. 2003;100:7569–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jongeneel CV. Searching the expressed sequence tag (EST) databases: panning for genes. Brief Bioinform. 2000;1:76–92 [DOI] [PubMed] [Google Scholar]
- 43.Moreno ME, Pe’rez-Conde C, Cámara C. Speciation of inorganic selenium in environmental matrices by flow injection analysis-hydride generation-atomic fluorescence spectrometry. Comparison of off-line, pseudo on-line and on-line extraction and reduction methods. J Anal At Spectrom. 2000;15:681–6 [Google Scholar]
- 44.Lawrence RA, Sunde RA, Schwartz GL, Hoekstra WG. Glutathione peroxidase activity in rat lens and other tissues in relation to dietary selenium intake. Exp Eye Res. 1974;18:563–9 [DOI] [PubMed] [Google Scholar]
- 45.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–54 [DOI] [PubMed] [Google Scholar]
- 46.Behne D, Hilmert H, Scheid S, Gessner H, Elger W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim Biophys Acta. 1988;966:12–21 [DOI] [PubMed] [Google Scholar]
- 47.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–24 [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, Lei XG. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal. 2011;14:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller AS, Klomann SD, Wolf NM, Schneider S, Schmidt R, Spielmann J, Stangl G, Eder K, Pallauf J. Redox regulation of protein tyrosine phosphatase 1B by manipulation of dietary selenium affects the triglyceride concentration in rat liver. J Nutr. 2008;138:2328–36 [DOI] [PubMed] [Google Scholar]
- 50.Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panee J, Stoytcheva ZR, Liu W, Berry MJ. Selenoprotein H is a redox-sensing high mobility group family DNA-binding protein that up-regulates genes involved in glutathione synthesis and phase II detoxification. J Biol Chem. 2007;282:23759–65 [DOI] [PubMed] [Google Scholar]
- 52.Seiderer J, Dambacher J, Kühnlein B, Pfennig S, Konrad A, Török HP, Haller D, Göke B, Ochsenkühn T, Lohse P, et al. The role of the selenoprotein S (SELS) gene -105G>A promoter polymorphism in inflammatory bowel disease and regulation of SELS gene expression in intestinal inflammation. Tissue Antigens. 2007;70:238–46 [DOI] [PubMed] [Google Scholar]
- 53.Gao Y, Walder K, Sunderland T, Kantham L, Feng HC, Quick M, Bishara N, de Silva A, Augert G, Tenne-Brown J, et al. Elevation in Tanis expression alters glucose metabolism and insulin sensitivity in H4IIE cells. Diabetes. 2003;52:929–34 [DOI] [PubMed] [Google Scholar]
- 54.Steinbrenner H, Speckmann B, Pinto A, Sies H. High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr. 2011;48:40–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005;184:455–65 [DOI] [PubMed] [Google Scholar]
- 56.Davis CD. Nutritional interactions: credentialing of molecular targets for cancer prevention. Exp Biol Med (Maywood). 2007;232:176–83 [PubMed] [Google Scholar]
- 57.NCBI BLAST. Internet web site [cited 2009]. Available from: http://blast.ncbi.nlm.nih.gov/Blast.cgi.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.