Abstract
The human diet contains β-carotene as the most abundant precursor of vitamin A, an essential nutrient for embryogenesis. Our laboratory previously showed the importance of β-carotene metabolism via β-carotene-15,15′-oxygenase (CMOI) to support mouse embryonic development. However, the mechanisms regulating embryonic acquisition and utilization of β-carotene from the maternal circulation via placenta remain unknown. We used wild-type (WT) and Lrat−/−Rbp−/− (L−/−R−/−) mice, the latter being a model of marginal vitamin A deficiency. Pregnant dams, fed a nonpurified diet sufficient in vitamin A throughout life, were i.p. supplemented with β-carotene or vehicle at 13.5 d postcoitum (dpc). Effects of this acute maternal supplementation on retinoid and β-carotene metabolism in maternal (serum, liver) and developing tissues (placenta, yolk sac, embryo) were investigated at 14.5 dpc. We showed that, upon supplementation, placental β-carotene concentrations were greater in L−/−R−/− than in WT mice. However, the retinoid (retinol and retinyl ester) concentrations remained unchanged in placenta (and in all other tissues analyzed) of both genotypes upon β-carotene administration. We also showed that upon a single i.p. β-carotene supplementation, placental LDL receptor-related protein (Lrp1) mRNA expression was lower in WT mice, and embryonic CmoI mRNA expression was greater in L−/−R−/− mice. Together, these data suggest a potential role of LRP1 in mediating the uptake of β-carotene across the placenta and that even a marginally impaired maternal vitamin A status may influence uptake and utilization of β-carotene by the placenta and the embryo.
Introduction
Vitamin A is an essential nutrient that is vital for normal embryogenesis (1–3), because its biologically active form, retinoic acid, regulates the transcription of hundreds of genes crucial for growth and development by binding to the nuclear receptor families of retinoic acid receptor and retinoid X receptor (4, 5). β-Carotene is the main vitamin A precursor, mostly found in green leafy vegetables, yellow- and orange-colored vegetables, and orange-colored fruits. The enzymatic cleavage of this provitamin A carotenoid by the action of β-carotene 15,15′ oxygenase (CMOI, also known as BCMOI) or β-carotene 9′,10′ oxygenase (CMOII, also known as BCDO2) gives rise to 2 or 1 molecule(s) of retinaldehyde, respectively (6). Retinaldehyde, in turn, can either be directly oxidized to form retinoic acid or be reduced to retinol and then esterified mainly by lecithin:retinol acyltransferase (LRAT) to generate retinyl esters, the tissue storage form of vitamin A (6). A number of studies support the notion that CMOI is the predominant enzyme generating retinoid (vitamin A and its derivatives) from β-carotene in vivo (6–8).
Although in Western countries, β-carotene contributes ~30% to vitamin A intake, an adequate vitamin A intake requires the contribution of both β-carotene and preformed vitamin A (mainly retinol and retinyl ester from meat and dairy products) (9). Hence, there is a risk of vitamin A insufficiency even in developed countries, especially in certain groups of people with a poor or highly restrictive dietary regimen, such as women of childbearing age or pregnant teenagers (9). On the other hand, β-carotene supplementation of infants and children as well as maternal supplementation before, during, and after pregnancy has been shown to improve neonatal development and growth and attenuate the incidence of premature birth, external birth defects, and infant morbidity and mortality associated with vitamin A deficiency, which is estimated to affect hundreds of millions of people (10). Therefore, furthering our understanding of the mechanisms that regulate retinoid production from β-carotene in the developing tissues in vivo is of utmost importance for human nutrition and health.
The importance of β-carotene metabolism during mammalian embryonic development is supported by a variety of evidence. First, placenta, yolk sac, and embryo express both CmoI and CmoII genes at different stages of development in mice (8, 11, 12), as do humans, in amniotic membranes and fetal heart (13–15). Second, intact β-carotene is present in the mammalian circulation and in humans, a direct correlation has been shown between maternal circulating β-carotene concentrations and those in infant plasma (16, 17). Third, by using a mouse model lacking both CMOI and retinol-binding protein (RBP), the sole specific carrier for retinol in the bloodstream, our laboratory recently demonstrated that intact β-carotene from maternal circulation can reach the developing tissues to be used in situ for de novo synthesis of retinoids, mainly via CMOI action (8). Because β-carotene is transported in the bloodstream in association with lipoproteins (18–20), it is likely that key players involved in lipoprotein uptake may also mediate tissue uptake of this provitamin A carotenoid. Most of the current knowledge regarding the enzymatic activity and the transcriptional regulation of the cleavage enzymes as well as the uptake of β-carotene pertains to adult tissues, such as intestine, liver, and adipose, and derives from studies that have mainly examined the effects of chronic administration of β-carotene (7, 21–25). The current manuscript provides novel insights into the unknown mechanisms that regulate β-carotene uptake and utilization by the developing tissues in response to a single maternal β-carotene supplementation of wild-type (WT)4 and double knockout mice lacking both Lrat and Rbp fed a nonpurified, vitamin A-sufficient diet.
Experimental Procedures
Mice and β-carotene supplementation.
WT and Lrat−/−Rbp−/− (L−/−R−/−double knockout mice (26) were used for our study, with the latter being a model of marginal vitamin A deficiency (26). All mice employed in this study were from a mixed genetic background (C57/BL6 × 129sv). Both diet and water were consumed ad libitum and mice were maintained on a 12-h-light/-dark cycle with the period of darkness between 1900 and 0700. Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (27) and were approved by the Rutgers University Institutional Committee on Animal Care.
WT and L−/−R−/−mice were fed a nonpurified diet (Supplemental Table 1; Prolab Isopro RMH3000 5p75; energy from protein, fat, and carbohydrates: 26, 14, and 60%, respectively; vitamin A: 9.98 μg/g of diet; β-carotene: from trace to 2.6 μg/g of diet) manufactured by LabDiet (W.F. Fisher and Son). Note that mice lacking RBP rely on dietary vitamin A to support normal embryonic development and do not breed if fed a diet containing <7.57 μg vitamin A/g diet (8, 28). To be consistent with the nutritional requirements of the mice with an RBP knockout background, which represents the majority of the genetically modified strains in our colony, we fed our mice a diet containing vitamin A levels higher than those recommended (29). Other laboratories use a similar concentration of vitamin A in the mouse diet and define this diet as “copious in vitamin A” (30). Furthermore, note that the diet used in this study was different (same manufacturer, different product) from the diets used in previously published papers from our laboratory. Three-month-old female mice were mated with males of the respective genotype. At the time of detecting a vaginal plug [set as 0.5 d postcoitum (dpc), the onset of gestation], females continued with this diet and were randomly assigned to the vehicle (veh)- or β-carotene (bc)–treated group. Following the study performed by Glise et al. (31), we added 50 mg of β-carotene in a 5-mL vehicle mixture of ethanol, cremophor (Sigma), and PBS (1:11:18 ratio) under red light, resulting in an emulsion of 2–5 g β-carotene/L based on the reading from the spectrophotometer at a wavelength of 450 nm. At 13.5 dpc, dams were given a 250-μL i.p. injection of this emulsion, which yielded a dose of ~20–50 μg β-carotene/g body weight. This resulted in serum β-carotene concentrations ranging from 4.3–12.3 μmol/L. Veh-assigned dams were injected with 250 μL of the vehicle mixture described above. All mice were killed at 14.5 dpc by CO2 asphyxiation between the hours of 0900 and 1100. Maternal serum and liver as well as placenta, yolk sac, and embryos were collected, frozen, and stored at −80°C until further analyses.
HPLC.
Measurements of serum and tissue (liver, placenta, yolk sac, and embryo) retinol, retinyl ester, and β-carotene concentrations were performed by reverse-phase HPLC analysis as previously described (8,32).
RNA extraction, cDNA synthesis, and qPCR.
RNA extraction, cDNA synthesis, and qPCR were performed as previously described (8). To determine changes in gene expression, the ΔΔCt method was used. All groups were expressed as fold of the control group (WT +veh). The primer list and amplicon size is reported in Supplemental Table 2.
Statistical analyses.
All the data were tested for normal distribution using the Shapiro-Wilk test. When the data were normally distributed, comparisons between genotype and supplementation were evaluated using a 2-way ANOVA for HLPC retinoids and qPCR followed by the Fisher’s least significance difference post hoc test. For serum retinyl ester concentrations, which were not normally distributed, comparisons among genotypes and supplementation groups were evaluated by the Kruskal Wallis test, followed by a Mann-Whitney test. For β-carotene concentrations, comparisons between the 2 genotypes were analyzed by Student’s t test. Comparisons between the 2 treatments within the WT strain for tissue retinyl ester concentrations were also analyzed by Student’s t test, given that retinyl esters are undetectable in the L−/−R−/− strain (26). Analyses were performed with SPSS statistical software (IBM SPSS Statistics, version 19). P < 0.05 was considered significant.
Results
Retinoid and β-carotene concentrations in maternal and developing tissues.
In agreement with a previous report from our laboratory (8), we showed that β-carotene, injected at 13.5 dpc in WT and L−/−R−/− dams fed a nonpurified diet, was detected at 14.5 dpc in maternal liver, placenta, yolk sac, and embryos by HPLC analysis (Table 1). Only the placental β-carotene concentration was greater in the L−/−R−/− +bc group than in the WT +bc group (P < 0.05). The β-carotene concentration did not differ between genotypes in other tissues (Table 1). Note that β-carotene was undetectable in serum and tissues of veh-treated mice, as previously reported [data not shown; (8)].
TABLE 1.
| Liver | Placenta | Yolk sac | Embryo | |
| nmol/g | nmol/g | nmol/g | nmol/g | |
| WT +bc | 1730 ± 780 | 15.9 ± 4.4 | 4.60 ± 2.4 | 0.34 ± 0.14 |
| n | 9 | 21 | 8 | 25 |
| L−/−R−/− +bc | 1320 ± 424 | 30.7 ± 6.1* | 2.70 ± 0.7 | 0.37 ± 0.14 |
| n | 4 | 6 | 6 | 6 |
| P value | 0.35 | <0.001 | 0.22 | 0.65 |
Data are mean ± SD. Analyses of placenta and embryos include more than one from each litter. Yolk sacs were analyzed as pools of 3–4 individual yolk sacs/litter and more than 1 pool/litter was analyzed. *Different from WT +bc, P < 0.05 (t test). bc, β-carotene treated; L−/−R−/−, Lrat−/−Rbp−/−; veh, vehicle treated; WT, wild-type.
bc concentrations in veh-treated groups were analyzed and were below the detection limit (<0.186 nmol/g).
β-Carotene supplementation did not affect maternal serum and hepatic retinoid (retinol and retinyl esters) concentrations compared with the veh-treated dams, regardless of the genotype (Table 2). The serum and hepatic retinol and retinyl ester concentrations of the L−/−R−/− mice were as previously reported (26).
TABLE 2.
Maternal serum and hepatic retinoid concentrations 24 h after veh or bc supplementation of WT and L−/−R−/− dams12
| Groups |
P value |
||||||
| Tissues | WT +veh | WT +bc | L−/−R−/− +veh | L−/−R−/− +bc | G | T | G x T |
| Serum, μmol/L | |||||||
| n | 6 | 8 | 7 | 7 | |||
| Retinol | 0.18 ± 0.03 | 0.24 ± 0.07 | 0.30 ± 0.10 | 0.20 ± 0.10 | 0.14 | 0.53 | 0.08 |
| Retinyl esters | n.d.–0.13 | n.d.–0.25 | n.d. | n.d. | n.a. | n.a. | n.a. |
| Liver, μmol/g | |||||||
| n | 6 | 9 | 7 | 4 | |||
| Retinol | 0.06 ± 0.02b | 0.10 ± 0.05b | 0.02 ± 0.01a | 0.02 ± 0.01a | <0.001 | 0.23 | 0.25 |
| Retinyl esters | 10.5 ± 2 | 11.4 ± 3 | n.d. | n.d. | n.a. | 0.52 | n.a. |
Data are mean ± SD or range (serum retinyl esters). bc, β-carotene treated; L−/−R−/−, Lrat−/−Rbp−/−; n.a., P value not present due to samples being below the detection limit; n.d., below detection limit (serum <0.004 nmol/L and tissues <3.5 nmol/g); veh, vehicle treated; WT, wild-type.
Statistical analysis by 2-way ANOVA with G (genotype) × T (treatment) as factors. Labeled means within a row without a common letter differ significantly, P < 0.05.
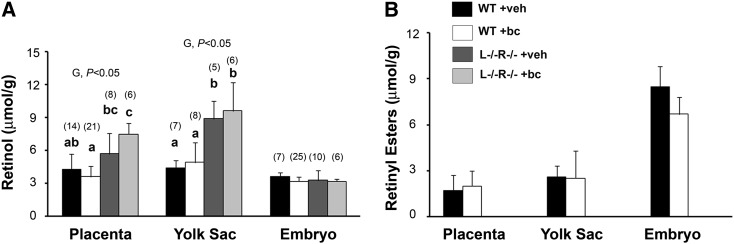
Maternal β-carotene supplementation in WT mice did not alter retinol or retinyl ester concentrations in embryonic or in extra-embryonic tissues compared with the WT +veh group (Fig. 1). Interestingly though, whereas the embryonic retinol concentration was similar between the 2 strains irrespective of the maternal supplementation (Fig. 1A), the yolk sac retinol concentration was greater in the L−/−R−/− mice than in the WT mice (P < 0.05) regardless of the maternal treatment (Fig. 1A). In the case of the L−/−R−/− strain, the placental retinol concentration did not differ upon maternal β-carotene supplementation. Furthermore, the retinol concentration was greater in L−/−R−/− +bc placenta than in WT +bc placenta (P < 0.05) (Fig. 1A). As expected, due to the lack of LRAT, retinyl esters were not detectable in developing tissues of the double knockout strain irrespective of treatment (Fig. 1B).
FIGURE 1.
Placental, yolk sac, and embryonic retinol (A) and retinyl ester (B) concentrations at 14.5 dpc, 24 h after veh or bc supplementation of WT and L−/−R−/− dams. Data are mean ± 95%CI. Sample size (n) analyzed for different groups and tissues is indicated in parentheses above the bar. Analyses of placenta and embryo include more than one for each litter. Yolk sacs were analyzed as pools of 3–4 individual yolk sacs/litter and more than 1 pool/litter was analyzed. Retinol and retinyl ester concentrations were obtained from the same sample. Statistical analysis was carried out by a 2-way ANOVA, with G (genotype) × T (treatment) as factors. Placental retinol concentrations were not normally distributed and analyzed by Kruskal Wallis. Differences in retinyl ester concentrations were analyzed by t test. Labeled means without a common letter within each tissue differ, P < 0.05. bc, β-carotene treated; dpc, days postcoitum; L−/−R−/−, Lrat−/−Rbp−/−; veh, vehicle treated; WT, wild type.
Taken together, the HPLC data indicate that under a normal maternal vitamin A status (e.g., in the WT mice), a single maternal administration of β-carotene does not perturb retinoid concentrations in maternal and developing tissues. However, a marginally impaired maternal vitamin A status, like that of the L−/−R−/− mice, may affect only placental uptake of β-carotene.
Expression of genes controlling β-carotene cleavage.
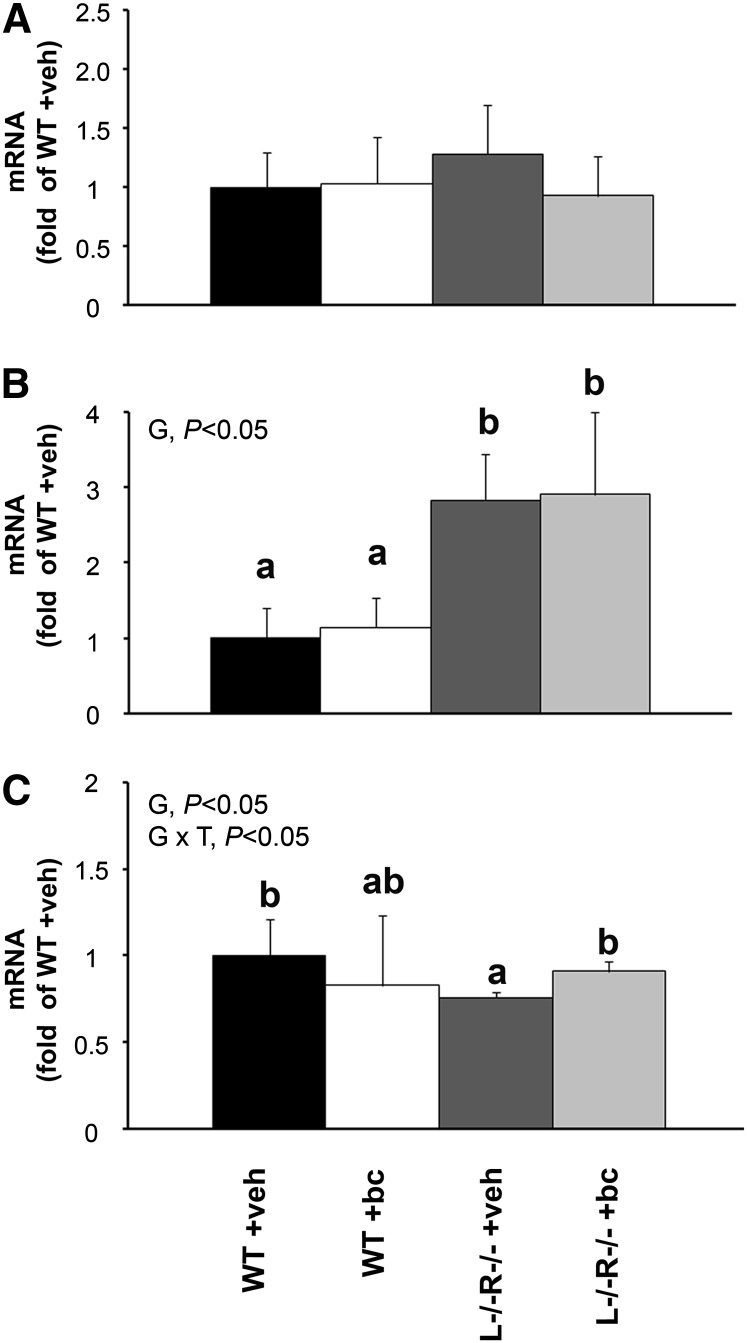
Maternal hepatic expression of CmoI 24 h following a single administration of β-carotene did not significantly differ between the experimental groups (Fig. 2A). Placental (Fig. 2B) and embryonic (Fig. 2C) expression following CmoI mRNA did not show any significant difference on β-carotene supplementation among the WT groups. Interestingly, placental CmoI expression was greater in the L−/−R−/− strain than in the WT mice (P < 0.05), even though no further upregulation was observed upon maternal β-carotene supplementation (Fig. 2B). In contrast to placenta, embryonic CmoI mRNA expression was lower in the L−/−R−/− +veh group than in the WT +veh group (P < 0.05) (Fig. 2C). However, in the double knockout strain, embryonic mRNA expression of CmoI was greater in L−/−R−/− +bc than in L−/−R−/− +veh (P < 0.05) (Fig. 2C).
FIGURE 2.
qPCR analysis of CmoI expression in maternal liver (A), placenta (B), and embryo (C) 24 h after veh or bc supplementation of WT and L−/−R−/− dams. Tissues from WT +veh set as a calibrator at 1. Data are mean ± SD fold of WT +veh. Sample size for different groups and tissues is n = 5–7. Statistical analysis was carried out by a 2-way ANOVA, with G (genotype) × T (treatment) as factors. Labeled means without a common letter within each tissue differ, P < 0.05. Figure represents data from one experiment out of 2 repeats. bc, β-carotene treated; L−/−R−/−, Lrat−/−Rbp−/−; veh, vehicle treated; WT, wild type.
Taken together, these results suggest that an acute administration of β-carotene does not perturb the provitamin A processing in placenta and embryo from dams with a normal vitamin A status, e.g., in the WT mice.
Placental uptake of β-carotene.
mRNA expression of LDL receptor-related protein 1 (Lrp1) was lower in the placenta of the WT +bc group than in the WT +veh group (P < 0.05) (Fig. 3). This downregulation did not take place in placenta from L−/−R−/− +bc dams (Fig. 3). However, placental expression of Lrp1 was lower in L−/−R−/− +veh group than in the WT +veh group (P < 0.05) (Fig. 3). Interestingly, in placenta from the L−/−R−/− +bc dams, mRNA expression of lipoprotein lipase (Lpl) was greater than in placenta from the L−/−R−/− +veh group (P < 0.05) as well as from WT +bc dams (P < 0.05) (Fig. 3). Expression of LDL receptor (Ldlr) and Scavenger receptor-b1 (Srb1) mRNA did not differ regardless of genotype and treatment (Fig. 3).
FIGURE 3.
qPCR analysis of placental Srb1, Ldlr, Lrp1, and Lpl mRNA expression 24 h after veh or bc supplementation of WT and L−/−R−/− dams. Tissues from WT +veh set as a calibrator at 1. Data are mean ± SD fold of WT +veh. Sample size for different groups and tissues is n = 5–7. Statistical analysis was carried out by a 2-way ANOVA, with G (genotype) × T (treatment) as factors. Labeled means without a common letter within each gene differ, P < 0.05. Figure represents data from one experiment of 2 repeats. bc, β-carotene treated; L−/−R−/−, Lrat−/−Rbp−/−; veh, vehicle treated; WT, wild-type.
Overall, these data suggest a potential role of LRP1 and LPL in mediating the uptake of maternal circulating intact β-carotene by the placenta.
Discussion
Recent studies from our laboratory have unequivocally shown the importance of β-carotene and its metabolism during mammalian embryonic development (8). The current manuscript expands this research by specifically evaluating the effects of a single maternal supplementation of β-carotene at mid-gestation, first, on retinoid concentrations in maternal and embryonic tissues; second, on provitamin A processing by maternal and developing tissues; and third, on placental β-carotene uptake. In addition to WT mice, we took advantage of mice lacking both Lrat and Rbp (L−/−R−/−) as a model of marginal vitamin A deficiency (26) to gain insights into whether the maternal vitamin A status would influence the above-mentioned metabolic processes. These mice are considered marginally vitamin A deficient, because the retinoid content of their tissues is limiting, even though when maintained on a regimen of sufficient vitamin A intake they may not show signs of overt deficiency of the vitamin.
Because developing tissues can metabolize β-carotene in situ (8) and chronic β-carotene intake has been shown to affect retinoid homeostasis in adult tissues (7, 33), we sought to investigate the effects of an acute maternal β-carotene administration on retinoid concentrations during mammalian development. The mouse embryo at mid-gestation, when the majority of the organogenesis is completed (34), can be considered “metabolically” active and capable of regulating its own retinoid metabolism (26, 35, 36). In addition, the genes for the β-carotene cleavage enzymes CMOI and CMOII (8) as well as the genes for proteins involved in tissue lipoprotein uptake (36) are expressed at this stage of development in both embryonic and extra-embryonic tissues. Hence, we chose to perform the maternal β-carotene supplementation at mid-gestation, because we hypothesized that at this stage of development, the embryo would also be capable of regulating its own β-carotene metabolism. We confirmed that intact β-carotene is taken up by both maternal (liver) and developing tissues (placenta, yolk sac, and embryo). A gradient of tissue β-carotene concentration was observed from the maternal liver to the embryo, with the former tissue being the one with the highest concentration of the provitamin A carotenoid. These results support the notion that the liver is the main site of β-carotene uptake and storage (21, 37, 38), and are in agreement with the literature suggesting that placenta and yolk sac act as a barrier to regulate the uptake and transfer of nutrients to the fetus, including retinoids and their carotenoid precursors (39). In contrast to previous reports of adult tissues of mice chronically fed with β-carotene (7, 33), our data showed that a single i.p. dose of β-carotene administered to the dams 24 h prior to killing did not affect retinoid homeostasis either in maternal or developing tissues of the WT mice. We therefore wondered whether this was the result of some regulatory mechanism aimed at downregulating β-carotene conversion into retinoid via CMOI action, under a normal vitamin A status, such as that of the WT mice. Data from the literature indicate that β-carotene supplementation regulates intestinal CmoI expression by a transcriptional feedback mechanism mediated by retinoic acid (25). Also, β-carotene supplementation has been shown to induce CmoI expression in inguinal white adipose tissue in a mouse model of vitamin A deficiency (24, 40), and feeding with lycopene, a nonprovitamin carotenoid, downregulated CmoI expression in adrenal gland and kidney of rats (41). Here, we demonstrate that a single i.p. β-carotene supplementation does not perturb the transcription of CmoI either in maternal (liver) or developing (placenta or embryo) tissues of WT mice. We hypothesize that this discrepancy may be due to the length of our supplementation protocol compared with previously published reports (i.e., acute vs. chronic). Nevertheless, our data do not rule out an effect of β-carotene availability on CMOI enzymatic activity (23).
The rather scarce knowledge regarding how tissues acquire β-carotene from the bloodstream pertains to adult tissues (19, 42, 43) and the molecular mechanisms that regulate the uptake of β-carotene at the placental barrier have not been investigated to date. β-Carotene circulates in the bloodstream in association with lipoprotein particles like chylomicrons and their remnants, VLDL, LDL, and even HDL (19, 20, 44). Therefore, its tissue uptake is postulated to be mediated by the same key regulators involved in lipoprotein uptake (19, 20, 44), such as SRB1, LDLR, LRP1, and LPL. We hypothesized that the β-carotene i.p. injected into the dams would transfer to the above-mentioned lipoproteins, which are all present in the bloodstream of constantly fed mice, like those used for this study, and thus would be available for placental and embryonic uptake. The transcriptional downregulation of Lrp1, in the placenta of supplemented WT dams, strongly suggests the involvement of this receptor in placental β-carotene uptake. Indeed, LRP1 has high affinity for apoE-containing lipoproteins, such as VLDL, HDL, and chylomicrons (45, 46). A similar effect on Lrp1 transcription was not observed on maternal liver (data not shown), suggesting a tissue-specific response. We interpret this downregulation as a feedback mechanism that prevents the placenta from acquiring excessive maternal circulating β-carotene when the dams are on a regimen of sufficient vitamin A intake. Placental LRP1 protein levels, however, did not change upon maternal β-carotene supplementation (data not shown). At the moment, we cannot exclude that changes in LRP1 protein levels could occur at earlier or later time points or that a prolonged maternal β-carotene supplementation is needed to induce an effect on placental LRP1 protein.
We previously demonstrated that placental LRP1 is the main player acting in concert with LPL and activated to respond to chronic changes in vitamin A content of the maternal diet (36). The failure to detect changes in WT placenta mRNA expression for Lpl as well as Srb1 and Ldlr in our experimental conditions does not rule out their possible involvement in β-carotene uptake at the placental barrier under different dietary conditions, such as prolonged maternal provitamin A supplementation, different regimens of maternal vitamin A intake, or in a feed-deprived state, for instance.
Once taken up by the placenta, β-carotene can be also transferred in its intact form to the embryo to be used as a “local” source of retinoic acid (8). By using qPCR analysis, we examined the above-mentioned genes in pools of mRNA from WT embryos to determine if they were involved in the uptake of β-carotene by the embryo per se. Our analysis did not show any significant difference in the embryonic mRNA expression of Lpl, Srb1, Lrp1, and Ldlr among our experimental groups (data not shown), suggesting that the regulation of β-carotene uptake may not be taking place at the embryonic level, at least when WT dams were administered a single dose of the provitamin A carotenoid. It remains to be established whether the transfer of β-carotene from placenta to embryo through the secretion of placental lipoproteins into the fetal circulation (47-49) could also be affected by β-carotene availability.
Due to their extremely low concentration of hepatic retinol stores, the lack of serum RBP and thus their increased susceptibility to develop signs of vitamin A deficiency, the L−/−R−/− mice can be considered a model of marginal vitamin A deficiency when maintained on a dietary regimen of sufficient vitamin A intake (26, 50). Only the placental β-carotene concentration was elevated in the L−/−R−/− mice compared with the WT mice, suggesting that the maternal vitamin A status may control uptake of intact β-carotene by this organ that regulates the exchange of gas and nutrients between mother and fetus (51). We previously showed that LPL facilitates placental uptake of postprandial retinoids (36). The upregulation of Lpl mRNA expression upon β-carotene supplementation supports our previous studies (36) and may highlight a critical role of LPL in mediating placental uptake of the provitamin A carotenoid in this strain. In the double knockout placenta, Lrp1 expression does not seem to be involved in responding to changes in β-carotene availability, as was seen in WT +bc. Furthermore, the baseline mRNA expression of Lrp1 in the double knockout placenta was significantly lower compared with those of WT mice. The reasons for these differences are unclear and currently under investigation.
Despite more β-carotene being taken up by the placenta of L−/−R−/− mice compared with WT mice, the placental retinol concentration did not change upon supplementation in this strain but was higher than in WT +bc. In addition, the placental mRNA expression of CmoI was elevated in the double knockout mice regardless of the maternal treatment. We interpret our data as the result of a compensatory mechanism that, under a marginal vitamin A-deficient status, such as that of the double knockout mice (50, 52), would maximize the conversion of the β-carotene acquired from the maternal circulation into retinol via CMOI action to ultimately increase the amount of retinol available for transfer to the embryo. This is in agreement with an earlier study in humans that suggested that β-carotene could be a precursor of retinol in placenta and that its conversion could be dependent on the nutritional status of the mother, being particularly efficient in a more depleted state (53). Nonetheless, the embryonic retinol concentration was similar among our experimental groups, regardless of genotype or treatment. We propose 2 possibilities to account for the difference in extra-embryonic retinol available for transfer toward the developing tissues compared with embryonic retinol concentration in the L−/−R−/− strain. First, because these mice lack RBP, the transport of retinol out of the placenta trophoblast toward the embryo could be impaired (36). Second, we previously showed that despite their inability to store vitamin A, increased retinoic acid oxidation via CYP26A1 and intracellular retinol efflux via STRA6 ensure adequate levels of retinoids in the developing tissues of the L−/−R−/− mice (26). So, these highly effective homeostatic mechanisms could result in similar embryonic retinol concentrations despite different metabolic pathways of tissue retinoid utilization.
Although more β-carotene was taken up by the placenta of the L−/−R−/− mice compared with WT, similar β-carotene concentrations were detected in WT and double knockout embryos from supplemented mothers. Interestingly, embryonic transcription of CmoI was upregulated upon maternal β-carotene treatment in the double knockout strain, suggesting that an increased rate of utilization of β-carotene in the embryo could account for the above-mentioned discrepancy. However, we cannot rule out that the placenta could transport β-carotene based on the need of the fetus. Indeed, although the L−/−R−/− mice could be considered marginally vitamin A deficient, they are viable and fertile and their embryos are phenotypically normal when fed a vitamin A–sufficient diet (26).
Finally, it is intriguing that in the absence of Lrat, as in the double knockout strain, the embryonic baseline mRNA expression of CmoI was lower than that in WT mice. Because we previously showed that Lrat embryonic mRNA expression was attenuated in the absence of CmoI (8), these data may suggest the existence of a reciprocal interference between CMOI and LRAT on the transcriptional regulation of each other. The molecular mechanisms and the biological significance of such interactions are unknown at the moment, but this effect seems to be tissue specific, because the mRNA baseline expression of CmoI was significantly elevated in placenta lacking LRAT compared with WT.
This study provides novel evidence that placental β-carotene uptake and its processing by developing tissues are influenced by β-carotene availability and maternal vitamin A status. It also identifies, for the first time, to our knowledge, LRP1 and LPL as potential molecular mediators of provitamin A uptake at the placenta-fetal barrier, at least in the fed state. These results further our knowledge on maternal-fetal nutrition and metabolism of this essential vitamin and may ultimately help in establishing adequate levels of β-carotene intake during pregnancy.
Supplementary Material
Acknowledgments
L.Q. and L.W. designed research; L.W., V.S., A.H., and E.S. conducted research; L.W. analyzed data; L.Q. and L.W. wrote the paper; and L.Q. had the primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by grants R01HD057493 and R01HD057493-02S1 from the U.S. NIH to L.Q.
Author disclosures: L. Wassef, V. Shete, A. Hong, E. Spiegler, and L. Quadro, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: bc, β-carotene treated; dpc, days postcoitum; L−/−R−/−, Lrat−/−Rbp−/−; veh, vehicle treated; WT, wild-type.
Literature Cited
- 1.Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morriss-Kay GM, Ward SJ. Retinoids and mammalian development. Int Rev Cytol. 1999;188:73–131 [DOI] [PubMed] [Google Scholar]
- 3.Zile MH. Vitamin A and embryonic development: an overview. J Nutr. 1998;128 Suppl 2:S455–8 [DOI] [PubMed] [Google Scholar]
- 4.Amann PM, Eichmuller SB, Schmidt J, Bazhin AV. Regulation of gene expression by retinoids. Curr Med Chem. 2011;18:1405–12 [DOI] [PubMed] [Google Scholar]
- 5.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30 [DOI] [PubMed] [Google Scholar]
- 6.Lobo GP, Amengual J, Palczewski G, Babino D, von Lintig J. Mammalian carotenoid-oxygenases: key players for carotenoid function and homeostasis. Biochim Biophys Acta. 2012;1821:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amengual J, Gouranton E, van Helden YG, Hessel S, Ribot J, Kramer E, Kiec-Wilk B, Razny U, Lietz G, Wyss A, et al. Beta-carotene reduces body adiposity of mice via BCMO1. PLoS ONE. 2011;6:e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YK, Wassef L, Chung S, Jiang H, Wyss A, Blaner WS, Quadro L. beta-Carotene and its cleavage enzyme beta-carotene-15,15'-oxygenase (CMOI) affect retinoid metabolism in developing tissues. FASEB J. 2011;25:1641–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK. Beta-carotene is an important vitamin A source for humans. J Nutr. 2010;140:S2268–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West KP, Jr, Christian P, Labrique AB, Rashid M, Shamim AA, Klemm RD, Massie AB, Mehra S, Schulze KJ, Ali H, et al. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: a cluster randomized trial. JAMA. 2011;305:1986–95 [DOI] [PubMed] [Google Scholar]
- 11.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15'-dioxygenase. J Biol Chem. 2001;276:6560–5 [DOI] [PubMed] [Google Scholar]
- 12.Paik J, During A, Harrison EH, Mendelsohn CL, Lai K, Blaner WS. Expression and characterization of a murine enzyme able to cleave beta-carotene. The formation of retinoids. J Biol Chem. 2001;276:32160–8 [DOI] [PubMed] [Google Scholar]
- 13.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9′,10'-monooxygenase in human tissues. J Histochem Cytochem. 2005;53:1403–12 [DOI] [PubMed] [Google Scholar]
- 14.Lindqvist A, Andersson S. Cell type-specific expression of beta-carotene 15,15'-mono-oxygenase in human tissues. J Histochem Cytochem. 2004;52:491–9 [DOI] [PubMed] [Google Scholar]
- 15.von Lintig J, Hessel S, Isken A, Kiefer C, Lampert JM, Voolstra O, Vogt K. Towards a better understanding of carotenoid metabolism in animals. Biochim Biophys Acta. 2005;1740:122–31 [DOI] [PubMed] [Google Scholar]
- 16.Yeum KJ, Ferland G, Patry J, Russell RM. Relationship of plasma carotenoids, retinol and tocopherols in mothers and newborn infants. J Am Coll Nutr. 1998;17:442–7 [DOI] [PubMed] [Google Scholar]
- 17.Scaife AR, McNeill G, Campbell DM, Martindale S, Devereux G, Seaton A. Maternal intake of antioxidant vitamins in pregnancy in relation to maternal and fetal plasma levels at delivery. Br J Nutr. 2006;95:771–8 [DOI] [PubMed] [Google Scholar]
- 18.During A, Hussain MM, Morel DW, Harrison EH. Carotenoid uptake and secretion by CaCo-2 cells: beta-carotene isomer selectivity and carotenoid interactions. J Lipid Res. 2002;43:1086–95 [DOI] [PubMed] [Google Scholar]
- 19.Harrison EH. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim Biophys Acta. 2012;1821:70–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribaya-Mercado JD, Lopez-Miranda J, Ordovas JM, Blanco MC, Fox JG, Russell RM. Distribution of beta-carotene and vitamin A in lipoprotein fractions of ferret serum. Effect of beta-carotene supplementation. Ann N Y Acad Sci. 1993;691:232–7 [DOI] [PubMed] [Google Scholar]
- 21.Gugger ET, Bierer TL, Henze TM, White WS, Erdman JW., Jr Beta-carotene uptake and tissue distribution in ferrets (Mustela putorius furo). J Nutr. 1992;122:115–9 [DOI] [PubMed] [Google Scholar]
- 22.Takitani K, Miyazaki H, Fukunishi S, Takaya R, Yoden A, Higuchi K, Tamai H. Altered expression of both beta-carotene 15,15' monooxygenase and lecithin:retinol acyltransferase in obese Zucker rats. J Nutr Sci Vitaminol (Tokyo). 2011;57:108–13 [DOI] [PubMed] [Google Scholar]
- 23.van Vliet T, van Vlissingen MF, van Schaik F, van den Berg H. beta-Carotene absorption and cleavage in rats is affected by the vitamin A concentration of the diet. J Nutr. 1996;126:499–508 [DOI] [PubMed] [Google Scholar]
- 24.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, et al. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15'-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283:4905–11 [DOI] [PubMed] [Google Scholar]
- 25.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YK, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin: retinol acyltransferase (LRAT) is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem. 2008;283:5611–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NRC. Guide for the care and use of laboratory animals. 7th ed. Washington, DC: National Academy Press; 1996.
- 28.Quadro L, Hamberger L, Gottesman ME, Colantuoni V, Ramakrishnan R, Blaner WS. Transplacental delivery of retinoid: the role of retinol-binding protein and lipoprotein retinyl ester. Am J Physiol Endocrinol Metab. 2004;286:E844–51 [DOI] [PubMed] [Google Scholar]
- 29.Ross AC. Diet in vitamin A research. Methods Mol Biol. 2010;652:295–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kane MA, Folias AE, Pingitore A, Perri M, Krois CR, Ryu JY, Cione E, Napoli JL. CrbpI modulates glucose homeostasis and pancreas 9-cis-retinoic acid concentrations. Mol Cell Biol. 2011;31:3277–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glise D, Riondel J, Favier A. Comparative distribution of beta-carotene and lycopene after intraperitoneal administration in mice. In Vivo. 1998;12:447–54 [PubMed] [Google Scholar]
- 32.Kim YK, Quadro L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol Biol. 2010;652:263–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fierce Y, de Morais Vieira M, Piantedosi R, Wyss A, Blaner WS, Paik J. In vitro and in vivo characterization of retinoid synthesis from beta-carotene. Arch Biochem Biophys. 2008;472:126–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossant J, Tam PPL. Mouse development: patterning, morphogenesis and organogenesis. San Diego: Academic; 2002. [Google Scholar]
- 35.Spiegler E, Kim YK, Wassef L, Shete V, Quadro L. Maternal-fetal transfer and metabolism of vitamin A and its precursor beta-carotene in the developing tissues. Biochim Biophys Acta. 2012;1821:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wassef L, Quadro L. Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem. 2011;286:32198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker RS. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10:542–51 [PubMed] [Google Scholar]
- 38.Shmarakov I, Fleshman MK, D'Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, Jr, von Lintig J, Rubin LP, Harrison EH, et al. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch Biochem Biophys. 2010;504:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marceau G, Gallot D, Lemery D, Sapin V. Metabolism of retinol during mammalian placental and embryonic development. Vitam Horm. 2007;75:97–115 [DOI] [PubMed] [Google Scholar]
- 40.Lobo GP, Amengual J, Li HN, Golczak M, Bonet ML, Palczewski K, von Lintig J. Beta,beta-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a beta,beta-carotene oxygenase 1-dependent manner. J Biol Chem. 2010;285:27891–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaripheh S, Nara TY, Nakamura MT, Erdman JW., Jr Dietary lycopene downregulates carotenoid 15,15'-monooxygenase and PPAR-gamma in selected rat tissues. J Nutr. 2006;136:932–8 [DOI] [PubMed] [Google Scholar]
- 42.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 2005;44:4517–25 [DOI] [PubMed] [Google Scholar]
- 44.Johnson EJ, Russell RM. Distribution of orally administered beta-carotene among lipoproteins in healthy men. Am J Clin Nutr. 1992;56:128–35 [DOI] [PubMed] [Google Scholar]
- 45.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–4 [DOI] [PubMed] [Google Scholar]
- 47.Rebholz SL, Burke KT, Yang Q, Tso P, Woollett LA. Dietary fat impacts fetal growth and metabolism: uptake of chylomicron remnant core lipids by the placenta. Am J Physiol Endocrinol Metab. 2011;301:E416–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woollett LA. Review: transport of maternal cholesterol to the fetal circulation. Placenta. 2011;32 Suppl 2:S218–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen EM, Lindegaard ML, Andersen CB, Damm P, Nielsen LB. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J Biol Chem. 2004;279:55271–6 [DOI] [PubMed] [Google Scholar]
- 50.Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–34 [DOI] [PubMed] [Google Scholar]
- 51.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda). 2005;20:180–93 [DOI] [PubMed] [Google Scholar]
- 52.Liu L, Tang XH, Gudas LJ. Homeostasis of retinol in lecithin: retinol acyltransferase gene knockout mice fed a high retinol diet. Biochem Pharmacol. 2008;75:2316–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dimenstein R, Trugo NM, Donangelo CM, Trugo LC, Anastacio AS. Effect of subadequate maternal vitamin-A status on placental transfer of retinol and beta-carotene to the human fetus. Biol Neonate. 1996;69:230–4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.