Abstract
Many human milk glycans inhibit pathogen binding to host receptors and their consumption by infants is associated with reduced risk of disease. Salmonella infection is more frequent among infants than among the general population, but the incidence is lower in breast-fed babies, suggesting that human milk could contain components that inhibit Salmonella. This study aimed to test whether human milk per se inhibits Salmonella invasion of human intestinal epithelial cells in vitro and, if so, to identify the milk components responsible for inhibition. Salmonella enterica serovar Typhimurium SL1344 (SL1344) invasion of FHs 74 Int and Caco-2 cells were the models of human intestinal epithelium infection. Internalization of fluorescein-5-isothiocyanate–labeled SL1344 into intestinal cells was measured by flow cytometry to quantify infection. Human milk and its fractions inhibited infection; the inhibitory activity localized to the high molecular weight glycans. Mucin 1 and mucin 4 were isolated to homogeneity. At 150 μg/L, a typical concentration in milk, human milk mucin 1 and mucin 4 inhibited SL1344 invasion of both target cell types. These mucins inhibited SL1344 invasion of epithelial cells in a dose-dependent manner. Thus, mucins may prove useful as a basis for developing novel oral prophylactic and therapeutic agents that inhibit infant diseases caused by Salmonella and related pathogens.
Introduction
Human milk is widely accepted as containing the optimum nutrients for most infants, while it simultaneously conveys immunologic and other health benefits (1). Breast-fed infants have lower risk of enteric disease than those artificially fed. A number of human milk glycans have been identified that contribute to the protection of infants through inhibition of pathogen binding to host cell membranes (2). Many of the most common human enteropathogens are inhibited by human milk glycans, but only limited information is available regarding inhibition of Salmonella species.
Salmonella is among the most commonly recognized enteric pathogens, estimated to cause 1.4 million illnesses and 400 deaths each year in the United States (3). Infection by Salmonella is more frequent in children <1 y of age than among older age groups (3, 4). The treatment for Salmonella infection is primarily antibiotic therapy, but resistant strains abound. Even when antibiotic therapy is effective against Salmonella, the treatment can also affect normal microbiota of the infant intestine, thereby inducing risk of secondary enteric disturbances (5). Thus, there is an urgent need to develop alternative safe and effective therapies against Salmonella infection in infants.
One promising approach is to search for molecules in human milk that specifically inhibit Salmonella invasion of human intestinal epithelial cells. The binding of an undefined clinical isolate Salmonella typhimurium to human HeLa epithelial cells was partially inhibited by a crude human milk glycan fraction; this inhibition was attributed to the free secretory component and lactoferrin of milk (6). We previously found that a mucin-associated molecule of human milk can bind to rotavirus and inhibit viral replication (7). Recently, a bovine milk molecule whose size by PAGE is similar to that of mucin 1 was reported to inhibit binding of enteric bacteria to Caco-2 cells (8). S. typhimurium specifically binds mucins of the intestinal mucosa (9). Human milk mucins competitively inhibit some types of enteropathogens binding to target receptor glycans on host cells (7, 10, 11). Thus, human milk glycans, and especially the milk mucins, are interesting candidates as potential inhibitors of Salmonella infection.
Human mucins are high-molecular weight glycoproteins whose typical size ranges from 200 to 2000 kDa and are primarily found in the extracellular glycocalyx region of extracellular matrix and in human milk. Their diverse functions include regulating cell signaling and transcription and modulating the binding of bacteria to the intestinal mucosa epithelium, including binding by both mutualists and pathogens (12). The glycans expressed on mucins include many common moieties that can act as cell surface targets for pathogens.
In the studies described herein, the specific hypothesis is that human milk mucins could inhibit Salmonella invasion of human intestinal epithelia. Two cell lines, FHs 74 Int (derived from normal human fetal intestine) and Caco-2 (derived from human colon adenocarcinoma), were used as models of the immature human intestinal epithelial cell. S. typhimurium is the most common of the Salmonella serovars that cause human infections (13). Therefore, Salmonella enterica serovar Typhimurium SL1344 (SL1344)6 invasion of FHs 74 Int and Caco-2 cell lines were developed as models of human salmonellosis. These models were used to determine whether human milk mucins affect SL1344 invasion of FHs 74 Int and Caco-2 cells in vitro, thereby indicating the potential utility of human milk mucins to protect infants from Salmonella infection.
Materials and Methods
Human milk.
Use of human milk was approved by the Institutional Review Boards of Massachusetts General Hospital. Human milk was collected with a breast pump from 40 healthy donors and stored at −20°C. This pooled milk from donors at different stages of lactation was tested as representative of human milk.
Bacterial strains and culture.
The invasive wild-type SL1344 (14) was obtained from the American Type Culture Collection (ATCC) and grown to the stationary phase in Luria-Bertoni medium at 37°C with constant shaking.
Cell lines and culture.
The normal small intestine epithelial cell line FHs 74 Int was obtained from ATCC and was cultured in Hybri-Care medium (ATCC) 10% FBS (Atlanta Biologicals) in the presence of 30 μg/L epidermal growth factor (Invitrogen) at 37°C in 5% CO2. The human epithelial colorectal adenocarcinoma cell line Caco-2 was obtained from ATCC and cultured in DMEM (Gibco-BRL)/10% FBS at 37°C in 5% CO2.
Isolation, purification, and analysis of mucins.
Pooled frozen human milk (40 donors) was thawed and centrifuged at 1000 × g for 40 min at 18°C to obtain cream and skim milk. The cream was washed 3 times with PBS to remove soluble protein. The cream was then resuspended in PBS and subjected to 3 cycles of freeze-thaw followed by sonication for 20 min to disrupt the milk fat globule. Milk fat globule membrane (MFGM) was collected as the pellet after centrifugation at 100,000 × g for 1 h. Whole milk, skim milk, and MFGM were freeze-dried and stored at −20°C for testing their biologic activity.
To isolate the human milk mucins, MFGM was dissolved in PBS with 8 mol/L urea and applied to a Sepharose CL-2B (GE Healthcare) column. Fractions containing mucin 1 and mucin 4 were collected and further purified over a Sepharose CL-4B (GE Healthcare) column. The mucin 1 and mucin 4 fractions were pooled, dialyzed, freeze-dried, and stored at −20°C for assessment of their ability to inhibit Salmonella. The identity and purity of mucins were evaluated by SDS-PAGE. Purified mucin 1 and mucin 4 were analyzed on 4–12% gradient SDS-PAGE gels stained by silver and periodic acid-Schiff (PAS) reagent. The identity of mucin 1 and mucin 4 were confirmed by Western-blot analysis against the mucin 1 antibody (a gift from Dr. Sandra Gendler, Mayo Clinic) or mucin 4 antibody (a gift from Dr. Surinder Batra, University of Nebraska Medical Center).
Fluorescence confocal microscopy.
The FHs 74 Int or Caco-2 cells were grown on polylysine-coated glass coverslips in 24-well cell culture plates to ~70% confluence. SL1344 was labeled with fluorescein-5-isothiocyanate (FITC) (Sigma) as previously described (8). Briefly, SL1344 (1012 CFU/L in PBS) were incubated with 100 μg of FITC for 30 min at room temperature and washed thrice with PBS. The epithelial cells were incubated with FITC-labeled SL1344 for 1 h at 37°C at a 100:1 bacteria/epithelial cell multiplicity of infection. The cells were washed with PBS to remove unattached SL1344 and then incubated in cell culture medium containing 100 mg/L of gentamicin (Sigma) for 1 h at 37°C. After gentamicin killing of extracellular bacteria, cells were washed by dipping the cover slip in PBS and the cells were fixed by dipping in 1% buffered formalin for 10 min at room temperature. Cells were rinsed twice and incubated with membrane-specific dye Vybrant DiI (Invitrogen) for 20 min according to the manufacturer’s instructions. After rinsing with PBS, a drop of mounting media was applied to a microscope slide and the inverted cover slip was carefully laid on the slide. The mounting media included 4′,6-diamidino-2-phenylindole stain and was left to harden overnight. Imaging analysis was performed with a Leica TCS SP5 inverted confocal laser-scanning microscope (Plan-Apochromat 63× oil immersion lens, 1.4 numerical aperture).
Invasion assay.
Intestinal epithelial cell lines, FHs 74 Int or Caco-2 cells were seeded onto 6-well plates at 106 cells/well. The cells were inoculated with suspensions of 1.0 × 108 CFU FITC-labeled SL1344, along with test samples or controls, and incubated for 1 h at 37°C, whereupon the intestinal epithelial cell monolayers were rinsed 5 times with fresh medium. The epithelial cells were then incubated for 1 h at 37°C in 5% CO2 with cell culture medium containing 100 mg/L of gentamicin to kill extracellular bacteria. After incubation, extracellular SL1344 was removed by thorough washing and the cells were fixed with 1% paraformaldehyde in PBS and analyzed for invasion as fluorescence intensity of FITC-labeled SL1344 by flow cytometry in a C6 Flow Cytometer system (Accuri Cytometers). Cells were harvested and fluorescence intensity analyzed by flow cytometry as previously described (15). The mean fluorescence intensity was set to detect 10,000 cells/sample, which was then multiplied by the number of cells within each well to give total fluorescence per well. Invasion of SL1344 was:
where Iexp = fluorescence intensity of well with epithelial cells, Salmonella, test sample; Ineg = fluorescence intensity of well with epithelial cells (background signal); and Ipos = fluorescence intensity of well with epithelial cells, Salmonella.
Inhibitor specificity.
Inhibition could result from the test molecule interacting with the target epithelial cells, the test molecule interacting with the pathogen, or both. As a first step toward investigating these possible mechanisms, epithelial cells (FHs 74 Int or Caco-2) or the pathogen SL1344 were individually preincubated with the inhibitor for 1 h prior to the invasion assay. An increase in inhibition by the test substance in either condition would suggest that the inhibitor interacts with the target epithelial cell or with the pathogen, respectively.
Statistical analysis.
Data are expressed as mean ± SD of at least 3 independent experiments. Relative inhibition by treatments was evaluated using 1-way ANOVA followed by Tukey’s post hoc test. The dose dependency used logistic dose response of nonlinear curve fit analysis; the half maximal inhibitory concentration (IC50) was calculated from each curve. The differences in IC50 between mucin 1 and mucin 4 were compared by Student’s t test for paired samples. All statistical analyses were conducted using the Originpro (OriginLab) and MATLAB (MathWorks). Differences were considered significant at P < 0.05.
Results
Preparation and analysis of mucin.
Two mucins, mucin 1 and mucin 4, were isolated from human MFGM, purified by size exclusion chromatography, and identified as mucin 1 and mucin 4 by SDS-PAGE and Western blot (Supplemental Fig. 1). Because these 2 mucins are glycoproteins, they stained with PAS reagent for carbohydrates and silver stain for proteins. PAGE with silver and PAS stains indicated that mucin 1 and mucin 4 were purified to >90%. The identities of mucin 1 and mucin 4 were confirmed by Western blot against authentic mucin 1 and mucin 4 antibodies.
Mucin inhibits Salmonella invasion of intestinal epithelial cells.
Confocal fluorescence microscopy indicates that FHs 74 Int and Caco-2 intestinal epithelial cells were invaded by FITC labeled SL1344 in vitro (Supplemental Fig. 2). FITC-labeled SL1344 invasion into epithelial intestinal cells was measured by flow cytometry to quantify inhibition of invasion by milk fractions (Supplemental Fig. 3). Human milk fractions inhibited SL1344 invasion of epithelial cells; the purified mucins displayed the highest specific activity of inhibition by several orders of magnitude. BSA, the negative control, did not inhibit invasion. Mucin 1 and mucin 4 were each found in human milk at concentrations of ∼150 μg/L, based on gravimetry of lyophilized purified fractions (data not shown). At these concentrations of 150 μg/L, mucin 1 and mucin 4 significantly inhibit SL1344 invasion of epithelial cells, whereas BSA, whole milk, skim milk, and MFGM at this concentration did not exhibit inhibitory activity (data not shown). Even at 1000 times this concentration (150 mg/L), inhibition was less than that of the mucins at 150 μg/L (Table 1); mucin 1 and mucin 4 showed much stronger inhibition of SL1344 invasion of FHs 74 Int cells than whole milk, skim milk, MFGM, or BSA (P < 0.05). Mucin 1 inhibited more strongly than mucin 4 (P < 0.05). Likewise, similar results were obtained in Caco-2 cells. Human milk mucins were more effective than the other human milk fractions to reduce invasion. Mucins and other human milk fractions did not reduce viability of either SL1344 or the epithelial FHs 74 Int and Caco-2 cells (data not shown) under the conditions of the assays for 3 h, the maximum incubation duration used in these studies. Mucins and other human milk fractions did not change the distribution of SL1344 in culture, indicating that decreased invasion was not due to agglutination. Altogether, these data indicate that human milk mucin 1 and mucin 4 account for essentially all of the inhibition of SL1344 invasion of epithelial cells by human milk.
TABLE 1.
Mucin 1 and mucin 4 inhibit SL1344 invasion of epithelial cells in vitro1,2
| SL1344 invasion, % |
||
| Inhibitor | of FHs 74 Int cells | of Caco-2 cells |
| BSA | 96 ± 6a | 96 ± 7a |
| Whole milk | 33 ± 7b | 36 ± 5b |
| Skim milk | 35 ± 8b | 42 ± 8b |
| MFGM | 28 ± 4b | 33 ± 4b |
| Mucin 4 | 20 ± 3c | 25 ± 3c |
| Mucin 1 | 16 ± 2d | 19 ± 2d |
Values are mean ± SD, = 3. Means in a column without a common letter differ, P < 0.05. MFGM, milk fat globule membrane SL1344, Salmonella enterica serovar Typhimurium SL1344.
BSA, whole milk, skim milk, and MFGM were tested at 150 mg/L. Mucin 1 and mucin 4 were tested at 150 g/L.
Mucins inhibit Salmonella invasion in a dose-dependent manner.
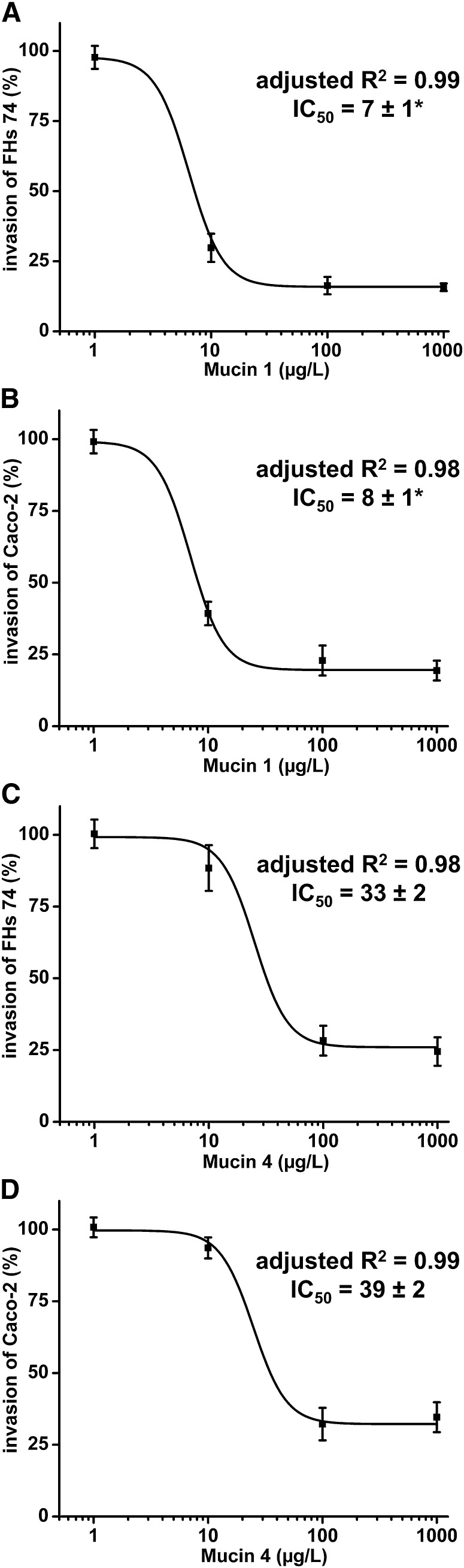
Both mucin 1 and mucin 4 inhibited Salmonella invasion at doses similar to those found in human milk. To determine the dose dependency of mucin inhibition, serial dilutions of purified mucin 1 and mucin 4 were tested for their relative ability to inhibit Salmonella infection of FHs 74 Int or Caco-2 cells. Mucin 1 inhibited SL1344 invasion of FHs 74 Int (Fig. 1A) and Caco-2 (Fig. 1B) cells in a dose-dependent manner (1–1000 μg/L); likewise, mucin 4 inhibition was also dose dependent in FHs 74 Int (Fig. 1C) and Caco-2 (Fig. 1D) cells. The inhibition strongly fit a logistic dose-response curve (adjusted R2 ≥ 0.98). The IC50 of mucin 1 was 7 ± 1 and 8 ± 1 μg/L for FHs 74 Int and Caco-2 cells, respectively. The corresponding IC50 for mucin 4 was 33 ± 2 and 39 ± 2 μg/L for FHs 74 Int and Caco-2 cells, respectively. Thus, in FHs 74 Int and Caco-2 cell models, the 2 mucins inhibit Salmonella, but mucin 1 inhibits this major serovar of Salmonella more strongly than mucin 4 for each comparison within a cell line (P < 0.001).
FIGURE 1.
Mucin 1 inhibits SL1344 invasion of FHs 74 Int (A) or Caco-2 (B) human intestinal epithelial cell lines and mucin 4 inhibits SL1344 invasion of FHs 74 Int (C) or Caco-2 (D) cells. Values are mean ± SD, n = 3. *Different from corresponding mucin 4, P < 0.001.
Mucin interaction with epithelial cells and SL134.
As a first step toward defining the mechanisms by which mucins inhibit the invasion of SL1344, either the epithelial cells or the Salmonella were separately preincubated with mucin 1 or mucin 4 for 1 h prior to performing the 1-h invasion assay (Table 2). The results were compared with those from the simultaneous incubation of mucins, epithelial cells, and SL1344 for 1 h only. Preincubation of either FHs 74 Int cells (host cell) or SL1344 (bacterial pathogen) with mucin 1 for 1 h followed by the simultaneous incubation of all together for 1 h reduced invasion relative to only the 1-h simultaneous incubation of all reagents (P < 0.05). Pretreatment also reduced invasion in the Caco-2 cell model. Preincubation with mucin 4 likewise further reduced infection. Thus, inhibition by mucin 1 and mucin 4 may be due to interaction with both the target epithelial cells and SL1344 bacteria.
TABLE 2.
Mucin 1 and mucin 4 interact with epithelial cells and SL13441ndash3
| Mucin 1 |
Mucin 4 |
|||
| Experimental group | FHs 74 Int | Caco-2 | FHs 74 Int | Caco-2 |
|
Invasion, % |
||||
| BSA | 96 ± 6a | 97 ± 7a | 100 ± 3a | 99 ± 5a |
| SL1344, epithelial cell mixed simultaneously | 17 ± 2b | 23 ± 5b | 29 ± 7b | 34 ± 7b |
| SL1344 pretreated with mucin | 8 ± 4c | 14 ± 4c | 19 ± 7c | 25 ± 6c |
| Epithelial cells pretreated with mucin | 10 ± 3c | 16 ± 3c | 22 ± 5c | 24 ± 6c |
Values are mean ± SD, = 3. Means in a column without a common letter differ, P < 0.05. SL1344, Salmonella enterica serovar Typhimurium SL1344.
Mucin 1, mucin 4, and BSA were tested at 150 g/L.
106 epithelial cells were inoculated with 1.0 × 108 FITC-labeled SL1344.
Discussion
Mucin 1 and mucin 4 are transmembrane glycoproteins with extensive N- and O- linked glycosylation (16, 17), containing highly sialylated glycan moieties (18, 19). Human milk mucin exhibits biological activity, including blocking transmission of human immunodeficiency virus from dendritic to T cells (11, 20) and binding Norwalk virus (16). The biological activities of mucins observed herein could reside in either specific carbohydrate moieties or peptide motifs. For example, sialic acid-containing carbohydrate moieties can act as key receptors for the adherence of major strains of S. typhimurium. Cell surface sialic acid is required for the adherence of S. typhimurium to Caco-2 epithelial cells in vitro (21). The mucin sialic acid is essential for bovine mucin 1 inhibition of pathogen binding to Caco-2 cells (8). Both mucin 1 and mucin 4 contain sialic acid. The ability of human milk mucins to inhibit SL1344 binding to epithelial cells is consistent with their carbohydrate moieties acting as competitive inhibitors of SL1344 binding to receptors on the epithelium. If this were so, the differences in inhibition observed between mucin 1 and mucin 4 could be due to differential glycosylation. On the other hand, the differences in inhibition that were observed could be due to differences in the protein backbone. For example, mucin 1 contains a sperm protein enterokinase and agrin (SEA) domain. Mucin 4 lacks the SEA domain, which may function in protein binding to carbohydrate moieties (22). If the SEA domain of mucin 1 participates in bacterial binding, this could account for the stronger inhibition of SL1344 by mucin 1 relative to mucin 4.
The mechanisms whereby Salmonella invade epithelial cells include 2 type III secretion systems: Salmonella pathogenicity island 1 allows Salmonella to invade the intestinal epithelium, whereas Salmonella pathogenicity island 2 promotes its survival within cells (23). There is a dearth of evidence supporting the specific interaction of human milk mucins with these systems. However, heavily glycosylated mucins inhibit binding to other receptors of epithelial cell binding. For example, mucin 1 and mucin 4 interact with galectin-3 (24, 25), a ligand found in epithelial cells, fibroblasts, dendritic cells, and inflammatory cells. Galectin-3 binds to LPS in intact bacteria and enhances bacterial interaction with host epithelial cells and macrophages (26). Thus, human milk mucins, by binding galectin 3, may inhibit LPS-mediated Salmonella binding to epithelial cells. This is supported by the increased inhibition of SL1344 invasion of epithelial cells by preincubation of the epithelial cells with the mucin. Various pili are expressed by Salmonella strains and binding of the pili to host target molecules is an essential step in pathogenesis. Many pili target specific glycans of the epithelial cell surface (27). Mucins have been demonstrated to bind to some Salmonella strains via mucin mannose interacting with pili adhesins (9). Other strains bind through interactions with other glycan motifs, but the binding specificity of most remains undefined. Thus, human milk mucins may inhibit Salmonella pathogenesis through the inhibition of as-yet-undefined binding by pili adhesins to epithelial cell surface glycans. This is consistent with the increased inhibition after preincubating the Salmonella with the human milk mucins. Altogether, human milk mucins may inhibit Salmonella invasion by binding to both the pathogens and epithelial cells but through different moieties of this large, highly glycosylated, complex molecule.
In this study, the target cells chosen to develop models of Salmonella invasion of human intestinal epithelial cells were undifferentiated Caco-2 and FHs 74 Int cells. Caco-2 cells, originally derived from colonic carcinoma, are classically chosen for in vitro models of Salmonella invasion assays (28). FHs 74 Int cells are derived from normal fetal small intestine and have epithelial features more similar to normal intact immature mucosa, including morphology that is epithelial like, reduction of doubling time by epidermal growth factor, and stimulation of growth by amniotic fluid and human milk that is tyrosine kinase dependent (29). FHs 74 Int cells are often used to model the immature infant intestinal epithelium (30). Infants are at higher risk for Salmonella infection than adults. Thus, the FHs 74 Int cell is a relevant model for studying inhibition of Salmonella invasion by human milk and yielded results that were consistent with those provided by the more widely used Caco-2 cell model.
Human milk contains many bioactive substances that function in protecting breast-fed infants. Only some human milk components have analogs in bovine milk and among them is mucin. Bovine milk mucin 1 inhibits binding of enteric bacteria to Caco-2 cells in vitro (8). The model used to test bovine milk mucin was a strain of Salmonella different from the SL1344 used herein and binding was to Caco-2 cells. That notwithstanding, the magnitude of inhibition by the bovine and human MFGM was roughly comparable. The mucin of bovine milk, which was assumed to be mucin 1, also displayed a specific activity of inhibition that was comparable with that of the human milk mucin 1. However, there is no mention of mucin 4 in bovine milk. Due to the known differences between bovine and human milk glycosylation for many other proteins, it seems likely that qualitative differences exist between the mucin 1 of human and bovine milk as well as potential qualitative differences in the types of mucins in each type of milk. For example, the molecular weight of bovine mucin 1 is <200 kDa by SDS-PAGE, and human milk mucin 1 is >250 kDa, consistent with greater glycosylation. In general, human milk components are more heavily glycosylated than analogous components of bovine milk (31). Moreover, the glycans of human milk are also qualitatively different in that human milk glycans tend to be more heavily fucosylated than bovine milk glycans (32). Many biologically active human milk glycans inhibit specific human pathogens in vitro and in vivo and reduce risk of enteric diseases in infants (2, 33–35). The data herein indicate that human milk mucin strongly inhibits Salmonella invasion of human epithelial cells, providing a basis for future comparisons of the structural basis of this inhibition by bovine milk mucin and each of the human milk mucins.
In summary, mucin 1 and mucin 4 were isolated, purified, and identified from human milk. To our knowledge, this is the first purified mucin 4 isolated from human milk. These mucins inhibit SL1344 invasion of human intestinal epithelium cells (FHs 74 Int and CaCo-2 cells) at the concentrations in milk and account for essentially all of this inhibitory activity of human milk. Mucin 1 is a stronger inhibitor than mucin 4. Therefore, human milk mucins are promising as potential prophylactic and therapeutic agents that inhibit infant diseases caused by Salmonella and perhaps other pathogens.
Supplementary Material
Acknowledgments
The authors thank Dr. Sandra J. Gendler for providing mucin 1 antibody, Dr. Surinder Batra for providing mucin 4 antibody, and Michele Busby for assistance with data statistical analysis. D.S.N. and B.L. designed research; B.L., Z.Y., C.C., and D.E.K. conducted the research; D.S.N. and B.L. analyzed data and wrote the manuscript; and D.S.N. and B.L. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grants HD013021 and AI075563 and by Abbott Nutrition.
Supplemental Figures 1–3 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: ATCC, American Type Culture Collection; FITC, fluorescein-5-isothiocyanate; IC50, half maximal inhibitory concentration; MFGM, milk fat globule membrane; PAS, periodic acid-Schiff; SL1344, Salmonella enterica serovar Typhimurium SL1344.
Literature Cited
- 1.Newburg DS. Bioactive components of human milk: evolution, efficiency, and protection. Adv Exp Med Biol. 2001;501:3–10 [DOI] [PubMed] [Google Scholar]
- 2.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr. 2005;25:37–58 [DOI] [PubMed] [Google Scholar]
- 3.Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, Cieslak PR, Deneen VC, Tauxe RV. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 2004;38 Suppl 3:S127–34 [DOI] [PubMed] [Google Scholar]
- 4.Jones TF, Ingram LA, Fullerton KE, Marcus R, Anderson BJ, McCarthy PV, Vugia D, Shiferaw B, Haubert N, Wedel S, et al. A case-control study of the epidemiology of sporadic Salmonella infection in infants. Pediatrics. 2006;118:2380–7 [DOI] [PubMed] [Google Scholar]
- 5.Savino F, Roana J, Mandras N, Tarasco V, Locatelli E, Tullio V. Faecal microbiota in breast-fed infants after antibiotic therapy. Acta Paediatr. 2011;100:75–8 [DOI] [PubMed] [Google Scholar]
- 6.Bessler HC, de Oliveira IR, Giugliano LG. Human milk glycoproteins inhibit the adherence of Salmonella typhimurium to HeLa cells. Microbiol Immunol. 2006;50:877–82 [DOI] [PubMed] [Google Scholar]
- 7.Yolken RH, Peterson JA, Vonderfecht SL, Fouts ET, Midthun K, Newburg DS. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90:1984–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker P, Sando L, Pearson R, Kongsuwan K, Tellam RL, Smith S. Bovine Muc1 inhibits binding of enteric bacteria to Caco-2 cells. Glycoconj J. 2010;27:89–97 [DOI] [PubMed] [Google Scholar]
- 9.Vimal DB, Khullar M, Gupta S, Ganguly NK. Intestinal mucins: the binding sites for Salmonella typhimurium. Mol Cell Biochem. 2000;204:107–17 [DOI] [PubMed] [Google Scholar]
- 10.Schroten H, Hanisch FG, Plogmann R, Hacker J, Uhlenbruck G, Nobis-Bosch R, Wahn V. Inhibition of adhesion of S-fimbriated Escherichia coli to buccal epithelial cells by human milk fat globule membrane components: a novel aspect of the protective function of mucins in the nonimmunoglobulin fraction. Infect Immun. 1992;60:2893–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruvoën-Clouet N, Mas E, Marionneau S, Guillon P, Lombardo D, Le Pendu J. Bile-salt-stimulated lipase and mucins from milk of 'secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem J. 2006;393:627–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baggesen DL, Wegener HC. Phage types of Salmonella enterica ssp. enterica serovar typhimurium isolated from production animals and humans in Denmark. Acta Vet Scand. 1994;35:349–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Cherayil BJ. Role of Toll-like receptor 4 in macrophage activation and tolerance during Salmonella enterica serovar Typhimurium infection. Infect Immun. 2003;71:4873–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara-Kaonga B, Pistole TG. A dual fluorescence flow cytometric analysis of bacterial adherence to mammalian host cells. J Microbiol Methods. 2007;69:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parry S, Hanisch FG, Leir SH, Sutton-Smith M, Morris HR, Dell A, Harris A. N-Glycosylation of the MUC1 mucin in epithelial cells and secretions. Glycobiology. 2006;16:623–34 [DOI] [PubMed] [Google Scholar]
- 17.Hull SR, Laine RA, Kaizu T, Rodriguez I, Carraway KL. Structures of the O-linked oligosaccharides of the major cell surface sialoglycoprotein of MAT-B1 and MAT-C1 ascites sublines of the 13762 rat mammary adenocarcinoma. J Biol Chem. 1984;259:4866–77 [PubMed] [Google Scholar]
- 18.Ho JJ, Cheng S, Kim YS. Access to peptide regions of a surface mucin (MUC1) is reduced by sialic acids. Biochem Biophys Res Commun. 1995;210:866–73 [DOI] [PubMed] [Google Scholar]
- 19.Carraway KL, Price-Schiavi SA, Komatsu M, Jepson S, Perez A, Carraway CA. Muc4/sialomucin complex in the mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:323–37 [DOI] [PubMed] [Google Scholar]
- 20.Saeland E, de Jong MA, Nabatov AA, Kalay H, Geijtenbeek TB, van Kooyk Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol Immunol. 2009;46:2309–16 [DOI] [PubMed] [Google Scholar]
- 21.Sakarya S, Gokturk C, Ozturk T, Ertugrul MB. Sialic acid is required for nonspecific adherence of Salmonella enterica ssp. enterica serovar Typhi on Caco-2 cells. FEMS Immunol Med Microbiol. 2010;58:330–5 [DOI] [PubMed] [Google Scholar]
- 22.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60 [DOI] [PubMed] [Google Scholar]
- 23.Galán JE. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–71 [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Guo X, Nash GB, Stone PC, Hilkens J, Rhodes JM, Yu LG. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009;69:6799–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, Batra SK. Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin Cancer Res. 2011;17:267–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17 [DOI] [PubMed] [Google Scholar]
- 27.De Greve H, Wyns L, Bouckaert J. Combining sites of bacterial fimbriae. Curr Opin Struct Biol. 2007;17:506–12 [DOI] [PubMed] [Google Scholar]
- 28.Giannasca KT, Giannasca PJ, Neutra MR. Adherence of Salmonella typhimurium to Caco-2 cells: identification of a glycoconjugate receptor. Infect Immun. 1996;64:135–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirai C, Ichiba H, Saito M, Shintaku H, Yamano T, Kusuda S. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J Pediatr Gastroenterol Nutr. 2002;34:524–8 [DOI] [PubMed] [Google Scholar]
- 30.Mirpuri J, Brazil JC, Berardinelli AJ, Nasr TR, Cooper K, Schnoor M, Lin PW, Parkos CA, Louis NA. Commensal Escherichia coli reduces epithelial apoptosis through IFN-alphaA-mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. J Immunol. 2010;184:7186–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudloff S, Kunz C. Protein and nonprotein nitrogen components in human milk, bovine milk, and infant formula: quantitative and qualitative aspects in infant nutrition. J Pediatr Gastroenterol Nutr. 1997;24:328–44 [DOI] [PubMed] [Google Scholar]
- 32.Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol. 2011;2:331–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coppa GV, Zampini L, Galeazzi T, Facinelli B, Ferrante L, Capretti R, Orazio G. Human milk oligosaccharides inhibit the adhesion to Caco-2 cells of diarrheal pathogens: Escherichia coli, Vibrio cholerae, and Salmonella fyris. Pediatr Res. 2006;59:377–82 [DOI] [PubMed] [Google Scholar]
- 34.Czirók E, Milch H, Nemeth K, Gado I. In vitro and in vivo (LD50) effects of human lactoferrin on bacteria. Acta Microbiol Hung. 1990;37:55–71 [PubMed] [Google Scholar]
- 35.Stevens CR, Millar TM, Clinch JG, Kanczler JM, Bodamyali T, Blake DR. Antibacterial properties of xanthine oxidase in human milk. Lancet. 2000;356:829–30 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.