Abstract
Little is known about the effects of different quantities of whey protein on exercise training–induced changes in body composition and indices of metabolic syndrome in middle-aged overweight and obese adults. Therefore, we examined the effects of consuming 0.8-MJ supplements with 0 (n = 126), 10 (n = 112), 20 (n = 44), or 30 (n = 45) g whey protein twice daily in conjunction with resistance (2 d/wk) and aerobic (1 d/wk) exercise training in a double-blind, randomized, placebo-controlled, community-based 9-mo study in men (n = 117) and women (n = 210); (age: 48 ± 7.9 y; BMI: 30.0 ± 2.8 kg/m2). Whey protein supplementation did not influence any of the following outcomes, some of which were affected by training. Among all participants, strength increased by 15 ± 12% (P < 0.001) and maximal oxygen uptake capacity (VO2max) increased by 9 ± 15% (P < 0.001). Body weight was unchanged (0.1 ± 3.7 kg, P = 0.80), lean body mass increased by 1.9 ± 2.8% (0.95 ± 1.3 kg, P < 0.001), and fat mass decreased by 2.6 ± 9.4% (−0.86 ± 3.1 kg, P = 0.001). Oral-glucose-tolerance testing showed that plasma glucose AUC was unchanged (−18.0 ± 170 mmol/L· 3 h, P = 0.16), insulin AUC decreased by 2.6 ± 32% (−7.5 ± 29 nmol/L· 3 h, P = 0.01), and HOMA-IR (0.2 ± 2.0, P = 0.81) and the insulin sensitivity index (0.3 ± 3.0, P = 0.63) were unchanged. Plasma concentrations of TG; total, LDL, and HDL cholesterol; C-reactive protein; plasminogen activator inhibitor-1; blood pressure; and waist circumference were unchanged. Whey protein supplementation did not affect exercise training–induced responses in body composition and indices of metabolic syndrome in middle-aged overweight and obese adults who maintained body weight.
Introduction
Metabolic syndrome is characterized by a combination of metabolic abnormalities that increase the risk of the development of diabetes and cardiovascular disease and include dyslipidemia (elevated TG and LDL cholesterol, and low HDL cholesterol), elevated blood pressure, insulin resistance or glucose intolerance, a prothrombotic state, and a proinflammatory state (1). Overweight, obesity, and physical inactivity are well-established risk factors for type 2 diabetes and cardiovascular comorbidities (2), which may be countered by improvements in body composition (decreased fat mass and increased lean body mass) (3, 4) and physical fitness (5–7). Exercise training is an effective way to increase lean body mass and reduce fat mass (3, 8), as well as to improve lipid-lipoprotein profile (particularly to increase HDL cholesterol) (5), blood pressure (6, 9), and insulin sensitivity (7) in weight-stable adults. Limited research suggests that the consumption of higher protein diets may enhance the exercise-induced improvements in body composition (10–12) and indices of metabolic syndrome (13).
Protein supplementation can be used to increase total protein intake. Studies evaluating the effects of higher protein diets and exercise training on body composition often use whey protein (13–15), which is 1 of the 2 primary proteins in milk and accounts for 20% of total milk protein (16). Evidence suggests that whey protein and its bioactive components exhibit hypolipidemic (17), insulinotrophic (18, 19), antihypertensive (20, 21), and antiinflammatory (22) properties. Furthermore, whey is considered a high-quality protein that contains all essential amino acids, making it a potent anabolic stimulus when coupled with resistance exercise (23). Findings from studies evaluating the effects of higher protein diets and resistance exercise training on body composition in overweight and obese middle-aged adults are conflicting (3, 10, 12, 15, 24, 25). Individual studies have reported no additional benefits of higher protein diets on exercise training–induced changes in body composition (3, 26), whereas a retrospective reassessment in 106 men and women showed gains in fat-free mass when >1.0 g protein · kg−1 · d−1 was consumed (10). In terms of whey protein supplementation, men who performed resistance training and who consumed >100 g whey protein/d (>2.0 g protein · kg−1 · d−1) experienced greater gains in lean body mass compared with those who received a casein supplement (12) or a placebo (25). On the other hand, men supplemented with 25–35 g/d (~1.0 g protein · kg−1 · d−1) did not enhance resistance training–induced increases in lean body mass and decreases in fat mass compared with a placebo supplement (13, 15). Collectively, these studies suggest that the quantity of supplemental whey protein may be an important factor influencing these body-composition and metabolic health responses, with intakes of whey protein >35 g/d, leading to total protein intakes >1.0 g protein · kg−1 · d−1 possibly being more effective. Limited studies have evaluated the effects of whey protein supplements in conjunction with exercise training on indices of metabolic syndrome (13), and studies that titrate different quantities of whey protein are needed.
The primary aims of this study were to examine the effectiveness of increased total dietary protein intake, through whey protein supplementation, under free-feeding conditions to enhance changes in body composition and positively change indices of metabolic syndrome with resistance and aerobic exercise training in overweight and obese middle-aged adults. We hypothesized that increased whey protein consumption would result in 1) enhanced training-induced gain of lean body mass and loss of fat mass and 2) enhanced reductions in waist circumference, blood lipids, blood pressure, and inflammation and increased indices of insulin sensitivity.
Participants and Methods
Participants.
Overweight and obese middle-aged men and women (aged 35–65 y) were recruited from the greater West Lafayette, IN, area. Inclusion criteria were as follows: body weight <300 lb (136 kg), BMI between 26 and 35 kg/m2, blood pressure <160/100 mm Hg, fasting plasma glucose <6.1 mmol/L, total cholesterol <6.7 mmol/L, LDL cholesterol <4.1 mmol/L, TG <4.5 mmol/L, no preexisting kidney or liver conditions, not currently or previously (past 6 mo) consuming a weight-loss diet or other special/nonbalanced diets, no weight loss/gain (±4.5 kg) within the past 6 mo, and <2 h/wk of habitual resistive or aerobic exercise training in the past 6 mo. Participants taking medications for elevated TG, reduced HDL cholesterol, or elevated blood pressure were included. The Purdue University Institutional Review Board approved the study protocol, which complied with the Helsinki Declaration as revised in 1983. All participants provided written informed consent and received monetary compensation for participation. A flow diagram of the recruitment and retention of participants is presented in Supplemental Figure 1.
Experimental design.
This intent-to-treat study was double-blind, placebo-controlled, community-based, and 36 wk in duration. After completing a 1-wk baseline period, participants were randomly assigned to 1 of 4 groups and were instructed to consume the assigned dietary supplements. All participants performed resistance (2 d/wk) and aerobic (1 d/wk) exercise for 36 wk at 1 of 5 local fitness facilities in the Lafayette/West Lafayette, IN, area. Details on the exercise training and testing can be found in the Online Supporting Materials. All measurements were taken pre- (baseline), mid- (wk 18), and post- (wk 36) intervention.
Whey protein supplementation and nutritional intakes.
During the 36-wk intervention period, each participant was instructed to consume one supplement sachet containing 0.8 MJ (200 kcal) and 0, 10, 20, or 30 g whey protein twice daily with breakfast and lunch mixed with the food or beverage of their preference. Daily supplemental whey protein intakes were 0, 20, 40, and 60 g/d, respectively. Supplements were in powder form and manufactured by Innovative Food Processors, Inc. (Supplemental Table 1). Participants were told that the supplements provided 1.7 MJ/d (400 kcal/d) of energy but were not counseled to purposefully alter their usual eating behaviors. Four-day food records (3 weekdays and 1 weekend day) were completed at wk 0, 18, and 36 to estimate daily energy intake and macronutrient composition (Nutritionist Pro, First DataBank version 1.3.36). The age- and sex-specific Schofield equations (27) were used to estimate basal metabolic rate, and food records that included the 1.7 MJ from the supplements at mid- and postintervention time points were considered valid if they fell within the previously established lower and upper 95% confidence limits (28). Data on 176 of the 220 participants (0 g/d: n = 72; 20 g/d: n = 65; 40 g/d: n = 16; and 60 g/d: n = 23) met this criterion and were used in the food record analyses.
To document group-specific differences in total protein intake, 24-h urinary urea nitrogen (UUN)10 was analyzed. Twenty-four-hour urine volumes (weight divided by specific gravity; Digital Probe Refractometer; Misco Products Division) were obtained from two 24-h urine collections, and aliquots were stored at −20°C for subsequent analyses of urea nitrogen (COBAS Integra 400; Roche Diagnostic Systems).
Assessment of dietary compensation.
Dietary compensation was determined by subtracting nonsupplement energy and macronutrient intakes at mid- and postintervention time points from baseline intakes. The dietary data were obtained from the 4-d food records completed at baseline, wk 18, and wk 36.
Compliance.
Participants completed daily supplement logs to document when the supplements were consumed and a weekly exercise training log for each exercise session, which included information pertaining to the amount of weight lifted, repetitions, and specific exercises completed for resistance exercise sessions and heart rate for the aerobic exercise sessions. On the basis of the self-reported daily supplement logs and weekly exercise training logs, participants were placed into 3 compliance categories for both supplementation (⩾80%, 50–79%, and <50% of supplements consumed) and exercise (⩾90%, 70–89%, and <90% of sessions completed).
Body composition and anthropometric measurements.
Body weight, lean body mass, and fat mass were determined by using dual-energy X-ray absorptiometry (LUNAR iDXA and Lunar enCORE software, version 11.2; GE Medical Systems). Waist circumference was measured in the standing position at the narrowest area between the lateral lower rib and the iliac crest. Hip measurement was taken at the largest circumference of the lower abdomen. The measurements were performed in triplicate, and mean values were reported.
Blood collection, lipoprotein profile, and renal function.
Following a 12-h overnight fast, blood samples were collected in tubes containing a clot activator to obtain serum or sodium heparin to obtain plasma (BD Vacutainer Brand; Becton, Dickinson and Co). Serum tubes were sent to Mid America Clinical Laboratories for lipoprotein profile (total and HDL cholesterol and TG) and renal function determination (albumin and creatinine). LDL cholesterol was calculated on the basis of the Friedewald equation (29). Glomerular filtration rate was estimated by using the Modification of Diet in Renal Disease equation (30). Plasma tubes were immediately placed on ice for 30 min, and centrifuged at 4°C for 10 min at 3000 × g. The plasma was then separated and stored in microcentrifuge tubes at −80°C for subsequent glucose, insulin, C-reactive protein (CRP), and plasminogen activator inhibitor-1 (PAI-1) analyses.
Blood pressure.
Sitting blood pressure was measured with an automated sphygmomanometer (Advantage 6014 Advanced Blood Pressure Monitor; American Diagnostic Corporation) after the participant rested in a sitting position for ≥10 min. The measurements were taken in duplicate on the same arm and averaged.
Glucose-tolerance assessment.
During the 3-h oral-glucose-tolerance test, participants consumed a sugar solution containing 75 g dextrose. Blood samples were collected at 0, 30, 60, 90, 120, 150, and 180 min, and AUC for glucose and insulin were determined using the trapezoidal rule (31). HOMA-IR and whole-body (composite) insulin sensitivity were calculated as previously described (32, 33). Plasma glucose concentration was measured by enzymatic colorimetry using an oxidase method on a COBAS Integra 400 analyzer (Roche Diagnostic Systems). Plasma insulin concentration was measured by an electrochemiluminescence immunoassay method on the Elecsys 2010 analyzer (Roche Diagnostic Systems).
Proinflammatory and prothrombotic markers.
Plasma CRP was measured on a COBAS Integra 400 analyzer (Roche Diagnostic Systems). Plasma PAI-1 was analyzed through enzyme immunoassay techniques by using an ELISA and the standard manufacturer’s protocol (PAI-1 Actibind; Technoclone GmbH). All samples from a given participant were tested in duplicate and analyzed within the same assay. The CV for CRP and plasma PAI-1 were 1.7% and 14.0%, respectively.
Metabolic syndrome criteria.
The number of participants who met the American Heart Association and National Heart, Lung, and Blood Institute criteria for metabolic syndrome (34) were documented pre-, mid-, and postintervention. A participant was defined as having metabolic syndrome if any 3 of the 5 following criteria were met: elevated waist circumference (population- and country-specific definitions), ≥≥TG ⩾1.7 mmol/L, HDL cholesterol <1.0 mmol/L in men and <1.3 mmol/L in women, systolic blood pressure ≥≥130 mm Hg and/or diastolic blood pressure ≥≥85 mm Hg, fasting glucose ≥≥5.6 mmol/L, or drug treatment for elevated TG, blood pressure, and/or reduced HDL cholesterol (34).
Metabolic measurements.
The participants reported to the laboratory after a 10-h overnight fast and reclined on a bed for 30 min to acclimate to room temperature. Indirect calorimetry was used to measure resting energy expenditure for the next 30 min. The first 10 min of data were excluded to reduce the variability in the initial adjustment period (MedGraphics Cardiopulmonary Diagnostics Systems; MedGraphics Corporation). Energy expenditure was estimated by using the Weir equation (35). Substrate oxidation (g/min) was estimated from the following equations (36): carbohydrate oxidation = 4.55 carbon dioxide production (VCO2) − 3.21 oxygen consumption (VO2) − 2.87 UUN; fat oxidation = 1.67 VO2 − 1.67 VCO2 − 1.92 UUN; protein oxidation = 6.25 UUN. VO2 and VCO2 are expressed in L/min, and UUN is expressed in g/min.
Appetite.
Participants completed 4-d appetite questionnaires assessing hunger, fullness, and desire to eat. The questionnaires were completed during every waking hour and daily hunger, fullness, and desire to eat total AUC were calculated (31). The questionnaires used a 13-point numerical rating scale [arbitrary units (AU)] with the left anchor “1” representing “not at all” and the right anchor “13” representing “extremely” (37). This type of assessment tool has been validated elsewhere (38).
Sample size and power calculations.
Power calculations were performed on the dietary protein–related differential change in lean body mass. Sixty-four participants per group were needed to statistically confirm a difference in lean body mass accretion of +0.8 kg between the control group and the 20-g/d group. Twenty participants per group were needed to statistically confirm differences in lean body mass accretion between the control group and the 40-g/d and the 60-g/d groups (P < 0.05, >90% power). This study was not powered to evaluate differences between the 20-, 40-, and 60-g/d groups.
Statistical analyses.
Analyses were performed by using data from participants who completed the entire 36-wk intervention (n = 188) or who dropped out after the midpoint but completed the wk 18 testing (n = 32) (Supplemental Figure 1). The appropriate transformation (i.e., log, square root, or reciprocal) was performed on data that were not normality distributed to meet the homogeneity of variance assumption needed to perform the ANOVA. A repeated-measures ANOVA was performed to determine the main effects of group and time and group-by-time interactions on all variables (n = 220 at pre-, , n = 220 at mid-, and n = 188 at postintervention). Dunnett’s test of multiple comparisons was used for post hoc analyses to detect differences between treatment groups and the control group. A Tukey-Kramer test was used to examine differences between time points within groups and with the overall group means. To examine the relationship between the presence or absence of metabolic syndrome between whey protein groups and over time, chi-square and McNemar’s tests were performed, respectively. On the basis of accumulating evidence that suggests that a change in insulin sensitivity is influenced by changes in body composition (39, 40), Pearson correlations were used to assess the relationship between changes in fat and lean body mass with changes in glucose and insulin AUC. Participants taking antihypertensive or antihyperlipidemic medications who either stopped taking the medication or if the dosage changed during the intervention were excluded from the blood pressure and blood lipid analyses, respectively.
Secondary analyses.
Because this was an intent-to-treat intervention, all participants who completed the entire 36-wk intervention or who dropped out after the midpoint but completed wk 18 testing were included in the original analyses; however, secondary statistical analyses were performed to assess the influence of compliance on the outcome variables. The mean compliance of each participant between wk 1–18 and 19–36 for the mid- and postintervention time points, respectively, was determined. Participants who achieved the highest compliance to both the supplementation (⩾80% consumed) and exercise sessions (⩾90% completed) were then included in repeated-measures ANOVA and post hoc (Tukey-Kramer and Dunnett’s) analyses.
All data are presented as means ± SD unless otherwise specified. Significance was accepted at P < 0.05. Data were analyzed by using PROC MIXED (SAS version 9.1.2; SAS Institute, Inc.).
Results
Participant characteristics and compliance
Characteristics of participants included in the analyses (n = 220; age: 48 ± 7.9 y; BMI: 30.0 ± 2.8 kg/m2) did not differ between the 4 groups at baseline (Supplemental Table 2). Of the 327 participants who started the study, 140 dropped out. Reasons included the following: too busy (n = 33), noncompliant with the supplements and/or exercise (n = 19), exercise-related injury (n = 1), medical issue (non–study related; n = 32), gaining weight/disliked supplements (n = 20), family issues/relocated (n = 25), cost of gas (n = 2), and no excuse provided (n = 8). Twenty-four-hour UUN output was greater in the 60-g/d group than in the 0-g/d group at the midpoint and end of the study, which is consistent with greater total protein intake (group-by-time, P < 0.05; Supplemental Table 3). During the first half of the study (baseline to wk 18), 89% of the participants consumed >50% of the supplements and 96% completed >80% of the exercise sessions (Supplemental Table 4). During the second half of the study, fewer participants were in the highest compliance category (supplements: >80%; exercise: >90%), whereas the number of participants increased in the lower compliance categories (supplements: 79–50% and <50%; exercise: 89–70% and <70%).
Whey protein supplementation
Except for total protein intake, whey protein supplementation did not affect any of the physiologic, metabolic, nutritional intake, body composition, or appetite responses or indices of metabolic syndrome over time as described below (i.e., there were no significant group-by-time interactions).
Physiologic and metabolic responses to training
Among all participants, whole-body strength (1 repetition maximum) increased 15 ± 12% (66 ± 49 kg, P < 0.001) and VO2max (maximal oxygen uptake capacity) increased by 9 ± 15% (3.0 ± 5.0 mL · kg−1 · min−1, P < 0.001) from pre- to postintervention (Supplemental Table 3). There was a trend for an increase in resting energy expenditure over time (pre- vs. postintervention; 0.6 ± 2.0 MJ/d, P = 0.08), with 62% of the change occurring between baseline and wk 18. Carbohydrate oxidation was lower at wk 18 (0.15 ± 0.09 g/min, P < 0.01) and wk 36 (0.17 ± 0.17 g/min, P < 0.01) vs. baseline (0.18 ± 0.09 g/min). Fat oxidation was higher (P < 0.01) at wk 36 (0.06 ± 0.05 g/min) vs. baseline (0.05 ± 0.04 g/min). Protein oxidation increased (P < 0.01) from baseline (0.01 ± 0.01 g/min) to wk 18 (0.04 ± 0.03 g/min) but decreased (P < 0.01) from wk 18 to wk 36 (0.01 ± 0.01 g/min).
Nutritional intakes
Nonsupplement intakes.
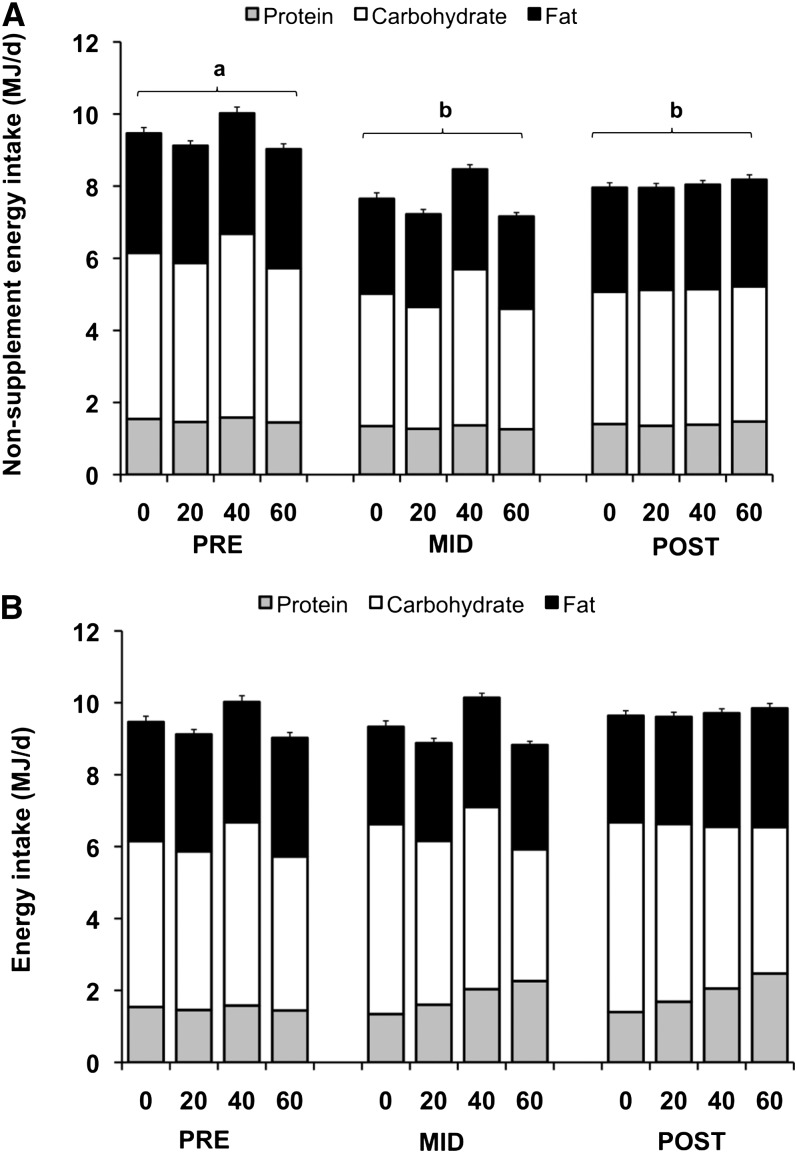
Among all groups, nonsupplement energy intake decreased from baseline to wk 18 (−1.8 ± 1.9 MJ/d, P < 0.001) and pre- to postintervention (−1.4 ± 2.2 MJ/d, P < 0.001); thus, the participants maintained body weight by compensating for the 1.7-MJ/d supplements (Fig. 1A, Supplemental Tables 5 and 6). The macronutrient distribution of nonsupplement energy intakes did not largely differ from baseline macronutrient distributions (Fig. 1A). The percentage of energy contribution from protein increased (16 ± 3% vs. 17 ± 4%, P < 0.01) and from carbohydrate decreased (48 ± 8% vs. 46 ± 8%, P = 0.03) from pre- to postintervention. The percentage of energy contribution from fat did not change (35 ± 6% vs. 36 ± 6%, P = 0.47).
FIGURE 1.
Contribution of nonsupplement macronutrient energy intake to daily nonsupplement energy intake (A) and total macronutrient energy intake to daily total energy intake (B) in overweight and obese adults at wk 0, 18, and 36 of the supplementation and exercise intervention. The x-axis represents the 4 supplement groups (0, 20, 40, and 60 g whey/d) at the pre-, mid-, and postintervention time points. Data are mean ± SE. n = 176 (n = 72, 0 g/d; n = 65, 20 g/d; n = 16, 40 g/d; n = 23, 60 g/d). Labeled time periods without a common letter had overall means that differ, P < 0.05.
Total energy and protein intakes (including supplements).
Total energy intakes were not different between time points or protein groups (Fig. 1B and Supplemental Tables 5 and 6). Total protein intake (g/d and g · kg−1 · d−1) decreased from pre- to postintervention in the 0-g/d group (P = 0.01) and increased (P < 0.001) from pre- to postintervention in the 20-, 40-, and 60-g/d groups (Fig. 2 and Supplemental Table 5). Over time, the percentage of energy from protein decreased in the 0-g/d group and increased in the 20-, 40-, and 60-g/d groups (P < 0.05). The relative contribution of total protein was lower in the 0-g/d group compared with the 20-, 40-, and 60-g/d groups (Supplemental Fig. 2).
FIGURE 2.
Total dietary protein intakes in overweight and obese adults at wk 0, 18, and 36 of the supplementation and exercise intervention. The x-axis represents the 4 supplement groups (0, 20, 40, and 60 g whey/d) at the pre-, mid-, and postintervention time points. Data are mean ± SE. n = 176 (n = 72, 0 g/d; n = 65, 20 g/d; n = 16, 40 g/d; n = 23, 60 g/d). *Different from 0 g/d (P < 0.05). Within a group, labeled means without a common letter differ (protein-by-week interaction P < 0.05).
Body composition
Among all participants, body weight from pre- to postintervention was unchanged (P = 0.80), whole-body lean body mass increased by 1.9 ± 2.8% (P < 0.001), and fat mass decreased by −2.6 ± 9.4% (P < 0.01) (Fig. 3 and Supplemental Table 7). Appendicular lean mass increased (0.5 ± 0.8 kg, P < 0.01) and appendicular fat mass decreased (−0.3 ± 1.0 kg, P < 0.01) over time (pre- to postintervention) (Supplemental Table 7).
FIGURE 3.
Changes in body weight, lean body mass, and fat mass in overweight and obese adults from the pre- to mid-, mid- to post-, and pre- to postintervention time points. Data are mean ± SE. n = 220 (pre- to mid-: n = 84, 0 g/d; n = 81, 20 g/d; n = 25, 40 g/d; n = 30, 60 g/d; mid- to post- and pre- to post-: n = 70, 0 g/d; n = 71, 20 g/d; n = 21, 40 g/d; n = 26, 60 g/d). Labeled means without a common letter differ, P < 0.05.
Indices of metabolic syndrome
Among all participants, waist circumference, waist-to-hip ratio, plasma lipid lipoprotein profile (total, LDL, and HDL cholesterol and TG), and blood pressure were unchanged (Supplemental Table 8). Glucose AUC was unchanged (−18.0 ± 170 mmol/L · 3 h, P = 0.16), insulin AUC decreased by 2.6 ± 32% (−7.5 ± 29 nmol/L · 3 h, P = 0.01), and HOMA-IR (0.2 ± 2.0, P = 0.81) and insulin sensitivity index (0.3 ± 3.0, P = 0.63) were unchanged (Supplemental Table 8). Change in insulin AUC, but not in glucose AUC, was associated with changes in body fat mass (r = 0.26, P < 0.001). Changes in lean body mass were associated with both changes in insulin (r = 0.16, P = 0.03) and glucose (r = 0.16, P = 0.03) AUC. CRP (−0.5 ± 6.0 mg/L; P = 0.14) and PAI-1 (−4.6 ± 47.0 μg/L, P = 0.29) were unchanged (Supplemental Table 8).
The number of participants that met the American Heart Association and National Heart, Lung, and Blood Institute criteria for metabolic syndrome was not affected by whey protein supplementation and did not change over time (baseline = 46%, wk 18 = 45%, and wk 36 = 47%).
Renal function
Among all participants, plasma albumin and creatinine and estimated glomerular filtration rate were within ranges of clinical normalcy (albumin: 32–52 g/L; creatinine: 53–114 μmol/L; glomerular filtration rate: >60 mL/min) at baseline and at wk 18 and wk 36.
Appetite
Desire to eat increased from mid- to postintervention (0.15 ± 1.1 AU, P = 0.02), and there was a trend for an increase in hunger from pre- to postintervention (0.15 ± 1.6 AU, P = 0.05). Fullness did not change (P = 0.25).
Secondary analysis of compliant participants
Overall, comparable results were achieved when only participants who achieved the highest compliance to both the supplementation and exercise sessions were included in the analyses (wk 0, n = 155; wk 18, n = 154; wk 36, n = 91). Similar to the original analyses, there was no effect of whey protein supplementation on any outcomes, except for UUN and dietary protein intakes. Findings for both nonsupplement and total nutritional intakes as well as body weight and composition were comparable to the primary analyses. However, a main effect of time for carbohydrate and fat oxidation was no longer present.
Discussion
This is the first study, to our knowledge, to examine the effect of different quantities of whey protein supplementation during 36 wk of exercise training on body composition and indices of metabolic syndrome in overweight and obese middle-aged adults. The primary findings were that increasing total protein intake through whey protein supplementation was not effective in enhancing exercise training–induced improvements in body composition and indices of metabolic syndrome. The participants compensated for the additional dietary energy from the supplements by reducing nonsupplement energy intakes, which led to weight maintenance. The increase in UUN with higher intakes of whey protein and increases in strength, aerobic capacity, and lean body mass support the success of this free-feeding, community-based intervention to increase total protein intakes and improve fitness and body composition over the long term.
The finding that exercise-induced increases in lean mass and decreases in fat mass were not altered with whey protein supplementation is consistent with other training studies in middle-aged adults (13, 15), but contrasts with findings in younger adults (12, 25). Training studies in young men showed an additional 2–6% increase in lean body mass with whey protein supplementation, but the participants consumed very high quantities of whey protein (>100 g/d for 6–10 wk) (12, 25). The studies in middle-aged men supplemented with 26–35 g whey protein/d for 12–14 wk and found no additional benefit. Because intakes of whey protein >100 g/d can be difficult to maintain over the long term, the current study examined consumption of whey protein ranging from 0 to 60 g/d. The current study also extended the intervention period to 36 wk to evaluate changes over a longer term. The results suggest that neither whey protein amount nor extending the supplementation period to 36 wk influenced the exercise-induced improvements in body composition in overweight and obese middle-aged adults.
Previous studies in 50–80-y-old adults indicated no difference in lean body mass gains between adults who consumed 1.2–1.6 g protein · kg−1 · d−1 compared with those who consumed close to the recommended dietary allowance (41) for protein (0.8–0.9 g protein · kg−1 · d−1) during a 12-wk progressive-resistance exercise program (3, 24). Total protein intakes in the current study were above the RDA (0.8 g protein · kg−1 · d−1), with intakes of 0.93, 1.13, 1.43, and 1.63 g · kg−1 · d−1 in the 0-, 20-, 40-, and 60-g/d groups, respectively. The lack of a lower protein group who consumed the RDA (0.8 g protein · kg−1 · d−1) for protein may account for the inability to detect a differential response between the 0-g/d group and the higher protein groups. Findings from some resistance-training studies indicated no change (42) or decreases in lean body mass (43) when older participants (>50 y) consumed 0.8 g protein · kg−1 · d−1, and a regression analysis combining findings from 6 studies supported these findings (10). Collectively, our results support a growing body of literature that suggests that a higher protein intake in middle-aged and older weight-stable adults does not enhance resistance training–induced gains in lean body mass when adequate dietary protein is consumed.
To date, only one study has examined the effect of whey protein supplementation during exercise training on indices of metabolic syndrome. Denysschen et al. (13) supplemented overweight hyperlipidemic men with 26.6 g whey protein/d during 12 wk of resistance training (3 d/wk). After the intervention, total cholesterol decreased, and TG and LDL and HDL cholesterol remained unchanged independent of whey protein. In the absence of exercise training, consumption of 54 g whey protein/d for 12 wk resulted in reductions in fasting TG, total and LDL cholesterol, insulin, and HOMA scores compared with a control group in overweight and obese adults; however, body composition was not altered (44). In the current study, blood lipids and lipoproteins were unaltered, whereas blood pressure decreased. Furthermore, a decreased insulin response (45) to an oral-glucose challenge was seen independent of whey protein intake and was associated with reductions in fat mass. The current study extends the previous findings to include a titration of whey protein quantities spanning 0–60 g/d and a more comprehensive evaluation of metabolic syndrome indices in combination with resistance and aerobic exercise training. Collectively, these findings suggest that, in the longer term, whey protein supplementation does not enhance exercise training–induced improvements in indices of metabolic syndrome and that changes in insulin sensitivity are likely a result of the reductions in fat mass in overweight and obese middle-aged adults. Although speculative, the lack of lipid- and cholesterol-lowering effects of whey protein during exercise training, specifically with the higher doses (40 and 60 g/d), may be a result of the participants having clinically normal lipoprotein profiles.
Studies that use protein supplementation as a means to increase total protein intake need to document dietary compensation because it can alter the daily energy and macronutrient intakes of participants. Dietary compensation data during long-term supplementation and exercise training are sparse, especially in overweight and obese middle-aged adults. In the current study, participants successfully compensated for the energy in the supplements, and as a result nonsupplement intakes of protein, carbohydrate, and fat decreased. Interestingly, the decrease was to a similar extent in all groups (−6%, −18%, and −12% for protein, carbohydrate, and fat, respectively) and did not largely change nonsupplement macronutrient distribution pre- and postintervention (Supplemental Table 5). This shows that participants did not preferentially compensate with a specific macronutrient. During resistance training, shorter-term supplementation studies have generally reported complete compensation for the supplemental energy and macronutrients (e.g., no increase in total protein intake), leading to no change in body weight in younger and middle-aged adults (12, 15) or a small increase in body weight (1.5 kg) that can be attributed to exercise-induced increases in lean body mass (2.3 kg) in young men (25). Middle-aged men were also shown to successfully compensate for the supplemental energy, but the resulting decrease in nonsupplemental protein was less than the protein content of the supplements, leading to increased total protein intakes (13). Our findings show that higher total protein intakes are achievable with supplementation as little as 20 g protein/d, and the impact of the supplements on dietary intakes is sustainable in the longer term.
The strengths of this study include using an exercise training program that effectively improved physiologic, metabolic, and anthropometric characteristics of the participants. The changes in fitness and body composition observed in this study are comparable to those in other community-based exercise training studies and include similar increases in aerobic capacity (7–19%) (46–48) and strength (8–39%) (49–51) and gains in lean body mass that were similar (50) or greater (51) than in other studies. Evidence from some (52–54), but not all (55), shorter-term studies (12 wk) suggests that consuming protein supplements immediately before or after resistance exercise sessions may be more effective in enhancing gains in lean body mass. Because the aim of the current study was to evaluate the effects of prolonged protein supplementation (9 mo) on gains in lean body mass, participants were instructed to consume the supplements with breakfast and lunch to maintain consistency with the timing of supplement consumption and to provide flexibility as to when they chose to perform the weekly exercise sessions. Future longer-term interventions should evaluate the effect of the timing of protein supplement intake on resistance exercise–induced lean body mass gains in middle-aged and older adults.
This study is limited in that it was not powered to statistically detect changes in indices of metabolic syndrome or sex differences. The original aim of the investigation was to evaluate the effect of whey protein supplementation during exercise training on changes in lean body mass. Therefore, power calculations were performed from available data on changes in lean body mass in men and women, and participants were recruited on the basis of BMI and not sex or the presence of metabolic syndrome. The lack of change in some of the indices of metabolic syndrome may be because <50% of the participants met the criteria for diagnosis of metabolic syndrome (34). Studies powered to evaluate the effect of whey protein and exercise training on sex-specific outcomes as well as on indices of metabolic syndrome are warranted. This study is also limited by the high dropout rate (43%). However, secondary analyses on compliant participants as well as analyses comparing completers vs. noncompleters (data not shown) showed no differences in any of the outcomes, suggesting that the dropout rate had a minor impact on the results.
In conclusion, whey protein supplementation that increased total protein intakes to as high as twice the RDA during exercise training did not enhance exercise-induced responses in strength, aerobic fitness, or body composition or indices of metabolic syndrome in weight-stable overweight and obese middle-aged adults. Although results from this study must be interpreted with caution due to the high dropout rate, this study supports the success of a community-based aerobic and resistance exercise training intervention to positively influence fitness and body composition and promote metabolic health.
Supplementary Material
Acknowledgments
W.W.C. and E.M.J. conceived and designed the experiment; E.M.W. and T.B.C. conducted the clinical portion of this study; E.M.W., T.B.C., V.M.K., E.M.J., and W.W.C. participated in sample analysis and data processing; E.M.W., L.P.S., E.L., and W.W.C. were involved in data analysis and interpretation; W.W.C. and E.M.W. wrote the manuscript; and all authors provided editorial input to finalize the manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by the U.S. Whey Protein Research Consortium and NIH T32AG025671 and UL1RR025761.
The trial was registered at clinicaltrials.gov as NCT00812409.
Supplemental Figures 1–3 and Tables 1–8 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: AU, arbitrary unit; CRP, C-reactive protein; PAI-1, plasminogen activator inhibitor-1; UUN, urinary urea nitrogen; VCO2, carbon dioxide production; VO2, oxygen consumption.
Literature Cited
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Cardiol Rev. 2005;13:322–7 [PubMed] [Google Scholar]
- 2.Sullivan PW, Morrato EH, Ghushchyan V, Wyatt HR, Hill JO. Obesity, inactivity, and the prevalence of diabetes and diabetes-related cardiovascular comorbidities in the U.S., 2000–2002. Diabetes Care. 2005;28:1599–603 [DOI] [PubMed] [Google Scholar]
- 3.Iglay HB, Thyfault JP, Apolzan JW, Campbell WW. Resistance training and dietary protein: effects on glucose tolerance and contents of skeletal muscle insulin signaling proteins in older persons. Am J Clin Nutr. 2007;85:1005–13 [DOI] [PubMed] [Google Scholar]
- 4.Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African-American and Caucasian women. J Gerontol A Biol Sci Med Sci. 2003;58:181–9 [DOI] [PubMed] [Google Scholar]
- 5.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001. Jun;33:S502–15; discussion S28–9 [DOI] [PubMed] [Google Scholar]
- 6.Kelley G, McClellan P. Antihypertensive effects of aerobic exercise. A brief meta-analytic review of randomized controlled trials. Am J Hypertens. 1994;7:115–9 [DOI] [PubMed] [Google Scholar]
- 7.American College of Sports Medicine and the American Diabetes Association: joint position statement Exercise and type 2 diabetes. Med Sci Sports Exerc. 2010;42:2282–303 [DOI] [PubMed] [Google Scholar]
- 8.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–88 [DOI] [PubMed] [Google Scholar]
- 9.Kelley G, Tran ZV. Aerobic exercise and normotensive adults: a meta-analysis. Med Sci Sports Exerc. 1995;27:1371–7 [PubMed] [Google Scholar]
- 10.Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr. 2007;26:696S–703S [DOI] [PubMed] [Google Scholar]
- 11.Phillips SM. Physiologic and molecular bases of muscle hypertrophy and atrophy: impact of resistance exercise on human skeletal muscle (protein and exercise dose effects). Appl Physiol Nutr Metab. 2009;34:403–10 [DOI] [PubMed] [Google Scholar]
- 12.Cribb PJ, Williams AD, Carey MF, Hayes A. The effect of whey isolate and resistance training on strength, body composition, and plasma glutamine. Int J Sport Nutr Exerc Metab. 2006;16:494–509 [DOI] [PubMed] [Google Scholar]
- 13.Denysschen CA, Burton HW, Horvath PJ, Leddy JJ, Browne RW. Resistance training with soy vs whey protein supplements in hyperlipidemic males. J Int Soc Sports Nutr. 2009;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes A, Cribb PJ. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care. 2008;11:40–4 [DOI] [PubMed] [Google Scholar]
- 15.Eliot KA, Knehans AW, Bemben DA, Witten MS, Carter J, Bemben MG. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging. 2008;12:208–12 [DOI] [PubMed] [Google Scholar]
- 16.Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr. 2007;26:713S–23S [DOI] [PubMed] [Google Scholar]
- 17.Kawase M, Hashimoto H, Hosoda M, Morita H, Hosono A. Effect of administration of fermented milk containing whey protein concentrate to rats and healthy men on serum lipids and blood pressure. J Dairy Sci. 2000;83:255–63 [DOI] [PubMed] [Google Scholar]
- 18.Frid AH, Nilsson M, Holst JJ, Bjorck IM. Effect of whey on blood glucose and insulin responses to composite breakfast and lunch meals in type 2 diabetic subjects. Am J Clin Nutr. 2005;82:69–75 [DOI] [PubMed] [Google Scholar]
- 19.Ostman EM, Liljeberg Elmstahl HG, Bjorck IM. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr. 2001;74:96–100 [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto N, Takano T. Antihypertensive peptides derived from milk proteins. Nahrung. 1999;43:159–64 [DOI] [PubMed] [Google Scholar]
- 21.Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 2003;77:326–30 [DOI] [PubMed] [Google Scholar]
- 22.Holmer-Jensen J, Karhu T, Mortensen LS, Pedersen SB, Herzig KH, Hermansen K. Differential effects of dietary protein sources on postprandial low-grade inflammation after a single high fat meal in obese non-diabetic subjects. Nutr J. 2011;10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips SM, Tang JE, Moore DR. The role of milk- and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J Am Coll Nutr. 2009;28:343–54 [DOI] [PubMed] [Google Scholar]
- 24.Campbell WW, Crim MC, Young VR, Joseph LJ, Evans WJ. Effects of resistance training and dietary protein intake on protein metabolism in older adults. Am J Physiol. 1995;268:E1143–53 [DOI] [PubMed] [Google Scholar]
- 25.Burke DG, Chilibeck PD, Davidson KS, Candow DG, Farthing J, Smith-Palmer T. The effect of whey protein supplementation with and without creatine monohydrate combined with resistance training on lean tissue mass and muscle strength. Int J Sport Nutr Exerc Metab. 2001;11:349–64 [DOI] [PubMed] [Google Scholar]
- 26.Campbell WW, Crim MC, Young VR, Evans WJ. Increased energy requirements and changes in body composition with resistance training in older adults. Am J Clin Nutr. 1994;60:167–75 [DOI] [PubMed] [Google Scholar]
- 27.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39 Suppl 1:5–41 [PubMed] [Google Scholar]
- 28.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate: a practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord. 2000;24:1119–30 [DOI] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70 [DOI] [PubMed] [Google Scholar]
- 31.Potteiger JA, Jacobsen DJ, Donnelly JE. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int J Obes Relat Metab Disord. 2002;26:87–9 [DOI] [PubMed] [Google Scholar]
- 32.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 33.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70 [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5 [DOI] [PubMed] [Google Scholar]
- 35.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–34 [DOI] [PubMed] [Google Scholar]
- 37.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24:794–800 [DOI] [PubMed] [Google Scholar]
- 38.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the 'Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–34 [DOI] [PubMed] [Google Scholar]
- 39.Belobrajdic DP, McIntosh GH, Owens JA. A high-whey-protein diet reduces body weight gain and alters insulin sensitivity relative to red meat in Wistar rats. J Nutr. 2004;134:1454–8 [DOI] [PubMed] [Google Scholar]
- 40.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, Alegiani SS, Raschetti R, Velussi M, Ferrannini E. Recurrence of cardiovascular events in patients with type 2 diabetes: epidemiology and risk factors. Diabetes Care. 2008;31:2154–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haub MD, Wells AM, Tarnopolsky MA, Campbell WW. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am J Clin Nutr. 2002;76:511–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell WW, Trappe TA, Jozsi AC, Kruskall LJ, Wolfe RR, Evans WJ. Dietary protein adequacy and lower body versus whole body resistive training in older humans. J Physiol. 2002;542:631–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr. 2010;104:716–23 [DOI] [PubMed] [Google Scholar]
- 45.Kubota N, Tobe K, Terauchi Y, Eto K, Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes. 2000;49:1880–9 [DOI] [PubMed] [Google Scholar]
- 46.Carroll S, Borkoles E, Polman R. Short-term effects of a non-dieting lifestyle intervention program on weight management, fitness, metabolic risk, and psychological well-being in obese premenopausal females with the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32:125–42 [DOI] [PubMed] [Google Scholar]
- 47.Hansen D, Dendale P, Jonkers RA, Beelen M, Manders RJ, Corluy L, Mullens A, Berger J, Meeusen R, van Loon LJ. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia. 2009;52:1789–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, Stone EJ, Rajab MW, Corso P. The effectiveness of interventions to increase physical activity: a systematic review. Am J Prev Med. 2002;22:73–107 [DOI] [PubMed] [Google Scholar]
- 49.de Vreede PL, Samson MM, van Meeteren NL, Duursma SA, Verhaar HJ. Functional-task exercise versus resistance strength exercise to improve daily function in older women: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:2–10 [DOI] [PubMed] [Google Scholar]
- 50.Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, Zimmet P. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002;25:1729–36 [DOI] [PubMed] [Google Scholar]
- 51.Jennings AE, Alberga A, Sigal RJ, Jay O, Boule NG, Kenny GP. The effect of exercise training on resting metabolic rate in type 2 diabetes mellitus. Med Sci Sports Exerc. 2009;41:1558–65 [DOI] [PubMed] [Google Scholar]
- 52.Josse AR, Tang JE, Tarnopolsky MA, Phillips SM. Body composition and strength changes in women with milk and resistance exercise. Med Sci Sports Exerc. 2010;42:1122–30 [DOI] [PubMed] [Google Scholar]
- 53.Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535:301–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips SM. The science of muscle hypertrophy: making dietary protein count. Proc Nutr Soc. 2011;•••:100–3 [DOI] [PubMed] [Google Scholar]
- 55.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89:608–16 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.