Abstract
The evidence relating prenatal supplementation with DHA to offspring neurological development is limited. We investigated the effect of prenatal DHA supplementation on infant brainstem auditory-evoked responses and visual- evoked potentials in a double-blind, randomized controlled trial in Cuernavaca, Mexico. Pregnant women were supplemented daily with 400 mg DHA or placebo from gestation wk 18–22 through delivery. DHA and placebo groups did not differ in maternal characteristics at randomization or infant characteristics at birth. Brainstem auditory-evoked responses were measured at 1 and 3 mo in 749 and 664 infants, respectively, and visual-evoked potentials were measured at 3 and 6 mo in 679 and 817 infants, respectively. Left-right brainstem auditory-evoked potentials were moderately correlated (range, 0.26–0.43; all P < 0.001) and left-right visual-evoked potentials were strongly correlated (range, 0.79–0.94; all P < 0.001) within any assessment. Correlations across visits were modest to moderate (range, 0.09–0.38; all P < 0.01). The offspring of DHA-supplemented women did not differ from those of control women with respect to any outcome measure (all comparisons P > 0.10). We conclude that DHA supplementation during pregnancy did not influence brainstem auditory-evoked responses at 1 and 3 mo or visual-evoked potentials at 3 and 6 mo.
Introduction
The first few years of life are critical to the development of the human brain’s structural and functional capacity. DHA is one of a class of long-chain PUFA proposed to be limiting to the growth and development of infants and young children (1). Maternal diets high in fish (a major source of DHA) are associated with increased offspring cognitive development (2, 3), but these associations are susceptible to confounding and the role of specific nutrients is not determinable.
Brainstem auditory-evoked responses and visual-evoked potentials are objective and reproducible measures of brain neurological functioning in early infancy (4–8). The brainstem auditory-evoked response reflects electrophysiological activity in the brainstem auditory pathway following acoustic stimulation. Visual-evoked potentials are the electrical responses of the brain to a visual stimulus. Previous studies of the effect of fish oil supplementation and other sources of DHA on infant cognitive development (9–11) and evoked potentials and acuity tests have yielded conflicting findings (12–18), perhaps related to the underlying nutritional intakes of the mothers, the source of DHA used in the trial (fish oil vs. isolated DHA), the delivered dose in the supplement (studies have provided doses ranging from 100 to 800 mg/d DHA), and the timing of the intervention (prenatal only, both prenatal and postnatal, postnatal only).
We conducted a large randomized, double-blind, placebo-controlled study among women with low dietary intakes of DHA of the effect of supplementation with 400 mg/d algal DHA from mid-pregnancy to delivery on the evoked potentials of their offspring.
Participants and Methods
Study population and setting.
We conducted an assessment of infants born to women recruited into the Prenatal DHA (omega-3 fatty acid) Supplements on Infant Growth and Development study conducted in Cuernavaca, Mexico. The full details of recruitment, inclusion, and exclusion criteria and randomization are provided elsewhere (19).
Women were assigned to receive 2 DHA (200 mg derived from an algal source; Martek Biosciences) or placebo (a mixture of corn and soy oil) capsules daily, from gestation wk 18–22 through parturition. Participants and members of the study team were unaware of the treatment scheme throughout the intervention period of the study.
Measurement of evoked potentials.
Parents were instructed to bathe the infant with mild, fragrance-free soap and to avoid using shampoos, gels, or lotions the evening prior to the study visit. On the day of the assessment, parents were encouraged to keep the child awake until the procedure was to start. The last feeding was to be at least 3 h prior to the appointment and the parents of formula-fed infants were asked to bring a prepared bottle. After check-in at the study center, located in the Mexican Social Security Institute Hospital no 2 in Cuernavaca, the mother or guardian was asked to feed the infant and allow the infant to sleep. Evoked potentials were measured in the hospital in a quiet room free of distractions. Once asleep, the infant was placed supine on a bed. Both visual-evoked potentials and brainstem auditory-evoked responses were recorded using a Sierra Wave instrument (Cadwell Laboratories). All staff underwent specific training.
Brainstem auditory-evoked responses, measured at 1 and 3 mo of age, were recorded between the vertex and the mastoid ipsilateral to the stimulated ear (A1, left, and A2, right). These 3 points were cleaned and conduction paste was used to affix standard gold cup electrodes in the appropriate locations. Electrode impedances were kept <5 kOhm. Acoustically shielded, TDH-39, 10-ohm speaker headphones (Cadwell Laboratories) were placed over the infant’s ears. In the first trial, the right ear was stimulated at 11.1 clicks/s for 1000 clicks at 80 dB to observe all the relevant waves of the auditory pathway. The second through fifth trials were of increasing intensity (25, 30, 40, and 60 dB) at 31.1 clicks/s for 1000 clicks. Two trials were conducted at each decibel level. The procedure was repeated in the left ear. Derived variables included the latency of waves I, III, and V and the interpeak latency of waves I-III, III-V, and I-V.
Visual-evoked potentials were measured via stimulus-synchronized electroencephalography at 3 and 6 mo. Visual-evoked potentials were recorded from the active electrode placed 1–2 cm above the occiput, with the reference electrode positioned in the center of the forehead. The ground electrode was fixed to the vertex, found by measuring the infant’s head from the right to the left tragus and placing the electrode at the midpoint distance on the scalp. These 3 points were thoroughly cleaned. Standard gold cup electrodes were affixed in the appropriate locations. Without dark adaptation or pupil dilation, visual-evoked potentials were elicited by light-emitting diode-stimulating goggles placed over the infant’s eyes. Each eye was stimulated independently at 1.1 Hz for a total of 100 stimuli, delivering an output intensity of 1500 cd/m2. Two trials were conducted. Normal visual-evoked potentials to diffuse light flashes are usually described as having 7 peaks that differ widely with respect to peak polarity, latency, and amplitude. We derived the following measures: the latency and magnitude of the major positive peak (P2), occurring between 50 and 100 milliseconds after the flash; the latency of the subsequent, large negative peak (N3), occurring between 100 and 250 milliseconds after the flash; and the latency of the negative wave (N1) preceding the major positive wave.
Other variables.
We obtained information on the mother, either at her home or at the hospital, by interview. We abstracted infant weight, length, and head circumference at birth from the delivery ward records.
Data analysis.
All statistical analyses were done using SAS 9.2 (SAS Institute). We compared data from the left and right sides and between the first and second administration by computing correlation coefficients. To reduce the impact of measurement error, we averaged the data from the left and right side at each time point. To evaluate the effectiveness of randomization, we compared control and intervention groups on selected baseline maternal characteristics and birth outcomes. To identify potential sources of selection bias, we also compared the final analytic sample with those infants lost to follow-up on these same characteristics. For these analyses, we used the Student’s t test for normally distributed, continuous variables and chi-square tests for categorical variables.
We compared the treatment groups using the Student’s t test. We assessed whether maternal DHA intakes were associated with infant-evoked responses in the placebo group using linear regression. We included in the analysis all infants who provided data at any time point. We considered P < 0.05 as significant. We did not adjust for multiple comparisons.
We examined 4 potential effect modifiers. We previously reported that parity modified the effect of DHA supplementation on weight and head circumference at birth, with a positive impact of DHA supplementation evident among offspring born to nulliparous women (19). We therefore tested for effect modification by maternal parity, categorized as nulliparous compared with other. We assessed whether head size might affect our estimates by categorizing head circumference, measured at birth, into tertiles. To assess whether the PUFA composition of the maternal diet during pregnancy modified the effect, we categorized the ratio of the (n-3):(n-6) fatty acids, as derived from intakes reported by the mother at randomization using a previously validated FFQ (20), into tertiles. To assess whether gestational age at birth, as reflective of infant maturation, might modify any associations, we categorized infants as being born preterm (<37 completed weeks) or not. We then developed interaction terms for each of these 4 variables with treatment assignment and tested the significance using P < 0.05.
Results
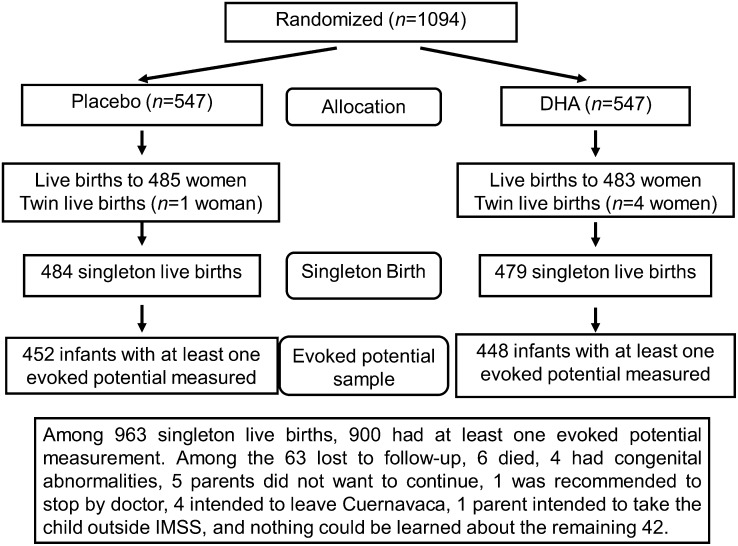
Figure 1 shows the trial profile. We obtained at least one measure of evoked potential from 900 singleton live births without congenital anomalies, 448 of whom were born to women who received DHA and 452 were born to women who received placebo. Overall, loss to follow-up of women who initially received treatment through 6 mo postpartum was 20.0% and was similar between the treatment groups (P = 0.8). Compliance, measured as the proportion of capsules consumed of those that were distributed, was 94% and was similar between the 2 groups (P = 0.6). We obtained all 4 evoked potentials from 572 infants (63.6%). The 572 infants were similar in many respects to the 328 infants who provided 1–3 evoked potentials, except that those with all 4 measures were less likely (34 v.s 42%; P < 0.05) to be first-born.
FIGURE 1.
Flow chart of study recruitment and follow-up.
Baseline demographics and birth outcomes.
The DHA and placebo groups were well matched on a wide range of maternal characteristics at randomization and offspring characteristics at delivery, and the women in the 2 groups did not significantly differ with respect to compliance with the study intervention (Table 1). Mean birth weight and length were 3220 g and 50.4 cm, respectively; the mean gestational age at birth was 39 wk and 9% of babies were born <37 wk gestation.
TABLE 1.
Selected characteristics of women who participated in a supplementation trial of 400 mg/d DHA vs. placebo during the second half of pregnancy and their infants, by treatment allocation12
| Placebo | DHA | P3 | |||
| n | n | ||||
| Maternal characteristics at randomization | |||||
| Age, y | 452 | 26.2 ± 4.6 | 448 | 26.4 ± 4.8 | 0.52 |
| Gestational age, wk | 452 | 20.6 ± 2.1 | 448 | 20.5 ± 2.0 | 0.74 |
| High school or more, % | 451 | 60.0 | 447 | 56.6 | 0.32 |
| Primiparous, % | 452 | 37.8 | 448 | 36.6 | 0.70 |
| Weight, kg | 452 | 63.3 ± 11.0 | 448 | 62.2 ± 11.4 | 0.13 |
| Height, cm | 452 | 155.4 ± 5.6 | 448 | 154.9 ± 5.7 | 0.14 |
| BMI, kg/m2 | 452 | 26.2 ± 4.2 | 448 | 25.9 ± 4.2 | 0.28 |
| (n-3):(n-6) Dietary fatty acid ratio | 452 | 12.0 ± 4.9 | 448 | 12.4 ± 5.7 | 0.28 |
| Compliance with study intervention | |||||
| Capsules consumed, n | 451 | 228 ± 41 | 446 | 228 ± 41 | 0.91 |
| Compliance,4 % | 451 | 95.0 ± 5.5 | 446 | 95.1 ± 5.6 | 0.77 |
| Compliance,5 % | 451 | 88.6 ± 10.1 | 446 | 88.6 ± 10.5 | 0.97 |
| Child characteristics at delivery | |||||
| Male, % | 452 | 52.0 | 448 | 52.9 | 0.78 |
| Weight, g | 452 | 3200 ± 460 | 448 | 3230 ± 430 | 0.52 |
| Length, cm | 452 | 50.3 ± 2.5 | 448 | 50.4 ± 2.2 | 0.77 |
| Head circumference, cm | 396 | 34.3 ± 1.7 | 386 | 34.4 ± 1.5 | 0.26 |
| Weight <2500 g, % | 452 | 5.3 | 448 | 3.8 | 0.28 |
| Gestational age, wk | 450 | 39.1 ± 1.7 | 447 | 39.1 ± 1.8 | 0.78 |
| Preterm (<37 wk), % | 450 | 8.2 | 447 | 9.4 | 0.54 |
Data are mean ± SD or %.
Includes women who were randomized to DHA or placebo, were followed through to delivery, and had a singleton child who provided at least one evoked potential.
value (by t test or chi-square test as appropriate).
Percentage of dose distributed calculated as 100 × (capsules consumed/capsules distributed).
Percentage of intended dose, calculated as 100 × (capsules consumed/days from randomization to delivery).
Brainstem auditory-evoked responses were measured at 1 and 3 mo in 749 (83.2%) and 664 (73.8%) infants, respectively. One rater obtained 630 measurements at 1 mo and 489 measurements at 3 mo and 2 additional raters obtained almost all of the rest. The within-visit correlation between the brainstem auditory-evoked response obtained on the left and right ears ranged from 0.26 (latency III at 1 mo) to 0.43 (interval I–III at 1 mo) and the between-visit correlation of the mean value from the 2 ears ranged from 0.09 (interval III–V) to 0.38 (latency III) (Supplemental Table 1), with no marked differences across raters.
Visual-evoked potentials were measured at 3 and 6 mo in 679 (75.4%) and 817 (90.8%) infants, respectively. One rater obtained 504 measurements at 3 mo and 581 measurements at 6 mo and 2 additional raters obtained almost all of the rest. The within-visit correlation between the visual-evoked potential obtained on the left and right eyes ranged from 0.79 (amplitude P1 at 3 mo) to 0.94 (latency P1 at 6 mo) and the between-visit correlation of the mean value from the 2 eyes ranged from 0.09 (amplitude P1) to 0.11 (latency P1) (Supplemental Table 2) with no marked differences across raters.
Evoked potentials in relation to maternal diet and treatment allocation.
There were no significant differences in any measure of brainstem auditory-evoked responses or visual-evoked potentials by treatment assignment of the mother (all P > 0.19) (Tables 2 and 3); no effect of DHA was observed when data from either the left or right ear or eye was analyzed in place of the mean value.No association was observed between maternal DHA intake and any measures of auditory or evoked potential (Supplemental Table 3). Two of the 80 interaction terms (10 evoked-potential variables across 2 ages at assessment and 4 potential sources of heterogeneity) were significant at P < 0.05 and none met a Bonferroni-corrected threshold.
TABLE 2.
Brainstem auditory-evoked responses at 1 and 3 mo among singleton children whose mothers participated in a supplementation trial of 400 mg/d DHA vs. placebo during the second half of pregnancy, by treatment allocation1
| Placebo | DHA | P2 | |
| One mo | n = 377 | n = 372 | |
| Age at measurement, d | 34.5 ± 5.5 | 34.2 ± 5.1 | 0.47 |
| Latency 1, ms | 1.63 ± 0.14 | 1.62 ± 0.16 | 0.36 |
| Latency 3, ms | 4.19 ± 0.33 | 4.18 ± 0.32 | 0.65 |
| Latency 5, ms | 6.55 ± 0.42 | 6.52 ± 0.48 | 0.35 |
| Interpeak latency 1–3, ms | 2.57 ± 0.36 | 2.56 ± 0.27 | 0.56 |
| Interpeak latency 3–5, ms | 2.37 ± 0.30 | 2.37 ± 0.34 | 0.89 |
| Interpeak latency 1–5, ms | 4.93 ± 0.36 | 4.91 ± 0.39 | 0.60 |
| Three mo | n = 334 | n = 330 | |
| Age at measurement, d | 94.2 ± 5.6 | 94.6 ± 5.5 | 0.41 |
| Latency 1, ms | 1.58 ± 0.15 | 1.58 ± 0.15 | 0.87 |
| Latency 3, ms | 4.02 ± 0.32 | 4.03 ± 0.33 | 0.59 |
| Latency 5, ms | 6.33 ± 0.40 | 6.29 ± 0.42 | 0.24 |
| Interpeak latency 1–3, ms | 2.44 ± 0.28 | 2.45 ± 0.28 | 0.66 |
| Interpeak latency 3–5, ms | 2.31 ± 0.35 | 2.28 ± 0.33 | 0.23 |
| Interpeak latency 1–5, ms | 4.75 ± 0.39 | 4.72 ± 0.39 | 0.34 |
Data are mean ± SD. Sample includes all infants measured at that age.
Significance of difference in means between treatment groups, by test.
TABLE 3.
Visual-evoked potentials at 3 and 6 mo among singleton children whose mothers participated in a supplementation trial of 400 mg/d DHA vs. placebo during the second half of pregnancy, by treatment allocation1
| Placebo | DHA | P2 | |
| Three mo | n = 342 | n = 337 | |
| Age at measurement, d | 94.0 ± 5.4 | 94.4 ± 5.2 | 0.25 |
| Latency N1, ms | 93.9 ± 17.1 | 94.2 ± 16.3 | 0.86 |
| Latency P1, ms | 126.3 ± 18.3 | 125.8 ± 17.5 | 0.72 |
| Amplitude P, μV | 8.14 ± 6.04 | 7.75 ± 5.97 | 0.40 |
| Latency N3, ms | 157.1 ± 24.1 | 154.8 ± 23.8 | 0.22 |
| Six mo | n = 410 | n = 407 | |
| Age at measurement, d | 185.5 ± 6.5 | 185.3 ± 5.9 | 0.76 |
| Latency N1, ms | 91.9 ± 15.1 | 90.5 ± 14.6 | 0.19 |
| Latency P1, ms | 123.5 ± 14.3 | 122.7 ± 14.6 | 0.39 |
| Amplitude P1, μV | 11.3 ± 6.9 | 11.2 ± 7.2 | 0.86 |
| Latency N3, ms | 154.9 ± 20.2 | 154.2 ± 19.9 | 0.63 |
Data are mean ± SD. Sample includes all infants measured at that age.
Significance of difference in means between treatment groups, by test.
Discussion
In a large, double-blind, randomized, placebo-controlled trial, we observed no effect of supplementation of pregnant women with 400 mg/d DHA in the second half of pregnancy on infant-evoked potentials. Furthermore, we observed no consistent heterogeneity across 4 prespecified variables that might have affected the relationship. Our study was conducted on a population with low dietary intakes of DHA and a high ratio of linoleic acid:alpha-linolenic acid (20) and provided DHA in amounts consistent with most recommendations (1). Compliance with the study intervention was high (19) and DHA supplementation was efficacious in raising DHA concentrations in maternal plasma, cord blood, and breast milk (21).
There was no differential response between the left and right ear or between the left and right eye. The brainstem audio-evoked responses were only modestly correlated across ears and within infants over time. Thus, even though we used the average of the left and right measures in our analysis to reduce the impact of measurement error, it is possible that measurement error attenuated our ability to detect any association. Other studies have shown that auditory responses differ by ear (22, 23), but we are not aware of studies that have examined differential response to DHA. The within-visit, between-eye correlations for visual-evoked potentials were very high, indicating high concurrent reproducibility. The lack of association of the visual-evoked potential at either age with DHA supplementation is therefore unlikely to be due to measurement error or noise. The correlations within infants over time were low. The poor reproducibility across the 1-, 3-, and 6-mo visits may be because different auditory and visual properties are measured at each age in the evolving brain; at no age was there any suggestion of an effect of DHA.
The potential for DHA to affect infant-evoked potentials derives from DHA’s role in neuronal development (1). Observational studies of dietary sources of DHA have generally shown positive associations between reported fish intake during pregnancy, or DHA as estimated from dietary reports, and offspring cognitive development (2, 3). In a Mexican sample (n = 76) of similar background to our own, Parra-Cabrera (24) identified dietary arachidonic acid as a predictor of infant brainstem auditory-evoked responses at 3 mo. We were unable to replicate these observations in our own, much larger sample. These studies are all limited by the potential for confounding by underlying factors that determine both fish intake and child development.
Supplementation studies have generally shown no effect on visual acuity, but sample sizes have been relatively small. Malcolm et al. (12) provided fish oil capsules to women from wk 15 through parturition and observed no effects on visual acuity. Innis and Friesen (13) provided 400 mg/d algal DHA or placebo from wk 16 through parturition to 135 women and observed that visual acuity at 2 mo was higher in the group assigned to DHA. In one small study (n = 30), Judge et al. (14) reported that acuity at 4 mo, but not at 6 mo, was enhanced among the offspring of women randomized to receive 200 mg/d DHA via fortified cereal bars. More recently, Smithers et al. (15) reported that visual-evoked potential acuity and latency at 4 mo were not related to a DHA-rich fish oil supplement providing 800 mg/d DHA. As in our study, supplementation was from mid-pregnancy through parturition. Our study, with ∼450 infants in each treatment group, the largest study to date, adds substantially to this growing database.
Other studies have suggested that postnatal supplementation with DHA is efficacious. Breast-fed children have greater visual and auditory acuity than formula-fed children (18, 25) and the addition of DHA to infant formula is associated with improved acuity (26). Supplementation of breast-feeding women has shown mixed results (27, 28). Our study did not examine this relationship, because DHA supplementation ended at parturition.
In conclusion, our study provides no support for the hypothesis that maternal intakes of DHA during pregnancy are causally related to infant-evoked potentials.
Supplementary Material
Acknowledgments
The authors acknowledge the guidance and contributions of Ricardo Uauy and Maria Makrides, who served as study consultants for the overall planning and implementation of the larger study, and the contributions of Clara Dominguez, who served as data manager for the study at the Instituto Nacional de Salud Pública, Cuernavaca, Mexico. Socorro Parra-Cabrera and Patricio Peirano trained the field team in collection of the evoked potentials. The authors also thank Mead-Johnson for formulating the active supplement and placebo capsules. A.D.S. designed and conducted research and wrote the paper; J.R. designed and conducted research; R.M. designed and conducted research; M.W. analyzed data; and U.R. designed and conducted research and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH (HD043099)and March of Dimes Foundation(6FY04-69).
This trial was registered at clinicaltrials. gov as NCT00646360
Supplemental Figures 1–3 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of content at http://jn.nutrition.org.
Literature Cited
- 1.Koletzko B, Lien E, Agostoni C, Bohles H, Campoy C, Cetin I, Decsi T, Dudenhausen JW, Dupont C, Forsyth S, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36:5–14 [DOI] [PubMed] [Google Scholar]
- 2.Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ Health Perspect. 2005;113:1376–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels JL, Longnecker MP, Rowland AS, Golding J. ALSPAC Study Team University of Bristol Institute of Child Health. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394–402 [DOI] [PubMed] [Google Scholar]
- 4.Taylor MJ, McCulloch DL. Visual evoked potentials in infants and children. J Clin Neurophysiol. 1992;9:357–72 [DOI] [PubMed] [Google Scholar]
- 5.Barnet AB, Friedman SL, Weiss IP, Ohlrich ES, Shanks B, Lodge A. VEP development in infancy and early childhood. A longitudinal study. Electroencephalogr Clin Neurophysiol. 1980;49:476–89 [DOI] [PubMed] [Google Scholar]
- 6.Benavente I, Tamargo P, Tajada N, Yuste V, Oliván MJ. Flash visually evoked potentials in the newborn and their maturation during the first six months of life. Doc Ophthalmol. 2005;110:255–63 [DOI] [PubMed] [Google Scholar]
- 7.Khedr EMH, Farghaly WMA, El-Din Amry S, Osman AAA. Neural maturation of breastfed and formula-fed infants. Acta Paediatr. 2004;93:734–8 [DOI] [PubMed] [Google Scholar]
- 8.Eldredge L, Salamy A. Functional auditory development in preterm and full term infants. Early Hum Dev. 1996;45:215–28 [DOI] [PubMed] [Google Scholar]
- 9.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics. 2003;111:e39–44 [DOI] [PubMed] [Google Scholar]
- 10.Campoy C, Escolano-Margarit MV, Ramos R, Parrilla-Roure M, Csábi G, Beyer J, Ramirez-Tortosa MC, Molloy AM, Decsi T, Koletzko BV. Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. Am J Clin Nutr. 2011;94 Suppl:S1880–8 [DOI] [PubMed] [Google Scholar]
- 11.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. DOMInO Investigative Team Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304:1675–83 [DOI] [PubMed] [Google Scholar]
- 12.Malcolm CA, McCulloch DL, Montgomery C, Shepherd A, Weaver LT. Maternal docosahexaenoic acid supplementation during pregnancy and visual evoked potential development in term infants: a double blind, prospective, randomised trial. Arch Dis Child Fetal Neonatal Ed. 2003;88:F383–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Innis SM, Friesen RW. Essential n-3 fatty acids in pregnant women and early visual acuity maturation in term infants. Am J Clin Nutr. 2008;87:548–57 [DOI] [PubMed] [Google Scholar]
- 14.Judge MP, Harel O, Lammi-Keefe CJ. A docosahexaenoic acid-functional food during pregnancy benefits infant visual acuity at four but not six months of age. Lipids. 2007;42:117–22 [DOI] [PubMed] [Google Scholar]
- 15.Smithers LG, Gibson RA, Makrides M. Maternal supplementation with docosahexaenoic acid during pregnancy does not affect early visual development in the infant: a randomized controlled trial. Am J Clin Nutr. 2011;93:1293–9 [DOI] [PubMed] [Google Scholar]
- 16.Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2009;81:151–8 [DOI] [PubMed] [Google Scholar]
- 17.Birch EE, Carlson SE, Hoffman DR, Fitzgerald-Gustafson KM, Fu VL, Drover JR, Castañeda YS, Minns L, Wheaton DK, Mundy D, et al. The DIAMOND (DHA Intake And Measurement Of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr. 2010;91:848–59 [DOI] [PubMed] [Google Scholar]
- 18.Unay B, Saricı SU, Ulas UH, Akın R, Alpay F, Gokcay E. Nutritional effects on auditory brainstem maturation in healthy term infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F177–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan U, Stein AD, Parra-Cabrera S, Wang M, Imhoff-Kunsch B, Juárez-Márquez S, Rivera J, Martorell R. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull. 2010; 31 Suppl 2:S108–16 [DOI] [PubMed] [Google Scholar]
- 20.Parra-Cabrera S, Stein AD, Wang M, Martorell R, Rivera J, Ramakrishnan U. Dietary intakes of polyunsaturated fatty acids among pregnant Mexican women. Matern Child Nutr. 2011;7:140–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imhoff-Kunsch B, Stein AD, Villalpando S, Martorell R, Ramakrishnan U. Docosahexaenoic acid supplementation from mid-pregnancy to parturition influenced breast milk fatty acid concentrations at 1 month postpartum in Mexican women. J Nutr. 2011;141:321–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockard JE, Stockard JJ, Coen RW. Auditory brain stem response variability in infants. Ear Hear. 1983;4:11–23 [DOI] [PubMed] [Google Scholar]
- 23.Sininger YS, Cone-Wesson B. Lateral asymmetry in the ABR of neonates: evidence and mechanisms. Hear Res. 2006;212:203–11 [DOI] [PubMed] [Google Scholar]
- 24.Parra-Cabrera S, Moreno-Macias H, Mendez-Ramirez I, Schnaas L, Romieu I. Maternal dietary omega fatty acid intake and auditory brainstem-evoked potentials in Mexican infants born at term: Cluster analysis. Early Hum Dev. 2008;84:51–7 [DOI] [PubMed] [Google Scholar]
- 25.Birch EE, Castañeda YS, Wheaton DH, Birch DG, Uauy RD, Hoffman DR. Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am J Clin Nutr. 2005;81:871–9 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman DR, Birch EE, Castañeda YS, Fawcett SL, Wheaton DH, Birch DG, Uauy R. Visual function in breast-fed term infants weaned to formula with or without long-chain polyunsaturates at 4 to 6 months: A randomized clinical trial. J Pediatr. 2003;142:669–77 [DOI] [PubMed] [Google Scholar]
- 27.Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Rozelle JC, Turcich MR, Llorente AM, Anderson RE, Heird WC. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J Clin Nutr. 2005;82:125–32 [DOI] [PubMed] [Google Scholar]
- 28.Morale SE, Hoffman DR, Castañeda YS, Wheaton DH, Burns RA, Birch EE. Duration of long-chain polyunsaturated fatty acids availability in the diet and visual acuity. Early Hum Dev. 2005;81:197–203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.