Abstract
Coadministration of retinoic acid (RA) and polyinosinic acid:polycytidylic acid (PIC) has been shown to cooperatively enhance the anti–tetanus toxoid (anti-TT) vaccine response in adult mice. Germinal center formation in the spleen is critical for a normal antibody response. Recent studies have identified Stimulated by Retinoic Acid-6 (Stra6) as the cell membrane receptor for retinol-binding protein (RBP) in many organs, including spleen. The objectives of the present studies were to test whether orally administered vitamin A (VA) itself, either alone or combined with RA, and/or treatment with PIC regulates Stra6 gene expression in mouse spleen and, concomitantly, antibody production. Eight-week-old C57BL/6 mice were immunized with TT. In an initial kinetic study, oral VA (6 mg/kg) increased anti-TT IgM and IgG production as well as splenic Stra6 mRNA expression. In treatment studies that were analyzed 9 d postimmunization, retinoids including VA, RA, VA and RA combined, and PIC significantly increased plasma anti-TT IgM and IgG (P < 0.05) and splenic Stra6 mRNA (P < 0.05). Treatments that included PIC elevated plasma anti-TT IgM and IgG concentrations >20-fold (P < 0.01). Immunohistochemistry of STRA6 protein in mouse spleen confirmed its increase after immunization and retinoid treatment. In conclusion, retinoid treatments that included VA, RA, VA and RA combined, and the combination of retinoid and PIC stimulated the expression of Stra6 in spleen, which potentially could increase the local uptake of retinol. Concomitantly, these treatments increased the systemic antigen-specific antibody response. The ability of oral retinoids to stimulate systemic immunity has implications for public health and therapeutic use of VA.
Introduction
Vitamin A (VA)6 was characterized as an antiinfective vitamin due to the observation that VA-deficient animals suffered from infections whereas VA-adequate animals survived (1). The antiinfective effect of VA is thought to be attributable to the important role of VA and its metabolites in innate and adaptive immunity. VA and its active metabolite, retinoic acid (RA), have been shown to modulate several indices of innate and adaptive immunity, such as dendritic cell maturation, cytokine production, T and B cell activation and differentiation, Th1/Th2 and Treg/Th17 cell balance, and antibody responses (2–9).
The roles of retinoids in immunity have been explored for developing strategies to improve vaccine efficiency. In adult mice, coadministration of RA and polyriboinosinic:polyribocytidylic acid (PIC), a Toll-like receptor-3 ligand and an inducer of IFN and other cytokines, cooperatively enhanced the anti-tetanus toxoid (anti-TT) antibody response (10). The combination of RA and PIC stimulated a robust increase in all of the anti-TT IgG isotypes. Similar responses were reported after treatment of neonatal mice with RA plus PIC at the time of immunization with TT (11). Whereas VA itself has been tested and shown to be effective in models of VA deficiency, there is little information on whether administration of VA promotes the antibody response in the VA-adequate state. This is also relevant, because strategies to improve child health through VA supplementation target entire populations, of which not all children are likely to be deficient.
The route of retinoid administration has varied among different studies, with some studies using administration by methods other than oral dosing. Although such routes may be effective, they are much less likely to be translated to human studies, and thus understanding the effects of retinoids delivered orally, on a variety of vaccine responses, is important for their use in public health or therapeutic treatments. VA itself, generally administered as retinyl palmitate, is the form used in micronutrient supplementation programs. Currently, there are few comparative studies on VA and RA with regard to their effectiveness in promoting innate immunity or antibody production.
Efficient vaccination with protein antigens such as TT depends on the germinal center (GC) reaction, in which antigen-activated B cells undergo maturation, class-switch recombination, and affinity maturation. Our laboratory provided the first evidence, in studies of adult mice (12), that RA and/or PIC can significantly promote the TT-induced GC reaction in spleen, which is a major location for the formation of GC after immunization with TT (12), through modulation of GC formation, isotype switching, and follicular dendritic cell network formation. On the basis of the positive effects of RA on processes occurring in spleen during the primary response to immunization, especially in the GC after immunization, it was of interest to determine if retinol (VA) is taken up into spleen, which could be important for the effective and efficient vaccine responses.
The major physiologic mediator of retinol uptake by cells into many tissues including spleen is Stimulated by Retinoic Acid-6 (Stra6), a widely expressed 74-kDa multi-transmembrane domain protein that functions as a cell-surface receptor for retinol bound to retinol-binding protein (RBP) (13, 14). Retinol removed from RBP after binding to Stra6 can be transported into cells and metabolized intracellularly (13). The Stra6 gene is expressed during embryonic development and in the adult brain, kidney, testis, female genital tract, and spleen and at lower quantities in heart and lung. It is worth noting that Stra6 is highly expressed in a subset of cells in the spleen (13). Stra6 was originally found to be an RA-stimulated gene in cancer cell lines (15). However, it can also be regulated by RA in normal tissues, because VA combined with RA (VARA) increased the uptake of retinol in the lung of neonatal rats within 6 h after dosing (16), whereas Stra6 mRNA expression increased 3~4 times (17). Previous studies have shown that the Stra6 gene can respond to immune stimulation. The expression of Stra6 mRNA in alloreactive T cells was upregulated by mesenteric lymph node–derived dendritic cells in a mixed lymphocyte culture (18) and in a dendritic cell line treated with LPS from Porphyromonas gingivalis (19). However, these studies were performed in culture and neither involved VA. Therefore, it was of interest to determine the response of Stra6 concomitant with vaccination and VA treatment in intact animals.
We hypothesized that orally administered VA, given alone and when combined with treatment with RA and/or PIC, will stimulate the expression of Stra6 in spleen, which may increase the retinol uptake into spleen, and these combinations may augment the antibody response after immunization.
Materials and Methods
Animal and diets.
Animal protocols were approved by the Institutional Animal Use and Care Committee of Pennsylvania State University. Eight-week-old female C57BL/6 mice were purchased from Charles River Laboratories. All mice were fed a nutritionally complete stock rodent diet (LabDiet 5001, which contains 22 IU vitamin A/g diet; Purina Mills) throughout the experimental period. The mice were housed 4–5 per cage with continuous access to food and water and a 12-h light/dark cycle.
Experiment design, immunization, and spleen and blood Collection.
Three experiments were conducted. In Expt. 1, we evaluated the kinetics of splenic Stra6 mRNA expression and antibody production after immunization with TT. Mice were randomly assigned to 9 groups. Group 1 (n = 4) served as a nonimmunized, nontreated control group (used to assess background), and groups 2–9 (n = 4/group) were immunized on d 0 with 100 μg i.p. TT, as previously described (10), and treated with either VA at a dose of 300 μg retinyl palmitate/mouse orally on the first day of the experiment or canola oil, which was also used as a vehicle for VA, as a placebo control. From d 1 to d 8 after immunization, the mice were fed the same dose of VA or oil each day. Mice were killed with carbon dioxide, and blood and spleen were collected on d 3, 5, 7, and 9 postimmunization. The syringes used for blood collection were treated with heparin. When data were analyzed, the anti-TT IgG and IgM concentrations in group 1 were subtracted as background, whereas the splenic Stra6 mRNA expression level was normalized as 1.00 in this group.
In Expt. 2, we tested the regulation of Stra6 mRNA in spleen and concomitant antibody production by various VA, RA, and PIC combinations. Mice were randomly assigned to 9 groups. Mice in group 1 (n = 2) were injected i.p. with PBS and fed canola oil as a nonimmunized and nontreated background group. Mice in groups 2–9 (n = 7/group) were immunized with TT, as in Expt. 1, and treated with VA, RA, and/or PIC given either individually or in combination. Treatments included VA orally (300 μg retinyl palmitate/mouse as described above), RA given orally at a dose of 37.5 μg/mouse, and/or 2 μg PIC administered i.p. once only on the first day of the experiment (d 0). From d 1 to 8 postimmunization, the mice were fed the same dose of VA and/or RA or oil each day. Spleen and blood samples were collected on d 8 postimmunization. For preparation of the RA dose, all-trans-RA (Sigma) was treated with ethanol and suspended in canola oil at 4 g/L, and the suspension was remixed just before each dose was delivered. TT (Connaught Laboratories) and PIC, stabilized with poly-l-lysine and carboxymethylcellulose, were used as previously described (10).
In Expt. 3, we analyzed the uptake of [3H]retinol into spleen with the use of samples from a VA kinetics study conducted in neonatal rats. The mothers of the pups were fed a VA-marginal diet (Research Diets) formulated to contain 0.35 mg retinol equivalents/kg diet. Pups (n = 3/group per time point) were orally administered a single dose containing 11,12-[3H]retinol, admixed as a tracer with the treatment dose, which was either oil as placebo or VA (6 mg/kg retinyl palmitate + RA added as 1/10 of VA on a molar basis and referred to as VARA), administered on postnatal day 4. Various tissues were collected at times from 1 h to 11 d after dose administration, including spleen, which was analyzed in this study. Spleens were extracted with ethanol and hexane by a method modified from previous reports (20–22), and extracts were dried and resolubilized in liquid scintillation fluid for analysis of 3H by liquid scintillation spectrometry, as previously described (23).
Determination of plasma anti-TT antibody (IgG and IgM) concentrations.
Serum samples in Expt. 1 and 2 were analyzed for anti-TT IgM and anti-TT IgG by ELISA as previously described (24). Measurements in a linear dose response range were compared with the standard curve derived from serially diluted pooled immune plasma to calculate the relative titers of anti-TT IgG or IgM, where 1 unit was defined as the dilution fold that produced 50% of the maximal optical density for the standard sample.
Stra6 expression in mouse spleen.
Immediately after dissection, one-half of the spleen was frozen in liquid nitrogen for later processing for gene mRNA analysis. The other half was embedded in Tissue-Tek OCT compound (Sakura Finetek) in tissue molds, and snap-frozen in dry ice. The frozen tissue and frozen blocks were stored at −80°C until use. For splenic Stra6 mRNA determination, total RNA was extracted from spleen samples of individual mice by using Trizol (Invitrogen), and cDNA was prepared by using reverse transcriptase. The equivalent of 0.05 μg RNA, as cDNA, was used for real-time qPCR analysis. The primer designed to detect Stra6 mRNA expression was mouse Stra6 (NM_009291), 5′-TGCAGAACATAGCAGCCAAC-3′ (forward), and 5′-TGGATTGTGGAGCAATGTGT-3′ (reverse). The mRNA expression level of each sample was normalized by calculating the mRNA-to-ribosomal 18S RNA ratio for each sample.
Splenic STRA6 protein level analysis using immunohistochemistry.
Frozen spleen tissue blocks were cut into 10-μm sections with a cryotome cryostat and mounted onto Superfrost plus (Fisher) slides. Sections were stored at −80°C for later use. Frozen tissue sections were warmed at room temperature, fixed in precooled acetone at −20°C for 10 min, and then placed at room temperature for >20 min. The slides were then washed in 10 mmol/L of PBS for 2 changes, 5 min each, and incubated in 0.3% H2O2 solution in PBS at room temperature for 10 min to block endogenous peroxidase activity. Sections were then washed in PBS again twice for 5 min each, and blocked with blocking buffer (10% FBS in PBS) in a humidified chamber at room temperature for 1 h. Afterward, sections were incubated with the primary anti-STRA6 antibody (made in goat; Sigma-Aldrich) in a humidified chamber overnight at 4°C. On the second day, sections were washed and incubated with Alexa Fluor-488–conjugated secondary antibody (Molecular Probes, Inc.) in 1% BSA in PBS in the dark for 30 min at room temperature. After washing in PBS, a drop of antifading fluorescent mounting medium (VECTASHIELD HardSet Mounting Medium with DAPI, Vector Laboratories) was dispensed onto the section to mount the tissue, and then the slides were cover-slipped. A negative control slide that was notincubated with primary antibody was included. Sections were viewed with an Olympus Fluoview 1000 Confocal Laser Scanning Microscope (Olympus America, Inc.). Images were analyzed by using Fluoview software.
Statistical analysis.
Data are reported as means ± SEM. When variances were unequal, data were transformed by log10 prior to statistical analysis. Differences between groups (P < 0.05) were determined by using 2-way ANOVA followed by Bonferroni post test in Prism software (GraphPad) or by 1-way ANOVA followed by Fisher’s protected least-significant-difference test using Origin software (OriginLab).
Results
Kinetics of splenic Stra6 expression and plasma anti-TT IgM and IgG concentrations after TT immunization, with and without oral VA treatment.
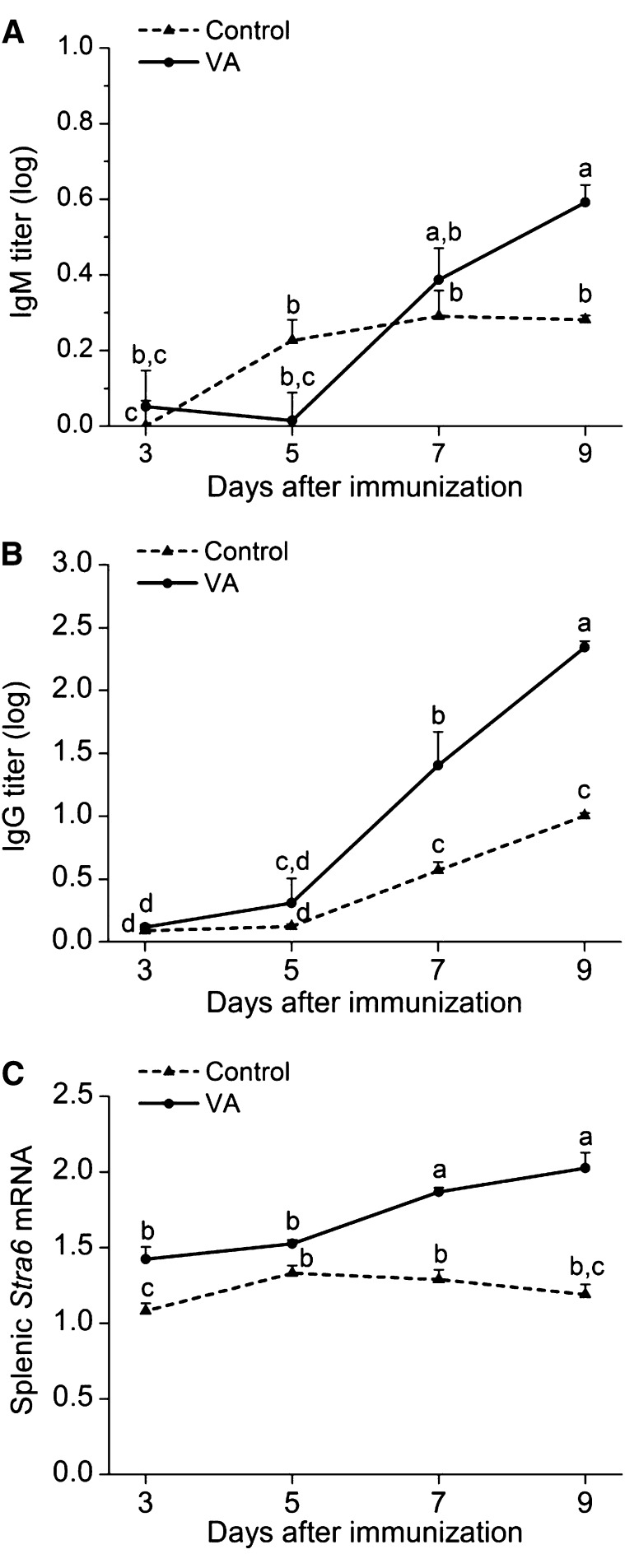
In Expt. 1, plasma anti-TT IgM concentrations (Fig. 1A) peaked on d 7 after priming with antigen, whereas anti-TT IgG (Fig. 1B) continued to increase up to d 9, the last day of the study. Stra6 mRNA expression in mouse spleen (Fig. 1C) was detectable at all times. Treatment with VA significantly (P < 0.05) augmented anti-TT IgM production on d 9, anti-TT IgG concentrations on d 7 and 9, and splenic Stra6 mRNA expression on d 3, 7, and 9.
FIGURE 1.
Kinetics of plasma anti-TT IgM (A) and IgG (B) concentrations and splenic Stra6 mRNA expression (C) after TT immunization and supplementation with VA in VA-adequate mice. Data are presented as means ± SEM for n = 4 mice/group. ELISA data were log10 transformed prior to statistical analysis. Stra6 mRNA results were normalized to results for 18S mRNA measured on the same samples. Treatment groups not sharing a letter are significantly different, P < 0.05. TT, tetanus toxoid; VA, vitamin A.
Retinoids alone and combined with PIC increased anti-TT IgM and IgG production and splenic Stra6 expression in TT-immunized mice.
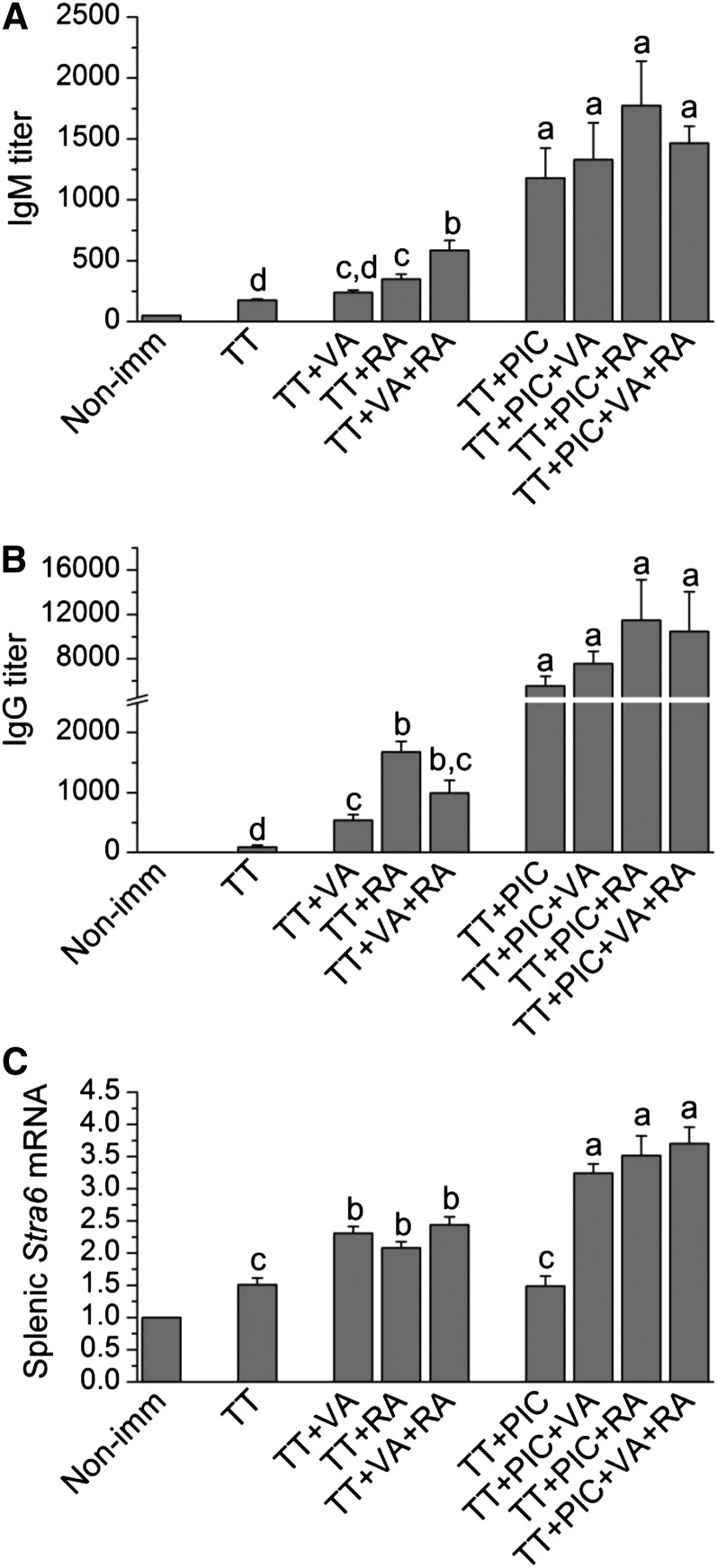
In Expt. 2, VA alone, RA alone, and the combination of VA and RA given orally all increased both antibody production measured by plasma anti-TT IgM (P < 0.05) and IgG (P < 0.01) antibody titers compared with those in TT-immunized mice without further treatment (Fig. 2A, ). For anti-TT IgM production, the combination of VA and RA resulted in significantly (P < 0.05) higher plasma concentrations compared with VA or RA alone (Fig. 2A).
FIGURE 2.
Plasma IgM (A) and IgG (B) and splenic Stra6 mRNA (C) in mice immunized with TT and treated with various combinations of VA and RA, each administered orally, and PIC administered i.p. Plasma IgM and IgG were determined on d 9 after TT priming. ELISA data were log10 transformed for statistical analysis, but untransformed data are shown. Stra6 mRNA results were normalized to 18S mRNA. Bars show means ± SEM, n = 2 in group 1 (not included in statistical analysis) and n = 7/group in group 2–9. Groups not sharing a letter are significantly different, P < 0.05. Non-imm, nonimmunized; PIC, polyinosinic acid:polycytidylic acid; RA, retinoic acid; TT, tetanus toxoid; VA, vitamin A.
Treatment with PIC alone augmented plasma anti-TT IgM and IgG concentrations over and above those by retinoid compounds alone (Fig 2A, B). In the presence of PIC, antibody titers on d 9 were not further increased by retinoids.
For Stra6, retinoid treatments, given alone or in combination, all had a significant (P < 0.05) effect. Whereas PIC did not elevate expression more than did TT alone, PIC in combination with retinoids increased Stra6 mRNA significantly (all P < 0.05 to P < 0.01). Expression levels were ~3 times the control value and more than twice that in mice immunized with TT but without further treatment (Fig. 2C).
STRA6 is expressed on the surface of splenic cells and is increased upon TT immunization and retinoid treatment.
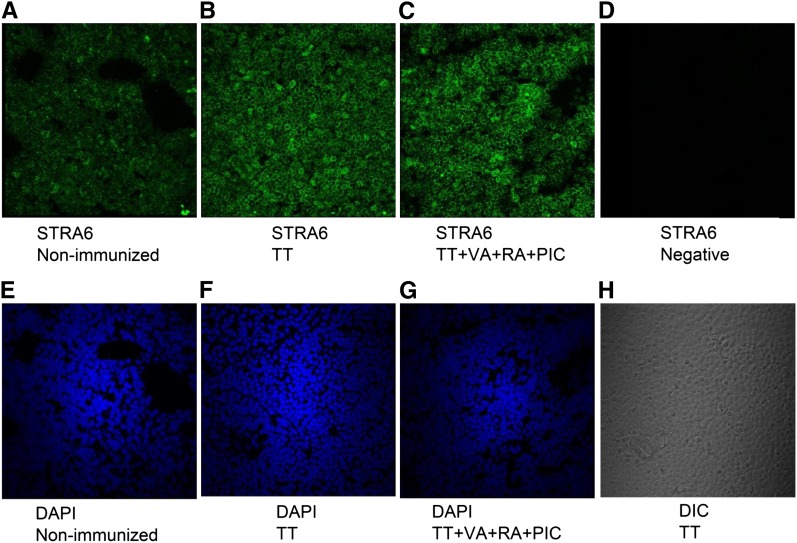
To explore the effects of immunization and retinoid and PIC treatments on splenic STRA6 protein expression, samples were selected from 3 groups for immunohistochemistry: nonimmunized control mice; nontreated, TT-immunized mice; and TT-immunized, PIC plus VA plus RA–treated mice. The staining results (Fig. 3) showed a pattern of STRA6 staining consistent with cell surface expression and of increased intensity after immunization with TT. The combination treatment (VA+RA+PIC) further slightly stimulated the expression as indicated by more intense staining. The staining was not highly localized to any particular splenic structure, suggesting that numerous cell types express STRA6 and can be regulated after retinoid treatment.
FIGURE 3.
Visualization of the expression of STRA6 protein in the spleen of nonimmunized, TT-immunized, and TT-immunized mice treated with VA+RA+PIC. (A–C) STRA6 protein, green signal; (D) a negative staining control for STRA6; (E–G) nuclear staining with DAPI, blue signal; (H) DIC image from the TT-immunized group as a reference. All panels represent 60× magnification. DAPI, 4′,6′-diamidino-2-phenylindole; DIC, differential interference contrast; PIC, polyinosinic acid:polycytidylic acid; RA, retinoic acid; STRA6, stimulated by retinoic acid-6; TT, tetanus toxoid; VA, vitamin A.
Retinol is associated with spleen tissue after oral administration.
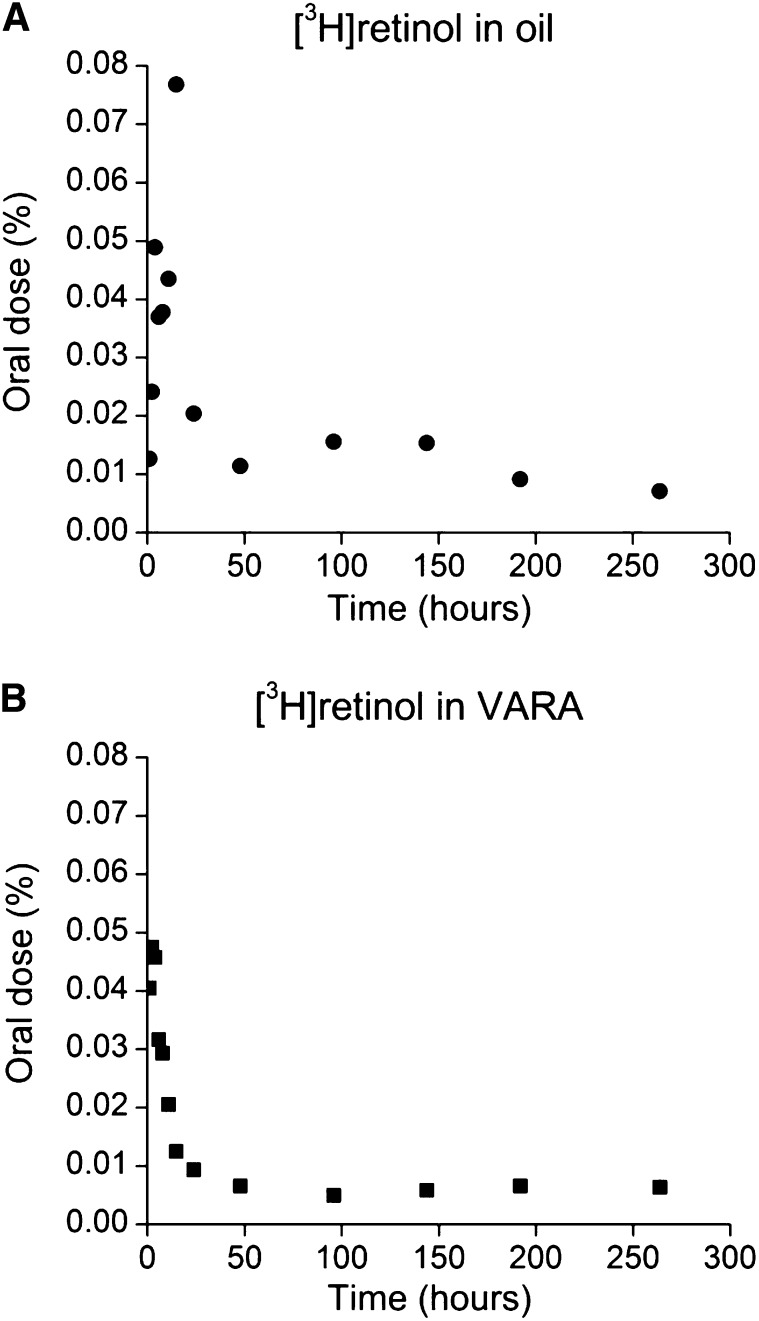
To determine if VA given orally is taken up by immune tissues, we examined spleens from a study of neonatal rats that were treated with [3H]retinol dissolved in a dose of VARA, or oil as placebo, in a kinetic study. Tissues were collected at times from 1 h to 11 d after dose administration, and the percentage of the oral dose of 3H in spleen was calculated at each time. Although only a small proportion of the oral [3H]retinol dose was present in spleen (Fig. 4A, ) at any given time, there was a peak in radioactivity at 15 h after dose administration in the oil group (Fig. 4A) and at 2–4 h in the VARA-treated group (Fig. 4B). By 48 h after dosing, the [3H]retinol decayed and then remained at a low level.
FIGURE 4.
Mean observed percentage of an oral dose of [3H]retinol in the spleen vs. time (hours after oral dose administration) of [3H]retinol administered in oil (A) or in VARA (B)–treated neonatal rats. Each point represents the mean of n = 3 rats. VARA, vitamin A combined with retinoic acid.
Discussion
Our previous research indicated that RA and the coadministration of RA and PIC could augment anti-TT antibody responses in adult and neonatal mice (10, 11) and regulate other immune responses occurring in spleen (10). Indications that Stra6 is highly expressed in a subset of cells in spleen (13) led us to hypothesize that the retinoid treatment(s) may upregulate the expression of Stra6 in the spleen, which could in turn increase the uptake of retinol into spleen, and thereby result in increased conversion into RA, to exert the functions of VA in regulating immune responses. Thus, our current study focused on VA supplementation, alone and combined with RA, and PIC due to its ability to induce a strong anti-TT antibody response (11, 12). We also focused on oral administration as being relevant to the use of VA and to the potential use of RA in clinical applications.
The results of our kinetic study (Fig. 1) showed that the cell membrane retinol receptor Stra6 is expressed even prior to immunization, and thus is constitutive in VA-adequate mice; nevertheless, the level of gene expression was increased by the treatments used in this study. Although the level of splenic Stra6 mRNA did not change dramatically in response to our treatments, the difference was significant, and it occurred ahead of the peaks of the anti-TT antibody responses. This may indicate that the uptake of retinol into spleen occurs before the peak antibody responses, when T cell–dependent antibody production by B cells is undergoing maturation. This is consistent with in vivo and cell culture studies showing that RA promotes cell maturation and heightens antibody production (5–12). There is also consistency in the regulation of splenic Stra6 expression and the level of antibody responses caused by different treatments. VA, RA, and the combination of VA and RA all enhanced the production of anti-TT IgM and IgG and increased splenic Stra6 mRNA expression. Treatments with PIC (VA+PIC, RA+PIC, VA+RA+PIC) all enhanced antibody responses and Stra6 expression to significantly higher levels.
The immunohistochemistry results confirmed the existence of STRA6 protein in spleen and showed that the expression was more intense on the cell surface. These visual results also suggest the stimulatory effects of TT immunization and coadministration of VA+RA+PIC on the expression level of STRA6 (Fig. 3), which is consistent with the Stra6 mRNA results in Figure 2. However, from the current results, we could not locate STRA6 protein expression to specific zones in the spleen. Future studies should focus more on this area of investigation and explore whether STRA6 is mainly expressed in the GC for the uptake of retinol into GC, to exert its function.
Our results from determination of [3H]retinol derived from an oral dose of VARA or oil in the spleen, conducted in spleen samples from a study of neonatal rats, provide evidence that orally administered retinol is taken up to some extent by this immune tissue. Although the percentage of the [3H]retinol dose was low at all times, there was a definite kinetic pattern, with a maximum content at 15 h in the oil group and at 2–4 h after oral dosing in the VARA group. In our present studies in immunized mice, VA was given daily from d 0 to 9 after immunization, and thus we would expect that a similar kinetic pattern would repeat itself on a daily basis after each dose. This potentially could lead to cumulatively higher amounts of VA after treatments with VA alone or VA combined with RA, or a sustained exposure due to multiple dosing.
A strength of our current research is that we explored the effects of VA at a supplemental dose on enhancing a clinically relevant vaccine response. Currently, VA supplementation is promoted by WHO/UNICEF as a strategy to improve child health and survival and is widely administered to children 6 mo to 5 y of age in parts of the world where VA deficiency exists (25). In our experimental studies conducted in well-nourished mice, supplemental VA changed the developing patterns of anti-TT antibody responses (primary response) significantly, as well as splenic Stra6 gene and protein expression. Although VA alone was not as effective as when it was combined with RA or with PIC, when given alone and in mice that were not VA deficient, VA modulated immune response. These results thus suggest the potential for VA to augment vaccine efficiency and immune responses. Although our results with Stra6 show coincidence rather than cause, they suggest that retinoid homeostasis, including uptake of retinol into cells, is modulated by immunization and retinoid supplementation. The uptake of retinol from plasma by immune tissues, which is indicated by our results showing a rapid uptake of [3H]retinol into spleen, may be part of an overall mechanism through which VA regulates the immune response.
Acknowledgments
L.T. and A.C.R. designed the research, wrote the manuscript, and have primary responsibility for the contents of the manuscript; and L.T. and A.E.W. conducted analyses. All authors read and approved the final manuscript.
Footnotes
Supported in part by NIH DK041479 and HD006692 (A.C.R.) and the Graduate Program in Nutrition, Pennsylvania State University.
Abbreviations used: GC, germinal center; PIC, polyinosinic acid:polycytidylic acid; RA, retinoic acid; RBP, retinol-binding protein; Stra6, stimulated by retinoic acid-6; TT, tetanus toxoid; VA, vitamin A; VARA, vitamin A combined with retinoic acid.
Literature Cited
- 1.Green HN, Mellany E. Vitamin A as an anti-infective agent. BMJ. 1928;2:691–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30 [DOI] [PubMed] [Google Scholar]
- 3.Ross AC. Vitamin A, retinoids and immune responses. In: Livrea MA, editor. Vitamin A and retinoids: an update of biological aspects and clinical applications. Basel (Switzerland): Birkhauser Verlag; 2000. p. 83–95.
- 4.Hengesbach LM, Hoag KA. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J Nutr. 2004;134:2653–9 [DOI] [PubMed] [Google Scholar]
- 5.Ertesvåg A, Naderi S, Blomhoff HK. Regulation of B cell proliferation and differentiation by retinoic acid. Semin Immunol. 2009;21:36–41 [DOI] [PubMed] [Google Scholar]
- 6.Chen Q, Ross AC. Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cell Immunol. 2007;249:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60 [DOI] [PubMed] [Google Scholar]
- 9.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21:8–13 [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci USA. 2005;102:13556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Ross AC. Toll-like receptor 3 ligand and retinoic acid enhance germinal center formation and increase the tetanus toxoid vaccine response. Clin Vaccine Immunol. 2009;16:1476–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding porotein mediates cellular uptake of vitamin A. Science. 2007;315:820–5 [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Kawaguchi R. The membrane receptor for plasma retinol-binding protein, a new type of cell-surface receptor. Int Rev Cell Mol Biol. 2011;288:1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, et al. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61:4197–205 [PubMed] [Google Scholar]
- 16.Ross AC, Ambalavanan N, Zolfaghari R, Li N-q. Vitamin A combined with retinoic acid increases retinol uptake and lung retinyl ester formation in a synergistic manner in neonatal rats. J Lipid Res. 2006;47:1844–51 [DOI] [PubMed] [Google Scholar]
- 17.Wu L, Ross AC. Acidic retinoids synergize with vitamin A to enhance retinol uptake and STRA6, LRAT, and CYP26B1 expression in neonatal lung. J Lipid Res. 2010;51:378–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TD, Terwey TH, Zakrzewski JL, Suh D, Kochman AA, Chen ME, King CG, Borsotti C, Grubin J, Smith OM, et al. Organ-derived dendritic cells have differential effects on alloreactive T cells. Blood. 2008;111:2929–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin H-K, Lee Y-H, Jeong S-Y, Na H-S, Park H-R, Chung J. Induction of signal transduction pathway genes in dendritic cells by lipopolysaccharides from Porphyromonas gingivalis and Escherichia coli. Int J Oral Biol. 2010;35:113–9 [Google Scholar]
- 20.Thompson JN, Erdody P, Brien R, Murray TK. Fluorometric determination of vitamin A in human blood and liver. Biochem Med. 1971;5:67–89 [DOI] [PubMed] [Google Scholar]
- 21.Ross AC, Zilversmit DB. Chylomicron remnant cholesteryl esters as the major constituent of very low density lipoproteins in plasma of cholesterol-fed rabbits. J Lipid Res. 1977;18:169–81 [PubMed] [Google Scholar]
- 22.Sheehe DM, Green JB, Green MH. Influence of dietary fat saturation on lipid absorption in the rat. Atherosclerosis. 1980;37:301–10 [DOI] [PubMed] [Google Scholar]
- 23.Lewis KC, Green MH, Green JB, Zech LA. Retinol metabolism in rats with low vitamin A status: a compartmental model. J Lipid Res. 1990;31:1535–48 [PubMed] [Google Scholar]
- 24.Kinoshita M, Ross A. Vitamin A status and immunoglobulin G subclasses in rats immunized with tetanus toxoid. FASEB J. 1993;7:1277–82 [DOI] [PubMed] [Google Scholar]
- 25.Guideline WHO. Vitamin A supplementation in infants and children 6–59 months of age. Geneva: World Heath Organization; 2011. [PubMed]