Abstract
Population exposure to multiple chemicals in air presents significant challenges for environmental public health. Air quality regulations distinguish criteria air pollutants (CAPs) (e.g., ozone, PM2.5) from hazardous air pollutants (HAPs)—187 chemicals which include carcinogens and others that are associated with respiratory, cardiovascular, neurological and numerous other non-cancer health effects. Evidence of the public’s cumulative exposure and the health effects of HAPs are quite limited. A multilevel model is used to assess differential exposure to HAP respiratory, neurological, and cancer hazards (2005) related to the Townsend Index of Socioeconomic Deprivation (TSI), after adjustment for regional population size and economic activity, and local population density. We found significant positive associations between tract TSI and respiratory and cancer HAP exposure hazards, and smaller effects for neurological HAPs. Tracts in the top quintile of TSI have between 38%–60% higher HAP exposure than the bottom quintile; increasing population size from the bottom quintile to the top quintile modifies HAP exposure hazard related to TSI, increasing cancer HAP exposure hazard by 6% to 20% and increasing respiratory HAP exposure hazard by 12% to 27%. This study demonstrates the value of social epidemiological methods for analyzing differential exposure and advancing cumulative risk assessment.
Keywords: cumulative risk assessment, socioeconomic deprivation, hazardous air pollution, respiratory health exposure hazards, social epidemiology, environmental epidemiology
1. Introduction
Air pollution is a major environmental public health issue in the United States directly affecting wellbeing and quality of life. Numerous studies attribute excess respiratory and cardiovascular morbidity and higher mortality rates to air pollution, particularly among urbanized places [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Air quality regulations in the United States from the beginning of the Clean Air Act in the early 1970s have separated criteria air pollutants (CAPs), which are ubiquitous chemicals in the environment (e.g., sulfur dioxide, ozone, and particulate matter (2.5 μm), from hazardous air pollutants (HAPs). HAPs comprise 187 chemicals including arsenic, lead, cadmium, pollutant gases, solvents, and pesticides many of which are thought to have no minimum threshold of exposure that can be considered safe [35]. While many State Implementation Plans employed under the Clean Air Act have made progress in reducing CAPs and other chemicals regulated as HAPs, evidence on environmental exposure to HAPs or their associated health effects is quite limited [36,37,38].
While acute exposures to high concentrations to HAPs are clearly dangerous, the cumulative health effects of low level chronic exposure are uncertain, given the multiple organs targeted by these metals and chemicals. Point source HAP emissions typically involve small amounts of dozens of chemicals, each with potentially different health effects based on the organ targeted by the chemical. For many of these chemicals small exposures are biologically capable of significant health effects. Similar to CAPs, ambient air exposure to HAPs is typically concentrated in urban and industrial areas and among adjacent populations.
HAPs have been problematic for both risk assessment methods and risk management policy. The cumulative burden of air pollution exposure among residential communities adjacent to industrial land use and point source emissions presents significant environmental justice policy issues for air quality management, and scientific challenges for risk assessment. One of the major challenges in environmental epidemiology and risk assessment in urban settings is the confounding of health effects associated with poverty with those related to environmental exposure. Complicating differential exposure among those of lower socioeconomic position is the potential for differential susceptibility to chemical exposures. The National Academy of Sciences has challenged the adequacy of risk assessment methods due to the potential for differential susceptibility, and cites the need to improve our understanding of population vulnerability to chemical exposure, particularly among populations subject to high levels of nonchemical stressors such as socioeconomic deprivation (SED) [39].
This paper seeks to contribute to risk assessment methods by demonstrating the value of incorporating social epidemiology in analyzing social gradients in HAP exposure related to urbanization and neighborhood SED. Developing better metrics and dose-response models relating to social gradients in exposure and health outcomes, particularly among vulnerable populations, can help advance cumulative risk assessment.
The study uses national emissions inventory data as modeled in the National Air Toxics Assessment in 2005 and includes health hazard exposure indices for chemicals related to risk of cancer risk and respiratory and neurological noncancerous health endpoints. We used a social determinant of health framework to develop a multilevel model to analyze how the baseline range HAP exposure hazards shifts with level of urbanization and related economic activity and degree of SED at the local (neighborhood) level. Urbanization is conceptualized as the concentration of residential population and economic activity, and is measured by local census tract population density, and tracts’ respective county population size, number employed and aggregate wage earned the tracts’ host county. The Townsend Index of Socioeconomic Deprivation (TSI) is used to measure SED at the census tract level [40]. Assessment of differential exposure relating to SED is strengthened by including known or suspected confounders and covariates relating to HAPs.
This study conceptualizes variation in local neighborhood chemical exposure to be decisively influenced by the population size in the region (with Host County of census tract as proxy) in combination with the economic activity generated in region (host county business metrics as proxy). How SED is generated and how it is geographically distribution SED is complicated [40,41,42,43]. While there is substantial rural poverty in the USA, greater numbers of households with SED are located in urban areas, often in ‘inner cities’. The key question is whether SED is confounded with HAP because of their common urban context, or whether SED works to modify HAP exposure.
Accordingly, in this study we tested the following hypotheses:
Hypothesis 1: The greater the population size and level of regional economic activity, the higher the level of HAP exposure hazard in the region.
Hypothesis 2: The greater the population size and level of regional economic activity, the higher the level of socioeconomic deprivation among the local neighborhoods.
Hypothesis 3: The higher the level of neighborhood socioeconomic deprivation, the higher the level of HAP exposure, after adjustment for population size and level of economic activity.
Hypothesis 1 identifies population size and level of economic activity as primary drivers of HAP exposure hazard. The second hypothesis addresses the necessary condition for SED to be confounded with HAP exposure hazard: their common relationship with the urban concentration of population and level of economic activity. As an alternative to a spurious relationship between SED and HAP exposure hazard, hypothesis 3 presents a SED differential exposure model. The geographic concentration of HAP exposure and higher SED households, it is argued, is related to “disinvestment” and public policies that work as a form of disenfranchisement among those that live in economically and politically weak urban places. In this model, neighborhood SED is directly related to HAP exposure after adjustment for urban concentration or level of economic activity.
This study evaluated evidence of “differential exposure” of HAP exposure hazard related to neighborhood SED, independent of U.S. geographic region and level of urbanization in terms of population size, density and regional economic activities. This research addresses the question: how does level of HAP respiratory, neurological, and cancer exposure hazard vary by regional population size and level of economic activity and local population density and SED. Is there evidence of differential HAP exposure related to localized SED, independent of the regional context of population level and economic activity, or is SED confounded with urbanization and HAP exposure? Among the unique contributions of this study are the application of a multilevel exposure model that examines the relationship of HAP exposure and neighborhood SED after adjustment for the level of urbanization, population density, and the regional level of economic activity that is monitored at the county level.
2. Research Design and Methods
The study design involves a retrospective, cross-sectional, population-based analysis of HAP exposure at the census tract level in 2005. Predictors of HAP exposure include census-tract neighborhood SED and urbanization as measured by population density at the census tract level, and two regional variables—county population size and county business activity. The study population consists of the 65,166 census tracts (99.6% of the universe of 65,443) and their respective 3121 United States (U.S.) counties (99.4% of the universe of 3141 counties) for which there is complete data available.
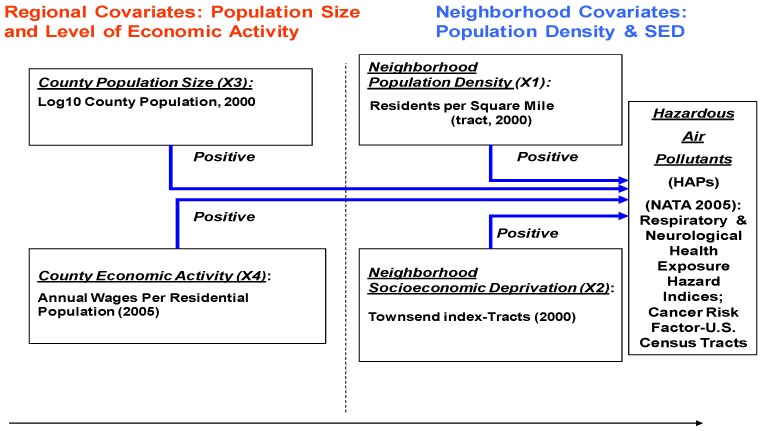
We assembled three databases to permit the specification of a multilevel HAP differential exposure model based on census tract, county, and regional criteria: (a) the National Air Toxics Assessment (NATA) HAP exposure data relating to health hazard exposure measures at the census tract level; (b) decennial census data (2000) on tract characteristics relating to population density and socioeconomic deprivation, and related demographics for tracts’ host counties, i.e., regional population; and (c) County Business Pattern survey data for all U.S. counties in 2005 on number employed and annual wages. Regarding the geographic dimension of the analysis, we used the Census Bureau’s categorization of States into nine regions to profile HAP exposure. Figure 1 summarizes the variables and indicators included in the multilevel exposure model used in this study.
Figure 1.
Multilevel Urban Market Exposure Model for HAPs: Differential Exposure Related to Neighborhood Socioeconomic Deprivation.
We examined the variation in modeled HAP exposure estimates from the NATA data regarding respiratory, neurological, and cancer-related exposure hazard indices and cancer risk factors, and give particular attention to the magnitude and upper tail of the distribution of HAP exposure: first at progressive deciles of census tracts related to population size, density, economic activity, and the Townsend Index, to better understand the social gradient of HAP exposure in the United States unadjusted for covariates; and then we tested for differential HAP exposure related to tract SED after adjustment for tract and county population size, density, and economic activity focusing on three HAP hazard exposure metrics (respiratory, neurological, and cancer) for 2005 using random intercept mixed models with maximum likelihood model estimation procedures.
2.1. Assessment of HAP Exposure
The U.S. Environmental Protection Agency (EPA) has been conducting a NATA and providing modeled estimates of ambient air concentration of progressively larger subsets of the chemicals classified as HAPs for 1999, 2002, and 2005, which will be the focus of this study. Among noncancer health endpoints, the U.S. EPA includes calculations of respiratory and neurological health hazard indices that quantify the ambient air concentration of selected HAPs targeting the lung and neurological functions, as the sum of chemical–specific health effects targeting a specific organ (e.g., the lung) [44]. These risk estimates are surrounded by substantial uncertainties associated with characterizing sources, exposures, and pollutant hazards, whereas cancer risk estimates are calculated with slope factors in toxicological databases for selected known or suspected carcinogens.
2.2. Measurement of Socioeconomic Deprivation
This paper focuses on the Townsend Index as the measure of SED [40]. Prior to specification of the Townsend Index, we conducted a sensitivity analysis of alternative measures of population size, economic activity and SED using the Index of Neighborhood Concentrated Disadvantage in contrast to the Townsend Index. We evaluated number employed and aggregate payroll of those employed with the respective county. All measures tested had significant positive relationships with HAP exposure hazards, however, the Townsend Index and Average Wages per Employee were more robust indicators (i.e., smaller variation) and thus thought to provide stronger tests of the potency of SED.
The four variables that comprise the TSI are:
Unemployment as a percentage of those aged 16 and over who are economically active.
Households without access to a car (car lease or ownership), as a percentage of all households.
Residential household renting, as a percentage of all households.
Percentage of households with “crowded housing,” i.e., the number of residents exceeds the number of rooms within the household.
The combination of each census tract’s respective standard deviates (or z scores) for these four variables forms the SED score for each tract, and represents its cumulative deviance from the average, positive or negative, among the universe of census tracts. The higher the Townsend Index score, the more deprived and disadvantaged an area is thought to be.
2.3. Measures of Urbanization and Economic Activity
This study focused on measures of population density at the census tract level and population size at the county level as a means of measuring level of urbanization.
The County Business Patternsdatabase provides the only source of annual, complete, and consistent county-level data for U.S. business establishments, with industry detail. Economic activity is measured by average annual wages per employee reported in the 2005 County Business Survey.
2.4. Multilevel Statistical Modeling
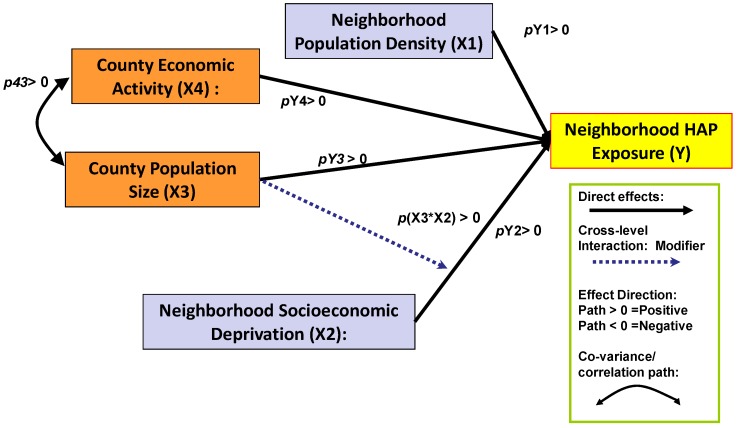
Figure 2 provides a view of the interrelationship of SED with HAP exposure and the regional population level-measured as county population—and the economic activity in the region. The figure illustrates four distinct direct effects—positive relationships between HAP exposure hazard and neighborhood (tract) population density, neighborhood SED (tract Townsend Index), and county population size and level of economic activity. The key issue remains whether SED is a confounder or effect modifier of the relationship of SED with HAP exposure. The diagram includes an interaction term signified by the pathway from county population size to SED. The empirical evidence in support of SED as a confounder versus an effect modifier will be found in the size, direction, and statistical significance of the pathway p(X3*X2), representing a conjoint effect of county population size and tract SED.
Figure 2.
Multilevel HAP Exposure Model: County-Level Population Size as Effect Modifier of Tract-Level Socioeconomic Deprivation on HAP Exposure Risk.
Multilevel HAP Exposure Equation
𝒀ij = γ00 + γ10 (Tract Population Density ij) TRACT LEVEL FIXED EFFECTS
+ γ11 (Tract Socioeconomic Deprivation ij)
+γ01 (County Population Size j) COUNTY LEVEL FIXED EFFECTS
+ γ02 (County Economic Activity j) CROSSLEVEL INTERACTION
+ γ12 (County Population Size j *Tract Socioeconomic Deprivation ij)
+ u0j + u0j (Tract Socioeconomic Deprivation ij)
+ rij COUNTY LEVEL RANDOM INTERCEPT
+γ03 (New England Region j) REGIONAL LEVEL FIXED EFFECTS
+γ04 (Middle Atlantic Region j) (Note: Mountain Region is excluded
+γ05 (East North Central Region j) and thus is reflected in the intercept) +γ06 (West North Central Region j)
+γ07 (South Atlantic Region j)
+γ08 (East South Central Region j)
+γ09 (West South Central Region j)
+γ0 10 (Pacific Region j)
It should be noted that the models’ explanation of the relationship of SED with HAP exposure represents a “state of exposure” that would be expected in the absence of aggressive HAP risk management policies at the State or local level. In practice, there are significant variations in state environmental policies regarding risk management of HAPs, as well as variation in the degree to which such policies are designed to meliorate disproportionate exposure burden among geographically concentrated vulnerable populations. The prevalence and success of such policies would be expected to work at attenuating the relationship of HAP exposure and SED. Nevertheless, this study advocates there is value in assessing the “signal” (i.e., covariate) representing the relationship between SED and HAP exposure at a national level, and attempts to adjust for the policy environment by introducing geographic region into the model.
Inclusion of geographic region in a multilevel model, adjusting for urbanization and economic activity and neighborhood SED, provides a means for improving the estimation of the model’s coefficients. It does this by reducing the model’s residual error by accounting for the spatial dependency of unexamined confounders of SED and HAPs. Most significant for this study would be adjustment for HAP enforcement policies which share certain commonalities among different regions in the United States. Equation (1) identifies the parameters of the model which we used to estimate HAP respiratory and neurological health hazards indices and cancer risk factor for 2005 using STATA version 11 [45].
3. Results
Table 1 shows descriptive statistics for population size, economic activity, TSI and exposure data used in this study. A breakdown of the U.S. population in 2000, by census tracts and counties according to geographic census division, is provided in Table A1 in the Appendix. Census tracts, as a geographic unit, are distributed disproportionately among urban counties; they are administrative units designed for the purpose of counting the population and conducting sample surveys of the population’s characteristics.
The contrast in the distribution in social and economic characteristics between tracts and counties illustrates that most census tracts are in urban areas while counties are predominantly rural. In spite of the skewed geographic distribution of the population in census tracts, there remains variation of economic activity both within and among urban and rural counties.
3.1. Variation in HAP Exposure Risk
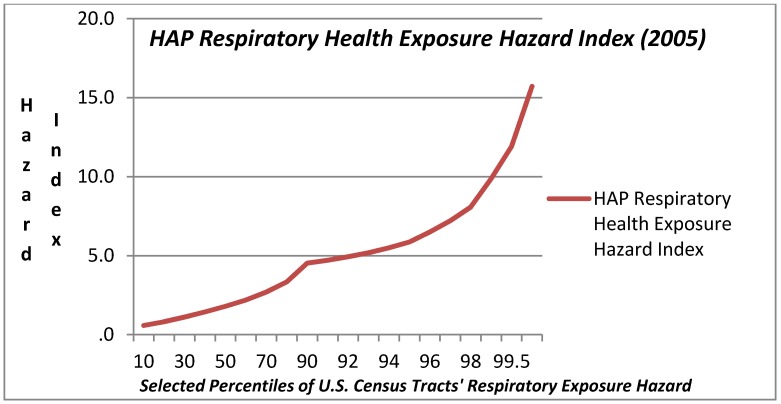
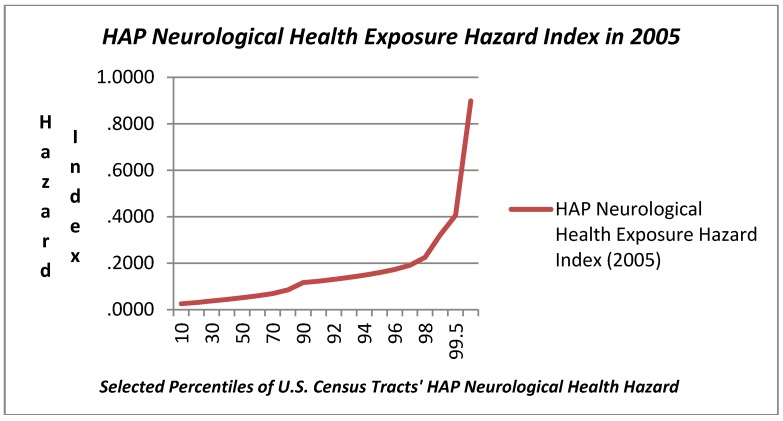
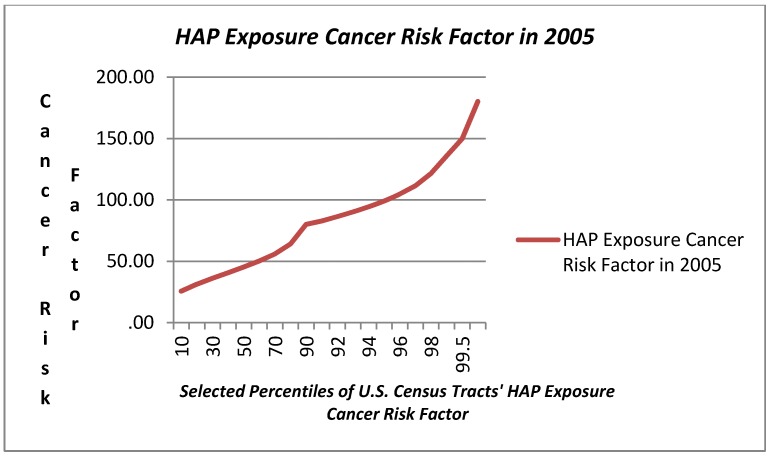
The range in modeled HAP exposure hazard ascertained in the NATA for 2005 related to respiratory, neurological, and cancer exposure hazard metrics is also reported in Table 1 and presented graphically in Figure 3, Figure 4, Figure 5. Respiratory exposure hazard, as modeled by the U.S. EPA in 2005, becomes a magnitude of genuine health risk at around the 75th percentile, and climbs three fold by the 99th percentile. HAP-related cancer risk increases progressively, tripling from the 5th to the 75th, and then increases by 2.3 magnitudes by the 99th percentile.
Table 1.
Descriptive Statistics for Study Variables: Tracts & Counties.
| Tract-Level Descriptive Statistics (N = 64,524) | Mean | Std. Dev. | Percentiles | ||||
|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 99th | |||
| Population Size of Tracts’ Host County [000s] | 1,049.71 | 1,906.97 | 16.87 | 95.79 | 401.61 | 1,003.16 | 9,937.74 |
| Density per Sq Mile Census Tract | 5,215.51 | 11,997.51 | 17.04 | 234.18 | 1,981.92 | 5,204.29 | 61,564.29 |
| Townsend Index-Tract | (0.00) | 3.08 | (3.06) | (2.11) | (0.99) | 1.14 | 10.90 |
| Number Employed in County, 2005 [000s] | 436.19 | 763.05 | 3.78 | 32.03 | 163.77 | 473.59 | 3,805.77 |
| Aggregate Wage Payroll (2005) [$000] | 19,016 | 34,796 | 97 | 955 | 5,861 | 18,893 | 162,202 |
| Avg. Wages per Employee in County 2005 [$000] | 36.05 | 9.42 | 23.51 | 29.26 | 34.97 | 40.81 | 66.37 |
| County-Level Descriptive Statistics (N = 3,138) | Mean | Std. Dev. | 5th | 25th | 50th | 75th | 99th |
| Population Size of Tracts’ Host County [000s] | 934.99 | 3,047.51 | 29.95 | 112.28 | 251.11 | 636.22 | 11,745.13 |
| Number Employed in County, 2005 [000s] | 364.03 | 1,330.98 | 4.77 | 21.69 | 65.36 | 195.73 | 5,507.85 |
| Aggregate Wage Payroll (2005) [$000] | 14,063 | 64,340 | 104 | 524 | 1,728 | 5,784 | 233,483 |
| Avg. Wages per Employee in County, 2005 [$000] | 27.71 | 6.90 | 19.26 | 23.44 | 26.64 | 30.63 | 50.26 |
| Tract-Level Outcomes: | Mean | Std. Dev. | 5th | 25th | 50th | 75th | 99th |
| Respiratory Health Hazard (2005) | 2.30 | 1.96 | 0.44 | 0.96 | 1.80 | 2.99 | 9.87 |
| Neurological Health Hazard (2005) | 0.07 | 0.10 | 0.02 | 0.03 | 0.05 | 0.08 | 0.32 |
| Cancer Risks per MM Population (2005) | 50.11 | 24.38 | 21.32 | 33.89 | 45.39 | 59.56 | 135.79 |
In light of the prominence of exposure in the upper tail of the distribution of exposure among census tracts, the plots in Figure 3, Figure 4, Figure 5 include a range of data points in the highest decile. The three graphs illustrate the very modest gradual increase in exposure hazard values up to the around the 70th percentile, where there is a slight inflexion in the data, and then a steeper rise in exposure until the 90th percentile where there is an exponential rise in the HAP estimates. The exposure curve for neurological health risks of HAPs is clearly different from that for respiratory and cancer HAP exposure hazard in that it is a flatter arc with less variation for census tracts below the 90th percentile. The HAP exposure curve for cancer, as compared with respiratory or neurological health exposure hazards shows a steeper arc in progressive risk from the 10th through the 80th percentile.
Figure 3.
Hazardous Air Pollution (HAP) Respiratory Health Exposure Hazard Index (2005) Values at Selected Percentiles for U.S. Census Tracts (U.S. E.P.A. National Air Toxics Assessment, 2005) [44].
Figure 4.
Hazardous Air Pollution (HAP) Neurological Health Exposure Hazard Index (2005) Values at Selected Percentiles for U.S. Census Tracts (U.S. E.P.A. National Air Toxics Assessment, 2005) [44].
Figure 5.
Hazardous Air Pollution (HAP) Cancer Risk Factor Exposure Hazard Index (2005) Values at Selected Percentiles for U.S. Census Tracts (U.S. E.P.A. National Air Toxics Assessment, 2005) [44].
3.2. HAP Chemical Exposure Hazard by Urbanization and Economic Activity
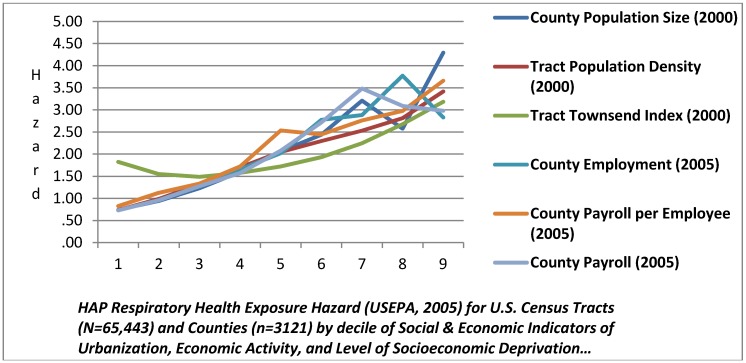
Table 2 summarizes the data regarding the three forms of HAP health exposure hazard under study and its unadjusted relationship to population characteristics of size, density, and economic activity. Based on a view of unadjusted HAP exposure hazard, there was strong evidence of differential exposure according to county population size and economic activity, and tract characteristics population density and SED. Table 2 illustrates the ratio of HAP exposure at selected percentiles using HAP exposure for the bottom quintile of census tracts nationally as the standard of comparison. We see that the top quintile related to tracts’ host county in terms of population size, employment, and payroll per employee, as well as census tract population density, has nearly three times the risk reported for the lowest quintile for respiratory HAP exposure. The risk progressively increases from the 80th to the 95th and 97th percentiles as compared to the lowest quintile, indicating a HAP exposure “dose-response” relationship. A similar dose-response pattern is found for the data on HAP cancer exposure hazards, though at lower magnitudes. The data for HAP neurological health exposure hazard do not manifest this dose-response increase in exposure hazard except for census tract population density and SED.
Figure 6 provides an additional view of these relationships by showing curve of the HAP respiratory health exposure hazard index at progressive deciles. We see a positive monotonic relationship between HAP respiratory exposure and county population size up to the 70th percentile, where there is an inflection and decline in exposure between the 70th and 80th percentile, and then a sharp increase in the exposure slope in the top quintile. Tract population density and county economic activity (private business payroll per employee) have positive monotonic relationships with no inflections in the exposure curve. Tract SED is generally flat in respiratory HAP exposure hazard through the 60th percentile, and then climbs exponentially. Similar Figures for Neurological and Cancer HAP data are provided in the Appendix.
Table 2.
The Social Gradient in HAP Exposure Hazard in U.S. (2005): Risk Ratios of 97th, 95th, and 80th Percentile to the Lowest Decile of Tract Population Size, Density, Economic Activity and SED.
| HAP Health Hazard Metrics | Ratio of HAP Exposure Risk at Selected Percentiles to Risk at 20th Percentile | County Population Size (2000) | Tract Population Density (2000) | Tract Townsend Index | County Employment (2005) | County Payroll per Employee (2005) |
|---|---|---|---|---|---|---|
| Respiratory Health Exposure Hazard | Ratio 80/20 | 2.73 | 2.85 | 1.73 | 3.93 | 2.65 |
| Ratio 95/20 | 2.81 | 4.51 | 2.94 | 4.39 | 3.23 | |
| Ratio 97/20 | 5.63 | 6.3 | 3.56 | 5.52 | 3.64 | |
| Neurological Health Exposure Hazard | Ratio 80/20 | 1.45 | 2.11 | 1.57 | 2.13 | 1.78 |
| Ratio 95/20 | 2.18 | 3.29 | 2.59 | 3.25 | 1.84 | |
| Ratio 97/20 | 1.91 | 4.21 | 2.82 | 2.26 | 1.76 | |
| Cancer Risk Factor | Ratio 80/20 | 1.82 | 1.85 | 1.38 | 2.17 | 1.83 |
| Ratio 95/20 | 1.99 | 2.63 | 2.01 | 2.48 | 2.11 | |
| Ratio 97/20 | 3.48 | 3.08 | 2.32 | 3.49 | 2.11 |
Figure 6.
Respiratory Exposure Hazard at Progressive Deciles of Social & Economic Characteristics of Tracts and Counties.
3.3. Differential HAP Exposure Related to Neighborhood SED: Multilevel Model Estimates
Table 3 presents the results from the three multilevel models for predictors of HAP respiratory, neurological, and cancer exposure hazards. SED as measured by the Townsend Index was highly significant and positively associated with all three categories of HAP exposure hazard, after adjustment of county population size and economic activity, and tract-level population density. The cross-level interaction of population size and census tract SED was also found to be highly significant and positively related to respiratory and cancer HAP exposure hazard. In addition, census tract population density was also highly significant and had a strong positive association in all six tests of HAP exposure hazard. County population size and level of economic activity were also highly significant with strong positive associations for respiratory and cancer HAP exposure hazard, however these factors were not found to be statistically significant in the case of neurological HAP exposure hazard. We observed earlier the relatively flat distribution of neurological HAP exposure hazard nationally, and multivariate analysis clarifies that this risk was not significantly related to regional levels of population size and economic activity. The general association of HAP exposure with the East and West coast in the U.S. provides a new perspective in light of the analysis of the regional effects shown in Table 4. The coefficients for the marginal effects of geographic region, after adjustment for county population size and economic activity, and neighborhood population density and SED, reveal a statistically significant and positive regional effect associated with the Pacific coast for respiratory and cancer HAP exposure hazard. In comparison, the coefficients for mid-Atlantic region were not statistically different from zero for respiratory and neurological HAP exposure hazards.
Table 3.
Multilevel Models of SED & HAP Exposure Hazard After Adjustment for County Population Size and Economic Activity and Tract Population Density: Respiratory, Neurological, and Cancer Exposure Hazards, 2005.
| HAP Exposure Hazards | Respiratory | Neurological | Cancer |
|---|---|---|---|
| Tract Population Density (Log 10 Density/Sq M) | 0.385 *** | 0.0121 *** | 5.797 *** |
| −0.00538 | −0.00057 | −0.0687 | |
| Townsend Index-Tract | 0.0905 *** | 0.00312 *** | 1.185 *** |
| −0.00138 | −0.000146 | −0.0176 | |
| County Population (2000) Log 10 | 0.247 *** | 0.00353 | 5.947 *** |
| −0.031 | −0.00334 | −0.297 | |
| Annual Wage per Employee in Tract’s County ’05 | 0.0170 *** | 0.000331 | 0.308 *** |
| −0.0026 | −0.00028 | −0.0247 | |
| Cross-level Tract SED & County Population Size | 0.0138 *** | −2.94 × 10−6 | 0.510 *** |
| −0.00079 | −8.41 × 10−5 | −0.0102 | |
| New England Region | 0.357 *** | −0.00992 | 1.792 * |
| −0.11 | −0.0118 | −0.983 | |
| Mid-Atlantic Region | −0.0684 | 0.00163 | 3.617 *** |
| −0.0823 | −0.00887 | −0.746 | |
| East North Central | −0.207 *** | 0.0132 * | 3.057 *** |
| −0.0633 | −0.00682 | −0.59 | |
| West North Central | −0.0189 | 0.000438 | 4.110 *** |
| −0.0609 | −0.00655 | −0.58 | |
| South Atlantic | 0.567 *** | −0.00275 | 10.68 *** |
| −0.0607 | −0.00653 | −0.57 | |
| East South Central | 0.166 ** | 0.0217 *** | 12.37 *** |
| −0.0657 | −0.00707 | −0.618 | |
| West South Central | −0.0179 | −0.00828 | 8.470 *** |
| −0.063 | −0.00678 | −0.595 | |
| Pacific Region | 0.854 *** | −0.00184 | 4.812 *** |
| −0.0812 | −0.00874 | −0.749 | |
| Constant | −1.372 *** | −0.00616 | −21.31 *** |
| West Region-Intercept | −0.127 | −0.0136 | −1.21 |
| Model Fit Statistics: | |||
| ll_0 | −85,224 | 67,237 | −252,964 |
| ll | −76,808 | 68,045 | −240,725 |
| df_m | 13 | 13 | 13 |
| Number Counties | 3,119 | 3,119 | 3,119 |
| rho | 0.485 | 0.494 | 0.294 |
| sigma_e | 0.755 | 0.08 | 9.706 |
| sigma_u | 0.733 | 0.0791 | 6.27 |
| chi2_c | 67,085 | 21,898 | 42,053 |
| ll_c | −110,351 | 57,096 | −261,752 |
| chi2 | 16,831 | 1,616 | 24,477 |
Standard errors below coefficients; coefficients *** p < 0.01, ** p < 0.05, * p < 0.1.
Although that region was estimated to account for an additional 3 to 4 cancer cases per million related to HAP exposure before adjustment. Statistically significant and high levels of cancer risk were found in the South Atlantic and both East and West South Central regions, after adjustment for population size, density, economic activity and SED.
Evaluation of the goodness of fit statistics for the six models indicate that all share a relatively good fit according to the chi square tests of −2 Log Likelihood, though the weakest model is HAP neurological exposure hazard and the strongest fit is for the HAP cancer exposure hazard. We found relatively strong intraclass correlation coefficients (rho) in each model confirming that exposure hazard for tracts within counties are closely related, as was expected, although this was less true for cancer exposure hazard than for respiratory and neurological exposure hazard.
Table 4 profiles the interrelationship of county population size and census tract SED in compounding HAP exposure hazard. These data demonstrate that HAP exposure hazard is not only related to neighborhood SED, but that county population size modifies the relationship of SED and HAP exposure.Effect modification associated with neighborhood SED is illustrated by comparing the three categories of HAP exposure hazard at different levels of SED within varying levels of county population size, holding constant the effect of county economic activity and census tract population density.
We find dose-response patterns of increased exposure hazard associated with increased levels of SED (20th, 80th, 95th, and 99th percentile) at the each level of county population size—the 20th, 80th, and 95th percentiles. For example, the increase in relative risk of HAP respiratory exposure for a census tract at the 80th percentile of SED compared with the 20th percentile of SED is 26% higher when that tract is in the 95th percentile of county population size as compared with the 80th percentile of county population size. The 99th percentile of SED is associated with 65% higher respiratory HAP exposure hazard when that tract is in the 95th percentile of county population size as compared with the 80th percentile of county population size. This pattern of compounded risk associated with the interaction of SED and county population size is evident for both respiratory and cancer HAP exposure hazard for both years. Thus, there is strong evidence of differential exposure to respiratory, neurological, and cancer-related HAPs related to socioeconomic deprivation, and this differential exposure associated with SED becomes even greater for tracts in the most populous counties in the United States concerning respiratory and cancer HAP exposure hazard.
Table 4.
Profile of SED Effect Modification of HAP Exposure Risk after Adjustment for County Population Size and Economic Activity and Tract Population Density: Respiratory, Neurological, and Cancer Exposure Hazards, 2005.
| Tract SED | Predicted HAP Exposure at 20th Percentile County Population Size | Predicted HAP Exposure at 80th Percentile County Population Size | Predicted HAP Exposure at 95th Percentile County Population Size | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Percentiles: | Resp | Neuro | Cancer | Resp | Neuro | Cancer | Resp | Neuro | Cancer |
| 20th | 1.43 | 0.06 | 38.80 | 1.73 | 0.06 | 45.75 | 1.73 | 0.06 | 44.01 |
| 80th | 1.77 | 0.07 | 42.65 | 2.11 | 0.07 | 51.04 | 2.38 | 0.08 | 58.91 |
| 95th | 2.16 | 0.08 | 46.91 | 2.54 | 0.09 | 56.89 | 3.09 | 0.09 | 75.36 |
| 99th | 2.53 | 0.10 | 50.97 | 2.95 | 0.10 | 62.46 | 3.77 | 0.11 | 91.04 |
| Ratio 80/20 | 1.25 | 1.23 | 1.10 | 1.23 | 1.21 | 1.12 | 1.37 | 1.20 | 1.34 |
| Ratio 95/20 | 1.52 | 1.48 | 1.21 | 1.47 | 1.45 | 1.24 | 1.79 | 1.43 | 1.71 |
| Ratio 99/20 | 1.77 | 1.73 | 1.31 | 1.709 | 1.671 | 1.365 | 2.18 | 1.65 | 2.07 |
| Progressive excess risk associated with increased SED, for census tracts at the 95th and 80th percentile of County population size: | Case Contrast Assumptions: Mid Atlantic Region; Median Tract Population Density & County Economic Activity; and varying levels of County Population Size and Tract SED | ||||||||
| Resp | Neuro | Cancer | |||||||
| Ratio 80/20 | 1.122 | 0.993 | 1.200 | ||||||
| Ratio 95/20 | 1.214 | 0.988 | 1.377 | ||||||
| Ratio 99/20 | 1.276 | 0.985 | 1.515 | ||||||
4. Discussion
There is extensive public health literature focusing on environmental inequality and environmental justice issues associated with the disproportionate impact of environmental contaminants and air pollutants [5,8,9,37,41,43,46,47,48,49,50,51,52,53,54,55,56]. Neighborhood characteristics relating to SED have been associated with differential exposure to air pollution, as well as related to neighborhood disparities in a range of health outcomes [2,5,14,42,47,49,57,58,59,60,61,62,63,64,65,66,67,68,69]. Neighborhood disparities in SED have been linked as both confounders and effect modifiers with health outcomes related to exposure to PM2.5 and ozone, though little research has been conducted relating to exposure to HAPs [37,60,70,71]. Prior epidemiologic research on SED and air pollution has tended to focus on urban places, with little attention to framing SED and chemical exposure in relation to both rural and urban America, perhaps due to greater availability of data for major cities [62]. Less clear is whether people of lower socioeconomic position were differentially exposed to HAPs across the spectrum of urban and rural places. Prior research has shown SED to be key social epidemiology variables and where there are significant gradients that explain variation in chemical exposure and health outcomes.
The substantive findings in this research are significant in that the SED differential exposure model directly relates increased HAP exposure to a social gradient of neighborhood SED, independent of the regional context of urbanization. The models suggests that there are serious incremental exposure hazards that accrue to progressive levels of socioeconomic deprivation in the United States, independent of urbanization, when we conceptualize urbanization in terms of population size and economic activity. Generalizing from the findings, the risk doubles in magnitude between the 80th percentile and 95th percentile, and is 2.6 greater at the 99th percentile of SED.
The other contribution of this research relates to cumulative risk assessment methods. It is generally recognized that the inability to quantify risks using comparative metrics accounting for nonchemical stressors—such as socioeconomic deprivation—has limited risk assessors’ abilities to assess cumulative impacts on a consistent, reliable basis, across different locations and time periods and populations. This research illustrates a population health model that enables quantification of the interaction between non-chemical stressors and chemical exposure, including measurement of exposure is modified in a dose-response curve related to SED.
There are two major threats to the validity of these findings. First, the underlying exposure models in the NATA assessments may have misclassified HAPs exposure. Certain regions of the country may be “undercounted” in terms of point source surveillance of facilities. Additionally, the population exposure model may have flaws. Among the most important uncertainties underlying these modeling results are the use of emission estimates from multiple sources (e.g., state- or industry-submitted, computed by EPA from activity data and emission factors, or developed by EPA test programs). According to the U.S. EPA, these estimates differ across geographic regions and source categories, and may vary substantially in quality. Additionally, the exposure model which is applied by the U.S. EPA nationally employs inputs and assumptions that may not be fully representative of particular local areas and the nature of their emissions and concentrations. Among additional caveats for the use of this data is that it projected exposure estimates for the median individual within each census tract;some individuals may have substantially higher or lower exposures than the median individual. The risk estimates are also limited to consideration of inhalation exposure but do not include emissions from indoor sources of air toxics. In addition, people receive substantial additional exposures to pollutants such as mercury, and dioxins that have bioaccumulated in food. The second source of bias is from “built-in” design effects that stem from the administrative organization and long-form sampling of census among tracts which would influence the underlying standard errors of the variables used in the SED measures.
On the other hand, these types of non-random measurement error do not appear to be of the type that would result in a design effect that would confound NATA’s exposure estimates with composite measures of population characteristics of census tracts. On the other side of the argument, there are good reasons for expecting that the magnitude of exposure in inner cities is undercounted given the NATA methodology and what is acknowledged to be excluded.
Given the above limitations, the results of this study demonstrate that HAP exposure was found to be related to the urban concentration of population and economic activity as well as the level of neighborhood socioeconomic deprivation. The size of a city matters as it pertains to both HAP exposure and prevalence of SED. We found relatively strong unadjusted bivariate relationships between HAPs and measures of population density, economic activity, and SED for all three measures of HAP risks in both years examined. SED clearly has two faces in the United States—urban and rural—and there is support for the notion that there are large rural areas with low levels of economic activity, high SED and low HAPs. Multivariate statistical analysis of the geographical distribution of HAPs presents a pattern of exposure hazard that is clearly associated with county population size, the county economy, and localized population density and SED within respective counties.
Differential chemical exposure among vulnerable populations raises questions about whether we would also find differential response to this exposure. The reference concentration value approach to risk assessment of chemical exposure assumes an underlying uniformity in human physiological response to toxic exposure [35,72,73,74,75]. Two aspects of this situation are worth emphasizing: first, there is clear evidence of differential chemical exposure among higher SED populations; second, the risk metrics that provide the basis for current standards as to what is safe may understate the health risks of HAPs for vulnerable populations. The role and relationship of nonchemical stressors, such as extreme poverty or socioeconomic deprivation, to multiple chemical exposures therefore represents a key issue in advancing cumulative risk assessment (CRA) and addressing environmental justice issues [35,39]. Social epidemiology is particularly suited to study differential exposure and differential susceptibility related to the interaction of chemical and nonchemical stressors in cumulative exposure and risk assessment.
Acknowledgments
The lead author would like to acknowledge the United States Environmental Protection Agency for its Environmental Justice Small Grant Program, and the financial support provided to Heart of Camden, Inc., for a community-based health risk assessment in Camden, NJ, for which the author served as Principle Investigator. The lead author would also like to acknowledge the support of NIEHS Center pilot grant ES03819 which provided significant assistance in conducting this research.
Appendix
Table A1.
Study Population: U.S. Census Tracts and Counties (2000).
| Census Division: | Counties (Pct) | Population (Pct) | Tracts (Pct) | |||
|---|---|---|---|---|---|---|
| New England | 67 | 2.1 | 14,238,888 | 4.8 | 3,203 | 4.9 |
| Middle Atlantic | 150 | 4.8 | 40,332,259 | 13.7 | 9,918 | 15.2 |
| East North Central | 437 | 13.9 | 46,031,860 | 15.7 | 11,328 | 17.4 |
| West North Central | 618 | 19.7 | 19,697,992 | 6.7 | 5,090 | 7.8 |
| South Atlantic | 589 | 18.8 | 55,182,959 | 18.8 | 10,781 | 16.5 |
| East South Central | 364 | 11.6 | 17,480,032 | 6.0 | 3,938 | 6.0 |
| West South Central | 470 | 15.0 | 33,281,974 | 11.3 | 7,104 | 10.9 |
| Mountain | 281 | 8.9 | 19,823,587 | 6.8 | 4,255 | 6.5 |
| Pacific | 165 | 5.3 | 47,610,448 | 16.2 | 9,549 | 14.7 |
| Total | 3,141 | 100.0 | 293,679,999 | 100.0 | 65,166 | 100.0 |
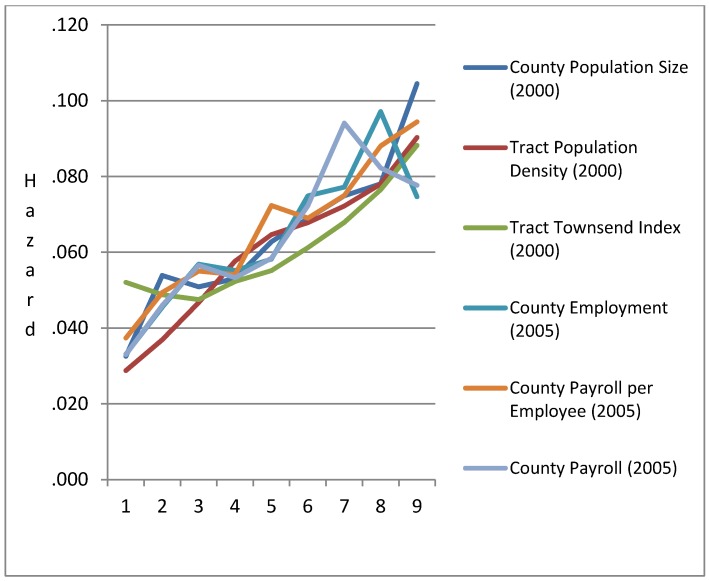
Figure A1.
Gradients in HAP Neurological Health Exposure Hazard (2005) at Progressive Deciles of Population Size, Density, Economic Activity, and Socioeconomic Deprivation.
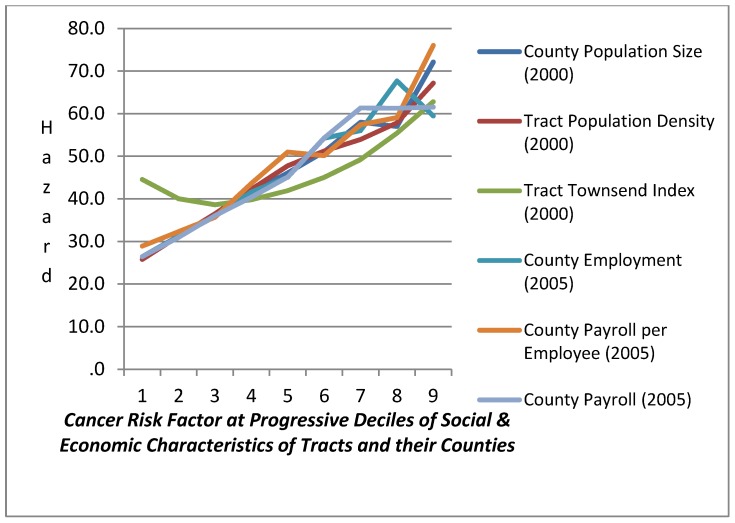
Figure A2.
Gradients in HAP Cancer Exposure Risk per MM (2005) at Progressive Deciles of Population Size, Density, Economic Activity, & Socioeconomic Deprivation.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Abbey D., Nishino N., McDonnell W., Burchette R., Knutsen S., Beeson L., Yang J. Long-term inhalable particles and other air pollutants related to mortality in non-smokers. Am. J. Respir. Crit. Care Med. 1992;159:373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 2.Anderson H.R., Atkinson R.W., Bremner S.A., Marston L. Particulate air pollution and hospital admissions for cardiorespiratory diseases: Are the elderly at greater risk? Eur. Pespir. J. 2003;21:39–46. doi: 10.1183/09031936.03.00402203. [DOI] [PubMed] [Google Scholar]
- 3.Bell M.L., Dominici F., Samet J.M. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16:436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell M.L., Ebisu K., Peng R.D., Samet J.M., Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am. J. Respir. Crit. Care Med. 2009;179:1115–1120. doi: 10.1164/rccm.200808-1240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell M.L., Ebisu K., Peng R.D., Walker J., Samet J.M., Zeger S.L., Dominici F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 U.S. counties, 1999-2005. Am. J. Epidemiol. 2008;168:1301–1310. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell M.L., Peng R.D., Dominici F., Samet J.M. Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide: Results for 126 United States urban counties, 1999-2005. Circulation. 2009;120:949–955. doi: 10.1161/CIRCULATIONAHA.109.851113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici F., Peng R.D., Bell M.L., Pham L., McDermott A., Zeger S.L., Samet J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. J. Am. Med. Assoc. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominici F. Revised Analysis of the National Morbidity Mortality Air Pollution Study: Part II. The Health Effects Institute; Cambridge, MA, USA: 2003. [Google Scholar]
- 9.Dominici F., McDermott A., Daniels M., Zeger S.L., Samet J.M. Revised analyses of the national morbidity, mortality, and air pollution study: Mortality among residents of 90 cities. J. Toxicol. Environ. Health Part A. 2005;68:1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- 10.Dominici F., McDermott A., Zeger S.L., Samet J.M. National maps of the effects of particulate matter on mortality: Exploring geographical variation. Environ. Health Perspect. 2003;111:39–44. doi: 10.1289/ehp.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eftim S.E., Samet J.M., Janes H., McDermott A., Dominici F. Fine particulate matter and mortality: A comparison of the six cities and American Cancer Society cohorts with a Medicare cohort. Epidemiology. 2008;19:209–216. doi: 10.1097/EDE.0b013e3181632c09. [DOI] [PubMed] [Google Scholar]
- 12.Peng R.D., Chang H.H., Bell M.L., McDermott A., Zeger S.L., Samet J.M., Dominici F. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. J. Am. Med. Assoc. 2008;299:2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samet J.M., Dominici F., Curriero F., Coursac I., Zeger S.L. Fine particulate air pollution and mortality in 20 U.S. cities: 1987-1994. N. Engl. J. Med. 2000;343:1742–1757. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 14.Samoli E., Peng R., Ramsay T., Pipikou M., Touloumi G., Dominici F., Burnett R., Cohen A., Krewski D., Samet J., Katsouyanni K. Acute effects of ambient particulate matter on mortality in Europe and North America: Results. Environ. Health Perspect. 2008;116:1480–1486. doi: 10.1289/ehp.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeger S.L., Dominici F., McDermott A., Samet J.M. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000-2005) Environ. Health Perspect. 2008;116:1614–1619. doi: 10.1289/ehp.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gauderman J.A., Vora H., McConnell R., Berhane K., Gwelliland F., Thomas D., Lurmann F., Avol E., Kunzli N., Jerrett M., Peters J. Effect of exposure to traffic on lung development from 10 to 18 years of age: A cohort study. Lancet. 2007;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 17.Dockery D., Pope C.A., Xu X., Spengler J., Ware J., Fay M., Ferris B., Speizer F. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 18.Pope C.A., Burnett R.T., Thruston G.D., Calle E., Thun M.J., Krewski D., Goldeski J. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2003;6:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 19.Pope C.A., Burnett R.T., Thun M.J., Calle E.E., Krewski D., Ito K., Thruston G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pope C.A., Dockery D., Schwartz J. Review of epidemiological evidence of health effects of particulate air pollution. Inhal. Toxicol. J. 1995;47:1–18. [Google Scholar]
- 21.Pope C.A., Dockery D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 22.Pope C.A., III, Muhlestein J.B., May H.T., Rehlund D.G., Anderson J.L., Horne B.D. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 23.Pope C.A., III., Thun M.J., Namboodiri M.M. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz J. Air pollution and daily mortality: A review and meta-analysis. Environ. Res. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz J. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environ. Health Perspect. 2000;108:563–568. doi: 10.1289/ehp.00108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz J. Is there harvesting association of airborne particles with daily deaths and hospital admissions? Epidemiology. 2001;12:55–61. doi: 10.1097/00001648-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz J. Lung function and chronic exposure to air pollution: A cross-sectional analysis of NHANES II. Environ. Res. 1989;50:309–321. doi: 10.1016/S0013-9351(89)80012-X. [DOI] [PubMed] [Google Scholar]
- 28.Wellenius G.A., Schwartz J., Mittleman M. Particulate air pollution and hospital admissions for congestive heart failure in seven U.S. cities. Am. J. Cardiol. 2006;97:404–408. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 29.Zanobetti A., Schwartz J. Cardiovascular damage by air borne particles: Are diabetics more susceptible? Epidemiology. 2002;13:588–592. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Zanobetti A., Schwartz J. The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environ. Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanobetti A., Schwartz J., Dockery D.W. Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environ. Health Perspect. 2000;108:1071–1077. doi: 10.1289/ehp.001081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanobetti A., Schwartz J., Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ. Health Perspect. 2000;108:841–845. doi: 10.1289/ehp.00108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenbaum D., Bachmann J., Krewski D., Samet J., White R., Wyzga R. Particulate air pollution standards and morbidity and mortality: Case study. Am. J. Epidemiol. 2001;154:78–90. doi: 10.1093/aje/154.12.S78. [DOI] [PubMed] [Google Scholar]
- 34.Wyzga R. Commentary on the HEI Reanalysis of the two cohort studies of particulate air pollution and mortality. J. Toxicol. Environ. Health Part A. 2003;66:1701–1704. doi: 10.1080/15287390306419. [DOI] [PubMed] [Google Scholar]
- 35.U.S. EPA. Concepts, Methods, and Data Sources for Cummulative Health Risk Assesment of Multiple Chemicals, Exposures and Effects: A Resource Document (Final Report) U.S. Environmental Protection Agency; Washington, DC, USA: 2007. [Google Scholar]
- 36.Laden F., Schwartz J., Speizer F.E., Dockery D.W. Reduction in fine particulate air pollution and mortality. Extended follow-up of Harvard Six Cities Study. Am. J. Respir. Crit. Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox M., Groopman J.D., Burke T.A. Evaluating cumulative risk assessment for environmental justice: A community case-study. Environ. Health Perspect. 2002;110:203–209. doi: 10.1289/ehp.02110s2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox M., Tran N.L., Groopman J.D., Burke T.A. Toxicological resources for cumulative risk: An example with hazardous air pollutants. Regul. Toxicol. Pharmacol. 2004;40:305–311. doi: 10.1016/j.yrtph.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 39.National Research Council. Science and Decisions: Advancing Risk Assessment. National Academies Press; Washington, DC, USA: 2008. [Google Scholar]
- 40.Townsend P., Phillimore P., Beattie A. Health and Deprivation: Inequality and the North. Croom Helm; London, UK: 1988. [Google Scholar]
- 41.Brown P. Race, class and environmental health: A review and systemisation of the literature. Environ. Res. 1995;69:15–30. doi: 10.1006/enrs.1995.1021. [DOI] [PubMed] [Google Scholar]
- 42.Anderson R.T., Sorlie P., Backlund J.N., Kaplan G.A. Mortality effects of community socioeconomic status. Epidemiology. 1997;8:42–47. doi: 10.1097/00001648-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Walker G., Mitchell G., Fairburn J., Smith G. Industrial pollution and social deprivation: evidence and complexity in evaluating and responding to environmental inequality. Local Environ. 2005;10:361–377. doi: 10.1080/13549830500160842. [DOI] [Google Scholar]
- 44.U.S. Environmental Protection Agency. 2005 National-Scale Air Toxics Assessment. Mar, 2011. [(accessed on 18 March 2012)]. Available online: http://www.epa.gov/ttn/atw/nata2005.
- 45.StataCorp. Stata Statistical Software, Release 11. StataCorp LP; College Station, TX, USA: 2009. [Google Scholar]
- 46.Bowen W. An analytical review of environmental justice research: What do I really know? Environ. Manag. 2002;29:3–15. doi: 10.1007/s00267-001-0037-8. [DOI] [PubMed] [Google Scholar]
- 47.Bard D. Exploring the joint effect of atmospheric pollution and socioeconomic status on selected health outcomes: An overview of the PAISARC project. Environ. Res. Lett. 2007;2 [Google Scholar]
- 48.Gwynn R., Thurston G.D. The burden of air pollution: Impacts among racial minorities environmental health perspectives. Inhaled Irrit. Allerg. 2001;109:501–506. doi: 10.1289/ehp.01109s4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellinger D.C., Leviton A., Waternaux C., Needleman H., Rabinowitz M. Low-level lead exposure, social class, and infant development. Neurotoxicol. Teratol. 1988;10:497–503. doi: 10.1016/0892-0362(88)90084-0. [DOI] [PubMed] [Google Scholar]
- 50.Weiss B., Bellinger D.C. Social ecology of children’s vulnerability to environmental pollutants. Environ. Health Perspect. 2006;114:1479–1485. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clougherty J.E., Levy J.I., Kubzansky L.D., Ryan P.B., Suglia S.F., Canner M.J., Wright R.J. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ. Health Perspect. 2007;115:1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.American Lung Association. Urban air pollution and health inequities: A workshop report. Environ. Health Perspect. 2001;109:357–373. doi: 10.1289/ehp.01109s3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheeler B. Health-related environmental indices and environmental equity in England and Wales. Environ. Plan. A. 2004;36:803–822. doi: 10.1068/a3691. [DOI] [Google Scholar]
- 54.Zimmerman R. Issues of classification in environmental equity: How we manage is how we measure. Fordham Urban Law Journal. 1994;21:633–669. [Google Scholar]
- 55.U.S. EPA. Ensuring Risk Reduction in Communities with Multiple Stressors: Environmental Justice and Cummulative Risks/Impacts. U.S. EPA; Washington, DC, USA: 2004. [Google Scholar]
- 56.Gee G.C., Payne-Sturges D.C. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ. Health Perspect. 2004;112:1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diez Roux A. The Examination of Neighborhood Effects on Health: Conceptual and Methodological Issues Related to the Presence of Multiple Levels of Organization. Oxford University Press; New York, NY, USA: 2003. [Google Scholar]
- 58.Macintyre S., Ellaway A. Ecological Approaches: Rediscovering the Role of Physical and Social Enviornment. Oxford University Press; New York, NY, USA: 2000. [Google Scholar]
- 59.O’Neil M.S., Jarret M., Kawachi I., Levy J.I., Cohen A.J. Health, wealth, and air pollution: Advancing theory and methods. Environ. Health Perspect. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drukker M., van Os J. Mediators of neighborhood socioeconomic deprivation and quality of life. Soc. Psychiatry Epidemiol. 2003;38:698–706. doi: 10.1007/s00127-003-0690-8. [DOI] [PubMed] [Google Scholar]
- 61.Augustin T., Glass T.A., James B., Schwartz B.S. Neighborhood psychosocial hazards and cardiovascular disease: The Baltimore Memory Study. Am. J. Public Health. 2008;98:1664–1670. doi: 10.2105/AJPH.2007.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eberhardt M.S., Pamuk E.R. The importance of place of residence: Examining health in rural and nonrural areas. Am. J. Public Health. 2004;94:1682–1686. doi: 10.2105/AJPH.94.10.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elreedy S., Krieger N., Ryan P., Sparrow D., Weiss S. Relations between individual and neighborhood-based measures of socioeconomic position and bone lead concentrations among community-exposed men: The Normative Aging Study. Am. J. Epidemiol. 1999;150:129–141. doi: 10.1093/oxfordjournals.aje.a009972. [DOI] [PubMed] [Google Scholar]
- 64.Geronimus A.T. Race, Ethnicity, and Health: A Public Health Reader. Josey-Bass; San Francisco, CA, USA: 2002. [Google Scholar]
- 65.Glass T.A. Neighborhoods and obesity in older adults: The Baltimore Memory Study. Am. J. Prev. Med. 2006;31:455–463. doi: 10.1016/j.amepre.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glass T.A., Bandeen-Roche K., McAtee M., Bolla K., Todd A.C., Schwartz B.S. The association of environmental lead exposure with cognitive function is modified by neighborhood psychosocial hazards. Am. J. Epidemiol. 2009;169:683–692. doi: 10.1093/aje/kwn390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwartz B.S., Glass T.A., Bolla K.I., Stewart W.F., Glass G., Rasmussen M., Bressler J., Shi W., Bandeen-Roche K. Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environ. Health Perspect. 2004;112:314–320. doi: 10.1289/ehp.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Katsouyanni K., Toulomi G., Samoli E., Gryparis A., LeTertre A., Monopolis Y., Rossi G., Zmirou D., Ballester F., Boumghar A., Anderson H.R. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12:521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 69.Katsouyanni K., Touloumi G., Spix C., Balducci F., Medina S., Rossi G., Wojtyniak B., Sunyer J., Bacharova L., Schouten J., Ponka A., Anderson H.R. Short term effects of ambient sulfur dioxide and particulate matter on mortality in 12 European cities: Results from time series data from the APHEA project. Br. Med. J. 1997;314:1658–1663. doi: 10.1136/bmj.314.7095.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janes H., Dominici F., Zeger S.L. Trends in air pollution and mortality: An approach to the assessment of unmeasured confounding. Epidemiology. 2007;18:416–423. doi: 10.1097/EDE.0b013e31806462e9. [DOI] [PubMed] [Google Scholar]
- 71.Hernan M.A. Causal knowledge as a prerequisite for confounding evaluation: An application to birth defects epidemiology. Am. J. Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 72.U.S. EPA. Supplementary Guidance for Conducting Health Risk Assesment of Chemical Mixtures. U.S. Environmental Protection Agency; Washington, DC, USA: 2000. [Google Scholar]
- 73.U.S. EPA. Guidance on Cummulative Risk Assesment of Pesticide Chemicals That Have a Common Mechanism of Toxicity. U.S. Environmental Protection Agency; Washington, DC, USA: 2002. [Google Scholar]
- 74.U.S. EPA. Framework for Cumulative Risk Assessment; EPA/630/P-02/001F. Risk Assessment Forum; Washington, DC, USA: 2003. [Google Scholar]
- 75.U.S. EPA. Guidelines for Exposure Assessment. U.S. Environmental Protection Agency; Washington, DC, USA: 1992. [Google Scholar]