Abstract
Objective
This study investigated a potential interaction between the triallelic polymorphism of the serotonin transporter gene (SLC6A4) promoter and the experience of childhood trauma on the number of problem eating behaviors.
Methods
The study sample was comprised of 439 (64.7% female) Caucasian college students (mean age = 22.49, SD = 6.12). Participants completed questionnaires that assessed eating problems and experience of trauma in childhood (ages 0-12) and donated cheek cells for 5-HTTLPR and rs25531 genotyping.
Results
Women carrying a lower expressing allele (i.e. LG or S) who were exposed to higher levels of childhood trauma reported significantly higher mean numbers of eating problems (gender × genotype × trauma interaction, p = .006).
Discussion
These results are consistent with findings that the lower expressing alleles of the SLC6A4 promoter are associated with increased sensitivity to the negative impact of childhood stressors on adult behavioral outcomes.
Keywords: SERT, maltreatment, early life stress, genotype by environment interaction
The serotonin system has long been known to be a key regulator of eating behavior (1.), and the evidence that serotonergic dysfunction plays a role in the neurobiology of eating disorders is substantial and growing (2.). Serotonin (5-HT) is a monoamine neurotransmitter synthesized from tryptophan, which is an essential amino acid found in most dietary proteins. Serotonin is known to be involved in mood regulation and mood is considered to be a key mediator in eating behavior (3.). Decreasing the availability of 5-HT by acute tryptophan depletion, in women with bulimia nervosa, increases the urge to binge eat and lowers mood (4-5.). Increasing the availability of synaptic 5-HT via selective serotonin reuptake inhibitor (SSRI) treatment has the side effect of loss of appetite and weight loss (6.). Serotonin also influences feeding behavior through its effects on gastric sensorimotor function (7.). Low 5-HT function is associated with mood dysregulation and impulsivity (8.), both of which are associated with eating and eating problems (4; 9.). Therefore, 5-HT system components that have a significant effect on overall 5-HT function are good candidates to influence eating behavior and risk for eating problems.
The serotonin transporter (SERT) is a transmembrane protein found in neurons that removes 5-HT from synapses via reuptake. The SERT is a critical element of the regulation of 5-HT system function and is therefore an excellent candidate for association with eating problems. The structural gene for SERT (SLC6A4; chromosome 17q11.1-q12) has a nearby promoter region polymorphism (5-HTTLPR) that affects transcriptional efficiency of the gene and has been widely studied (10.). Lower cerebral SERT binding is associated with higher body mass index (BMI) (11.). A recent meta-analysis of 15 studies examining the potential association between the commonly studied 5-HTTLPR genotype that affects SERT transcriptional efficiency and eating disorder found convergent evidence that the lower functioning allele (S) is associated with a significantly increased risk for eating disorders, especially with Anorexia Nervosa (12.).
There is growing evidence to support the position that there may be gender differences in the associations between 5-HTTLPR genotype and eating behaviors. Prevalence rates of eating disorders and eating problems are significantly higher for women than men (13.); and the relations between 5-HTTLPR genotype and 5-HT system function also differ by gender (14.). Such gender differences may manifest in emotional reactivity and increase vulnerability to emotional eating. Women homozygous for the S allele of the 5-HTTLPR have greater heart rate reactivity when exposed to a social stressor than women with other genotypes or men with any genotype (15.); and in a non-clinical sample of women who had engaged in binge eating, the S allele was associated with higher levels of binge eating and state anxiety (16.). Conversely, the higher expressing allele (LA) of the triallelic (5-HTTLPR plus rs25531) may be associated with “inhibited/compulsive” traits in women with eating disorders (17.). Clearly, the 5-HTTLPR polymorphism is a good candidate for association with eating disorders and problems.
The experience of childhood trauma increases risk for several health-risk behaviors, including eating problems (18-19.), and the association between abuse and eating disorders may be mediated by emotional disturbances (20.). Individuals with a lower expressing 5-HTTLPR genotype (S allele carriers) may be particularly sensitive to early life stresses, which may increase vulnerability to problems later in life (21-22.), although there is some controversy about these findings (23-27.). Women who have experienced childhood trauma and have the lower expressing genotype of 5-HTTLPR are quicker to recognize facial expressions of fear and anger (28.). Such sensitivity to negative emotions is thought to be a risk factor for psychiatric disorders with an emotional component. A substantial and growing empirical literature examining potential mechanisms of the sensitivity of the lower expressing genetic variant of the 5-HT transporter in non-human animal models supports the position that the effects of 5-HTTLPR genotype are conditional on exposure to early life stresses (29.).
The relations among genetic polymorphisms in SLC6A4, gender, childhood trauma and eating problems have been studied, but are not yet fully characterized. Richardson, et al. (30.) reported that experiencing of child abuse and carrying the S allele of 5-HTTLPR was more common in a group of women with bulimia-spectrum disorder and other psychiatric comorbidity (i.e. major depressive disorder, anxiety disorder and/or substance use disorder). Such results are consistent with the notion that genotypes associated with lower SERT expression, when coupled with the experience of childhood adversity, increase vulnerability to psychiatric disorders including eating disorders. Such an increase in risk for eating problems may be at least partially mediated by an increase in impulsivity in those women with lower SERT expression who have experienced childhood trauma (31-32.).
Despite some successes in identifying genetic polymorphisms associated with psychiatric disorders, the rate of discovery has not kept pace with expectations (33.). Two main perspectives have emerged to address this apparent shortcoming of current research into the genetic basis of psychiatric disorders. One perspective is that study sample sizes are just too small to identify small genetic effects; whereas another perspective is that psychiatric diagnoses are not good phenotypes for genetic analyses because they produce groups of patients that are heterogeneous with respect to their genetic influences. Although we see merit in both positions, our perspective is more consistent with the latter, and therefore our approach utilizes strong candidate genes with common variants; and rather than diagnoses we study quantitative traits in non-clinical populations. By using non-clinical populations we are able to increase our sample sizes and by using quantitative measures of eating problems rather than dichotomous categorizations we can further increase our statistical power to detect likely small genetic effects. We focus on both men and women to be better able to characterize potential gender differences.
In the present study, we tested the hypothesis that exposure to childhood trauma would be associated with more eating problems in adulthood for those individuals who carried one or more alleles associated with low SERT expression (i.e. LG, S). We further hypothesized that the pattern of this association would differ for men and women.
Materials and Methods
Participants and basic procedure
College students from a small Midwestern university were recruited via posters and brief in-class presentations that described the study. To control for potential population stratification, we report only analyses conducted on data collected from self-reported Caucasians (N = 439; 64.7% female). The mean age of the sample was 22.49 (SD = 6.12) with 85% of the sample being between the ages of 18 and 25. The local institutional review board approved the study and all participants gave informed consent prior to their participation. Participants completed questionnaires assessing impulsivity and health-risk behaviors, computer tasks assessing impulsivity and decision-making and donated buccal cell samples for genotyping. Participants were remunerated $20 for approximately 2 hr of their time. Only data directly relevant to the questions under study are reported here; other analyses are reported elsewhere (34-35.).
DNA Extraction and Genotyping
The DNA extraction and genotyping conditions and procedures are standard and are reported elsewhere (34.). We re-genotyped 38 randomly chosen DNA samples and found no discrepancies in genotype calls.
Measures
The Traumatic Antecedent Questionnaire (TAQ) is a 42-item self-report questionnaire that assesses an individual’s personal positive and negative experiences using a 4-point intensity scale (from 0 = “never or not at all” to 3 = “often or very much”) and across four life stages (ages 0-6, 7-12-13-18, adult) (36.). Although the TAQ is not a well-known scale for assessing trauma, higher scores for the negative experience scales in childhood have been observed in psychiatric patients relative to controls (36.). Further characterization of the reliability and validity of the TAQ should be pursued. For our purposes, the TAQ provided the means to divide our sample into groups that had experienced relatively different exposure to childhood trauma. We used a slightly modified version of the TAQ whereby we assessed only two life stages (0-12 and 13-18). Therefore, we are unable to differentiate early and late childhood. In this study, we report only scores from the negative experience scales representing the Trauma factor (physical abuse [e.g. “I was beaten, kicked or punched by someone close to me”], sexual abuse [e.g. “Someone (older) touched me sexually, against my wishes or tried to make me touch them”], witnessing [e.g. “I witnessed physical violence in my family”] and other traumas [e.g. “I was involved in a serious accident”]; (36.)) from childhood (ages 0-12). The internal consistency among these four scales is acceptable (Cronbach’s α = .79 in this study). We constructed a two-level variable representing levels of trauma by summing the scores for four subscales and then using a score of 2 as a cut point for the Low (n = 370) and High (n = 64) groups. We chose this cut point because we reasoned that “high” trauma should be relatively low (15%) in frequency, but not so low to make examining interaction effects impossible. This rate appears to be broadly consistent with estimates of physical and sexual maltreatment (37.).
The Eating Attitudes Test (EAT-26) is a 26 item instrument that measures characteristics and concerns of eating disorders (38.). Four factors derived from scores on 16 of the items have been shown to be a better fit than the original three factors of Dieting, Bulimia and Food preoccupation (39.). Self-perception of body shape, Dieting, Awareness of food contents and Food preoccupation comprise the four factors that we calculated. The total score is reliable (in terms of internal consistency, Cronbach’s α = .87 in this study). Our eating problems assessment also included four yes/no items regarding binge eating (“Have you gone on eating binges where you feel that you may not be able to stop?”), purging (“Have you ever made yourself sick (vomited) to control your weight or shape?” and “Have you ever used laxatives, diet pills or diuretics (water pills) to control your weight or shape?”), and history of eating disorder treatment (“Have you ever been treated for an eating disorder?”).
The Barratt Impulsiveness Scale (BIS-11) is a 30-item self-report instrument that uses a 4-point Likert scale from Rarely/Never to Almost Always (40.). A total score is calculated by summing three subscale scores. The subscales are Motor (“I act on the spur of the moment”), Attentional (“I have outside thoughts when thinking”) and Nonplanning (“I plan trips well ahead of time” reverse scored). The total score is reliable (in terms of internal consistency, Cronbach’s α = .86 in this study).
Statistical Analysis
Analyses of covariance (ANCOVA) using age as a covariate were performed using the univariate GLM procedure to test associations between the triallelic 5-HTTLPR genotype (LA/LA [i.e. higher expression] vs. all other genotypes [i.e. lower expression]), gender, childhood trauma (Low vs. High) and their interactions first on EAT-26 Total score and then on its four subscales scores and on BIS-11 scores. A two-tailed alpha of 0.05 is appropriate to determine significance in the analyses of the full EAT-26. Follow-up analyses of the EAT-26 subscales should be evaluated using a Bonferroni corrected significance level of 0.05/4 = 0.0125; although it should be recognized that this is a conservative approach especially since these subscales are significantly correlated with each other and are summed to create the total score. All statistical analyses were conducted using IBM SPSS (version 19).
Results
Descriptive Statistics
Genotype frequencies for 5-HTTLPR (L/L = 143, L/S = 201, S/S = 92) and rs25531 (A/A = 379, A/G = 54, G/G = 3) were in Hardy-Weinberg equilibrium (χ2=1.85, df = 1, p > .05 and χ 2=0.49, df = 1, p > .05, respectively). Allele frequencies for 5-HTTLPR (L = .56, S = .44) and rs25531 (A = .93, G = .07) were consistent with those reported for Caucasian populations (41.).
We focused on the trauma factor of the TAQ, which is a sum of scores on the physical abuse, sexual abuse, witnessing and other trauma subscales for ages 0-12 (36.). Higher scores indicate self-report of greater exposure to traumatic experiences. Men (M = 0.33, SD = 0.54) had higher scores than women (M = 0.18, SD = 0.44) on the Traumatic Antecedent Questionnaire Childhood (ages 0-12) Physical Abuse scale F (1, 430) = 9.56, p = .002, partial η 2=.022. Men (M = 0.07, SD = 0.23) had lower scores than women (M = 0.17, SD = 0.47) on the TAQ Childhood Sexual Abuse scale F (1, 430) = 7.23, p < .001, partial η2=.017. Men (M = .28, SD = 0.39) and women (M = 0.33, SD = 0.49) did not differ on the TAQ Childhood Witnessing scale F (1, 430) = 0.97, p =.32, partial η2=.002. Men (M = 0.35, SD = 0.41) had higher scores than women (M = 0.24, SD = 0.31) on the TAQ Childhood Other Traumas scale F (1, 430) = 9.58, p = .002, partial η2=.022. As with most clinical screening instruments, the trauma factor score distribution was highly positively skewed and leptokurtic (M = 0.96, SD = 1.34, Skewness = 2.97, SE = 0.12, Kurtosis = 12.34, SE = 0.23). We split the sample into “high” and “low” groups based on this score. We reasoned that the “high” trauma group should be relatively small, so we examined the score distribution and determined that a natural break would create childhood trauma groups of reasonable size(i.e. “low” [score = o-2; 85% of participants] and “high” [score greater than 2]. Trauma classification was independent of gender (χ2=0.07, df = 1, p = .79) and genotype (χ2=0.09, df = 1, p = .77).
To reduce the influence of outliers on mean EAT-26 scores, we Winsorized the scores by converting all scores that were greater than 32 to a score of 33.
General Linear Models
In a univariate General Linear Model with EAT-26 total score as the dependent variable and age as a covariate, there were significant main effects for gender (partial η2 = 0.028), and triallelic 5-HTTLPR genotype (partial η2 = 0.021), but not for childhood trauma (see Table 1). Women (M = 12.71, SD = 8.37) had higher mean scores than men (M = 8.34, SD = 6.30). Those with lower expression alleles (M= 11.65, SD = 7.96) had higher mean EAT-26 Total scores than those with higher expression alleles (M = 9.94, SD = 7.93). There were significant interaction effects for the two-way genotype by childhood trauma interaction (partial η2 = 0.012), and the three-way gender by genotype by childhood trauma interaction (partial η2 = 0.018). No other interaction effects were statistically significant. Figure 1 shows the mean EAT-26 Total score (±se) for groups defined by gender, genotype and childhood trauma. Post hoc pair-wise comparisons indicate that the only significant effect of triallelic 5-HTTLPR genotype was in the female, high trauma group, F(1, 421) = 15.04, p < .001, partial η2 = 0.034.
Table 1.
F and p-values from general linear models for EAT-26 Total Scores and its four factor subscales.
| EAT-26 Total |
Self-perception of Body Shape |
Dieting | Awareness of Food Contents |
Food Preoccupation |
BIS-11 Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Source | F | p | F | p | F | p | F | p | F | p | F | p |
| 1. Gender | 12.02 | .001 | 12.22 | .001 | 10.33 | .001 | 1.57 | .211 | 2.98 | .085 | 4.05 | .045 |
| 2. Genotype | 8.90 | .003 | 8.67 | .003 | 3.28 | .071 | 3.11 | .079 | 2.47 | .117 | 4.69 | .031 |
| 3. Trauma | 0.12 | .729 | 0.14 | .710 | 0.19 | .665 | 0.08 | .783 | 5.51 | .019 | 17.17 | .000 |
| 1 × 2 | 2.18 | .140 | 2.14 | .144 | 5.09 | .025 | 0.99 | .319 | 7.66 | .006 | 1.07 | .302 |
| 1 × 3 | 0.12 | .733 | 0.45 | .502 | 2.71 | .101 | .391 | .532 | .052 | .820 | 0.26 | .612 |
| 2 × 3 | 5.13 | .024 | 6.37 | .012 | 2.71 | .101 | 1.38 | .241 | 2.76 | .097 | 5.35 | .021 |
| 1 × 2 × 3 | 7.78 | .006 | 5.53 | .019 | 9.72 | .002 | 3.26 | .072 | 8.05 | .005 | 1.84 | .175 |
| Adj. R2 | 0.114 | .114 | .108 | .035 | .107 | .048 | ||||||
Note: Age was included as a covariate in all models. For all, df = 1, 421. Significant p-values are shown in bold.
Figure 1.
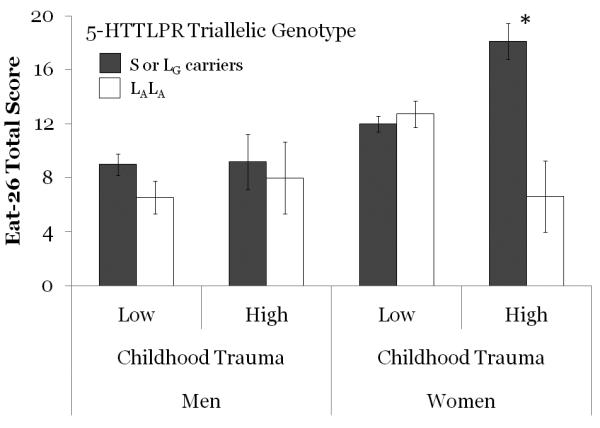
Mean number of eating problems (i.e. EAT-26 Total scores, ± s.e.) for groups defined by gender, level of exposure to childhood trauma and 5-HTTLPR triallelic genotype. * p < .001
We then conducted a multivariate GLM with scores on the four EAT-26 subscales (Self-perception of Body Shape, Dieting, Awareness of Food Contents and Food Preoccupation; (39.)) as dependent variables and with age as a covariate.
For the Self-perception of Body Shape subscale, there were significant main effects only for gender (partial η2 = 0.028), and triallelic 5-HTTLPR genotype (partial η2 = 0.020; see Table 1). Women (M = 2.62, SD = 2.40) had higher mean scores than men (M = 1.19, SD = 1.90). Those with lower expression alleles (M= 2.25, SD = 2.35) had higher mean scores than those with higher expression alleles (M = 1.78, SD = 2.26). There was a significant two-way interaction effect for genotype × trauma (partial η2 =0.015); and a trend for the three-way interaction (partial η2 = 0.013).
For the Dieting subscale, there were significant main effects only for gender (partial η2 = 0.024). Women (M = 3.01, SD = 3.07) had higher mean scores than men (M = 1.50, SD = 1.98). There was a non-significant trend (after Bonferroni correction) for an interaction between gender and genotype (partial η2 = 0.012). There was a significant interaction effect for the three-way gender by genotype by childhood trauma interaction (partial η2 = 0.023). No other effects were statistically significant.
For the Food Preoccupation scale, none of the main effects were significant. There was a significant two-way gender × genotype interaction effect (partial η2 = 0.018). There was also a significant three-way interaction effect (partial η2 = 0.019).
For the Awareness of Food Contents there were no significant main effects or interaction effects. Table 2 presents the descriptive statistics for the four EAT-26 subscales for groups defined by gender, genotype and childhood trauma.
Table 2.
Descriptive statistics for the four EAT-26 factor subscales and percent reporting specific types of eating problems for groups defined by gender, 5-HTTLPR triallelic genotype and experience of childhood trauma.
| Self- perception of body shape |
Dieting | Awareness of food contents |
Food Pre- occupation |
Eating Binges |
Vomiteda | Used Laxativesb |
Treated for ED |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Gender | Triallelic 5-HTTLPR Genotype |
Childhood Trauma |
N | Mean | SD | Mean | SD | Mean | SD | Mean | SD | % | % | % | % |
| Male | LALA | Low | 38 | 0.82 | 1.49 | 1.18 | 1.67 | 1.08 | 1.55 | 0.68 | 1.07 | 8.57 | 2.63 | 2.63 | 0.00 |
| High | 8 | 1.13 | 2.03 | 2.13 | 3.44 | 1.38 | 2.20 | 1.75 | 2.71 | 25.00 | 0.00 | 12.50 | 0.00 | ||
| S or LG | Low | 89 | 1.29 | 1.88 | 1.57 | 1.93 | 1.47 | 1.65 | 0.64 | 1.20 | 8.99 | 6.74 | 11.24 | 1.12 | |
| High | 14 | 1.64 | 2.76 | 1.50 | 2.07 | 1.64 | 2.31 | 1.21 | 1.97 | 28.57 | 14.29 | 7.14 | 0.00 | ||
|
|
|||||||||||||||
| Female | LALA | Low | 61 | 2.61 | 2.50 | 3.05 | 3.10 | 1.92 | 2.38 | 1.25 | 1.93 | 11.48 | 24.59 | 29.51 | 1.64 |
| High | 8 | 0.75 | 0.89 | 1.13 | 0.99 | 0.75 | 1.04 | 0.63 | 1.41 | 12.50 | 0.00 | 0.00 | 0.00 | ||
| S or LG | Low | 179 | 2.45 | 2.25 | 2.72 | 2.75 | 1.82 | 1.99 | 1.18 | 1.62 | 10.06 | 11.17 | 21.23 | 3.91 | |
| High | 33 | 4.00 | 2.70 | 5.00 | 4.10 | 2.70 | 2.77 | 3.21 | 3.43 | 47.06 | 36.36 | 63.64 | 21.21 | ||
|
|
|||||||||||||||
| Total | 430 | 2.12 | 2.34 | 2.49 | 2.83 | 1.73 | 2.05 | 1.19 | 1.89 | ||||||
Note: For 5-HTTLPR triallelic genotype LALA is the higher expression genotype; all others (i.e. carriers of S or LG alleles) are lower expression genotypes.
To control weight or shape.
Or used diet pills, diuretics to control weight or shape. ED = eating disorder.
We then ran a GLM with the same independent variables and covariate with the BIS-11 as the dependent variable. Examining this model should provide insight into whether the effects seen with the eating problem factors are consistent with an interpretation that impulsivity is a potential mediator. There were marginally significant main effects for gender (partial η2 = 0.010), and triallelic 5-HTTLPR genotype (partial η2 = 0.011). There was a significant main effect of childhood trauma (partial η2 = 0.039; see Table 1). Women (M = 63.93, SD = 11.37) had lower mean scores than men (M = 66.97, SD = 10.63). Those with lower expression alleles (M= 64.69, SD = 10.74) had lower mean scores than those with higher expression alleles (M = 65.77, SD = 12.42). Those who experienced lower levels of childhood trauma (M = 64.14, SD = 10.70) had lower mean scores than those who experienced high levels of trauma (M = 69.87, SD = 12.82). There was a significant two-way interaction effect for genotype × trauma (partial η2 =0.013). Post hoc analyses of the effect of 5-HTTLPR triallelic genotype indicated that the only statistically significant difference was that, in men who had experienced High levels of childhood trauma, those with the higher expression alleles had higher BIS-11 Total scores than those with lower function alleles (partial η2 = 0.014; see Figure 2).
Figure 2.
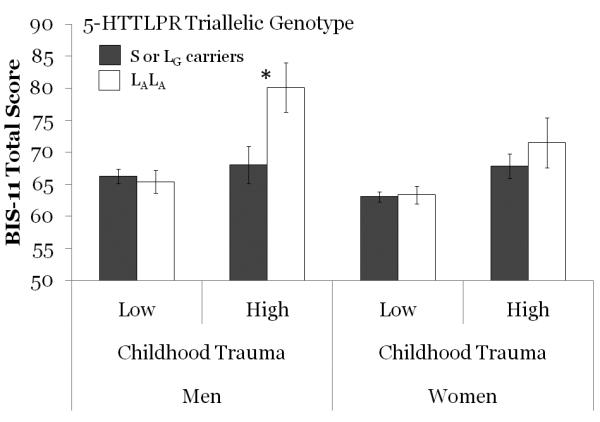
Mean impulsivity score (i.e. BIS-11 Total, ± s.e.) for groups defined by gender, level of exposure to childhood trauma and 5-HTTLPR triallelic genotype. * p = .013
Table 3 presents bivariate correlations among scores on EAT-26, Traumatic Antecedent Questionnaire and BIS-11. Only correlation coefficients significant at p<.05 are shown for clarity of presentation. Scores on subscales for each instrument are significantly inter-correlated and are shown in boxes.
Table 3.
Bivariate correlations among scores on the EAT-26, Traumatic Antecedent Questionnaire and the BIS-11 for men and women.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Eat-26 Total | 0.83 | 0.83 | 0.68 | 0.60 | 0.18 | −0.17 | |||||||
| 2. Self-Perception of Body Shape | 0.82 | 0.75 | 0.38 | 0.61 | |||||||||
| 3. Dieting | 0.91 | 0.76 | 0.52 | 0.49 | 0.18 | ||||||||
| 4. Awareness of Food Contents | 0.76 | 0.47 | 0.69 | 0.18 | 0.17 | −0.19 | −0.24 | ||||||
| 5. Food Preoccupation | 0.64 | 0.54 | 0.60 | 0.33 | 0.17 | 0.18 | |||||||
| 6. Physical Abuse | 0.19 | 0.28 | 0.67 | 0.59 | 0.17 | 0.21 | |||||||
| 7. Sexual Abuse | 0.18 | 0.17 | 0.17 | 0.19 | 0.55 | 0.39 | 0.45 | ||||||
| 8. Witnessing | 0.13 | 0.23 | 0.63 | 0.49 | 0.53 | ||||||||
| 9. Other Traumas | 0.21 | 0.46 | 0.49 | 0.53 | 0.20 | ||||||||
| 10. BIS-11 Total | −0.20 | 0.21 | 0.19 | 0.15 | 0.21 | 0.23 | 0.76 | 0.82 | 0.78 | ||||
| 11. Attentional | 0.15 | −0.18 | 0.17 | 0.13 | 0.13 | 0.21 | 0.22 | 0.79 | 0.43 | 0.41 | |||
| 12. Nonplanning | −0.18 | 0.13 | 0.13 | 0.12 | 0.15 | 0.85 | 0.52 | 0.45 | |||||
| 13. Motor | −0.12 | 0.22 | 0.20 | 0.20 | 0.19 | 0.82 | 0.49 | 0.53 |
Note: Men above diagonal (N=155), Women below (N=284). Only those correlation coefficients significant at p<.05 are shown. Boxes indicate inter-correlations for scales.
Discussion
Our goal for this study was to determine whether the well studied 5-HTTLPR triallelic genotype was associated with eating problems in a non-clinical sample of men and women, and whether the association was moderated by the experience of childhood trauma or by gender. Consistent with our hypothesis, we found that women carrying lower expression alleles (LG or S) who reported being exposed to higher levels of childhood trauma reported a significantly higher mean number of eating problems than other participants. Women in that high risk group were also more likely to report having been treated for an eating disorder and to endorse bulimia-specific symptoms (i.e. binge eating, and controlling weight by vomiting and/or the use of laxatives or diuretics). In addition to this genotype × gender × trauma interaction, our analysis also identified a main effect of the 5-HTTLPR triallelic genotype on eating problems, such that the lower expression alleles were associated with higher mean numbers of eating problems. This finding is consistent with other studies indicating that the S allele of 5-HTTLPR is a risk factor for eating disorders. It is also consistent with the somewhat controversial finding that the S allele appears to confer vulnerability to adult behavioral problems especially when it is coupled with the experience of early childhood adversity. An interaction between 5-HTTLPR triallelic genotype and childhood trauma on eating problems was not observed in men.
When we examined the four factor subscales of the EAT-26 with the same, full factorial, general linear model, we observed that the pattern of results seen in the total score was generally replicated in Self-perception of Body Shape, Dieting and Food Preoccupation. None of the main (gender, genotype, and trauma) or interaction effects was significantly associated with Awareness of Food Contents Scores. In response to a reviewer’s comment, we re-analyzed the data with the more commonly seen three factor subscales (Dieting; Bulimia and Food Preoccupation and Oral Control). The pattern of results was the same in both analyses for the Dieting subscales and for the Food Preoccupation/Bulimia and Food Preoccupation subscales because of the substantial overlap in item representation (data not shown). For Oral Control, however, the regression model was not significant F (8, 421) = 1.26, p = .262. Therefore, it seems that the Self-Perception of Body Shape factor may represent an aspect of eating problems not examined in the traditional three factor extraction.
The Food preoccupation subscale of the EAT-26 was most correlated with reports of childhood traumatic experiences and impulsivity. This factor is comprised of scores on four items: 1) I find myself preoccupied with food. 2) I feel that food controls my life. 3) I give too much time and thought to food. 4) I have gone on eating binges where I feel I am not able to stop (39.). For women, scores on the Food Preoccupation subscale were positively correlated with all measures of childhood trauma, BIS-11 Total and all BIS-11 subscale scores. For men, scores on the Food Preoccupation factor were not correlated with any of the measures of childhood trauma, but they were correlated with BIS-11 Total score and the BIS-11 Nonplanning subscale. This pattern of correlations characterizes reduced behavioral control over eating that, particularly in women, appears to be related to the experience of childhood trauma. If this pattern of findings is confirmed, it seems that prevention and treatment efforts for women who have experienced childhood trauma should focus on counteracting preoccupation with food.
The Awareness of Food Contents factor scores are negatively correlated with BIS-11 Total score and Motor score in men and with all BIS-11 scores in women. Those with high Motor impulsivity (i.e. acting without thinking) appear to be reporting that they do not seem to be very concerned about the type of foods they are eating. Examination of the subscales for both the EAT-26 and the BIS-11 revealed that certain associations may be obscured when only examining the total scale scores. For example, impulsivity was negatively correlated with Awareness of Food Contents and positively associated with Food Preoccupation and so summing them to obtain the EAT-26 total score eliminated the correlation between EAT-26 and impulsivity.
Women with lower expressing 5-HTTLPR alleles, who had reported high levels of childhood trauma, did not have elevated scores on impulsivity as measured by the BIS-11 Total score. It seems then that other psychological constructs, such as anxiety or affect are required to explain the elevation of eating problems observed in that group (42.). This pattern of results is consistent with a recent study that found the relationship between depressive feelings and emotional eating was strongest in girls with the S allele of 5-HTTLPR (43.). For men who reported high levels of childhood trauma the higher expressing 5-HTTLPR allele was associated with higher impulsivity scores than those with the lower expressing alleles. This pattern is different from that seen for eating problems. It may be that men with the higher expressing alleles are at risk for other problems because of their risky decision making (34; 44.), relatively lower amygdala activation in response to emotional stimuli (45-46.), and slow rate of learning from punishment (47.). These men do not appear to be at elevated risk for eating problems, but may be of particular interest for potential involvement in other risky behaviors. While it may be that the lack of effect could be due to relatively lower statistical power (i.e. relatively few men in the High Trauma exposure condition (23.)), the pattern of mean EAT-26 scores for men does not resemble the pattern observed in women. The EAT-26 may better assess relevant variation in eating problems in women than in men. Alternatively, the phenotypic expression of risk vulnerability in men might be revealed in other health-risk behaviors. However, it must be noted that because of the rather small number of men who had higher expressing 5-HTTLPR alleles and reported experiencing High levels of childhood trauma (n=8; see Table 2), the gender specificity of this result should be interpreted with some caution.
Women with lower expressing 5-HTTLPR alleles, who have experienced childhood trauma appear to be at especially high risk for eating problems and therefore screening efforts may consider collecting genotype information along with information about the experience of childhood physical and sexual abuse as well as witnessing and exposure to other traumas in the context of risk assessment. If 5-HTTLPR S allele carriers are less likely to respond to SSRI treatment for eating disorders (48.), then knowing a patient’s genotype and childhood trauma history may indicate another treatment approach, such as cognitive behavioral therapy.
This study has several strengths. Our sample size is relatively large for this type of study and our sample is relatively ethnically homogeneous. We tested straightforward hypotheses and purposefully conducted a limited number of statistical tests to conserve statistical power. We used well known instruments to assess eating problems and impulsivity. We assayed the well known 5-HTTLPR and rs25531 polymorphisms and grouped participants based on the current understanding of the influence of these loci on SLC6A4 expression. Our results are consistent with several other studies.
This study has some limitations. One is that individuals with different 5-HTTLPR genotypes may interpret events differently and there may be a bias such that those homozygous for higher expression alleles may report fewer traumatic experiences because their subjective interpretation of an event might lead them to label it as non-traumatic. However, in this study, genotype and level of trauma reported were independent (χ2=0.09, df = 1, p = .77). There are other genetic polymorphisms that affect SLC6A4 expression and function and we did not assay all of them. So, it is likely that we are not capturing all of the genetic variation relevant to the question under consideration. We did not assess reward sensitivity, affect regulation, or history of anxiety or mood disorder. The connection between eating disorder symptoms and depression symptoms in women appears to be especially important (49.), so future studies should definitely assess mood disorder symptoms as well as eating problems. We also did not assess other aspects of impulsivity that might be relevant for eating problems, such as delay discounting or response inhibition. We also did not assess height and weight of participants, so we are unable to examine questions regarding obesity. Additionally, we focused on the self-report of trauma during a single, rather broad age range (age 0-12), so our findings may not generalize to trauma experienced outside of that age range.
In this study we assessed eating problems, childhood trauma, impulsivity and genotypes that influence a key regulator of 5-HT function in a non-clinical sample. Our results suggest that in women who have experienced high levels of childhood trauma, lower expressing 5-HTTLPR alleles are associated with elevated risk for eating problems. Further, we have shown that impulsivity is unlikely to mediate this association. Finer grain analyses with different aspects of impulsivity, and more detailed assessments of childhood trauma and eating problem, and other genotypes in both clinical and non-clinical populations may help to better characterize the genetic architecture of eating problems.
Acknowledgements
The authors would like to thank Melissa K. Lehmann and Christa C. Christ for their work in the lab; and Krista B. Highland, Christa C. Christ and Matthew Brady for comments on an earlier version of this manuscript.
Supported by R15MH077654-01A1 (NIMH) and P20 RR016479 (NCRR).
Footnotes
None of the authors has any financial disclosures to make or conflicts of interest to declare.
References
- 1.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioakimidis I, Zandian M, Ulbl F, Bergh C, Leon M, Sodersten P. How eating affects mood. Physiol Behav. 2011;103:290–294. doi: 10.1016/j.physbeh.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Bruce KR, Steiger H, Young SN, Kin NM, Israel M, Levesque M. Impact of acute tryptophan depletion on mood and eating-related urges in bulimic and nonbulimic women. J Psychiatry Neurosci. 2009;34:376–382. [PMC free article] [PubMed] [Google Scholar]
- 5.Kaye WH, Gendall KA, Fernstrom MH, Fernstrom JD, McConaha CW, Weltzin TE. Effects of acute tryptophan depletion on mood in bulimia nervosa. Biol Psychiatry. 2000;47:151–157. doi: 10.1016/s0006-3223(99)00108-0. [DOI] [PubMed] [Google Scholar]
- 6.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 7.Geeraerts B, Van Oudenhove L, Boesmans W, Vos R, Vanden Berghe P, Tack J. Influence of acute tryptophan depletion on gastric sensorimotor function in humans. Am J Physiol Gastrointest Liver Physiol. 2011;300:G228–235. doi: 10.1152/ajpgi.00020.2010. [DOI] [PubMed] [Google Scholar]
- 8.Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychol Bull. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou R, Mogg K, Bradley BP, Moss-Morris R, Peveler R, Roefs A. External eating, impulsivity and attentional bias to food cues. Appetite. 2011;56:424–427. doi: 10.1016/j.appet.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 10.Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erritzoe D, Frokjaer VG, Haahr MT, Kalbitzer J, Svarer C, Holst KK, et al. Cerebral serotonin transporter binding is inversely related to body mass index. Neuroimage. 2010;52:284–289. doi: 10.1016/j.neuroimage.2010.03.086. [DOI] [PubMed] [Google Scholar]
- 12.Calati R, De Ronchi D, Bellini M, Serretti A. The 5-HTTLPR polymorphism and eating disorders: a meta-analysis. Int J Eat Disord. 2011;44:191–199. doi: 10.1002/eat.20811. [DOI] [PubMed] [Google Scholar]
- 13.Miller CA, Golden NH. An introduction to eating disorders: clinical presentation, epidemiology, and prognosis. Nutr Clin Pract. 2010;25:110–115. doi: 10.1177/0884533609357566. [DOI] [PubMed] [Google Scholar]
- 14.Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- 15.Way BM, Taylor SE. A polymorphism in the serotonin transporter gene moderates cardiovascular reactivity to psychosocial stress. Psychosom Med. 2011;73:310–317. doi: 10.1097/PSY.0b013e31821195ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akkermann K, Nordquist N, Oreland L, Harro J. Serotonin transporter gene promoter polymorphism affects the severity of binge eating in general population. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:111–114. doi: 10.1016/j.pnpbp.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Steiger H, Richardson J, Schmitz N, Joober R, Israel M, Bruce KR, et al. Association of trait-defined, eating-disorder sub-phenotypes with (biallelic and triallelic) 5HTTLPR variations. J Psychiatr Res. 2009;43:1086–1094. doi: 10.1016/j.jpsychires.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Ericsson NS, Keel PK, Holland L, Selby EA, Verona E, Cougle JR, et al. Parental disorders, childhood abuse, and binge eating in a large community sample. Int J Eat Disord. 2011 doi: 10.1002/eat.20938. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JG, Cohen P, Kasen S, Brook JS. Childhood adversities associated with risk for eating disorders or weight problems during adolescence or early adulthood. Am J Psychiatry. 2002;159:394–400. doi: 10.1176/appi.ajp.159.3.394. [DOI] [PubMed] [Google Scholar]
- 20.Mazzeo SE, Espelage DL. Association between childhood physical and emotional abuse and disordered eating behaviors in female undergraduates: An investigation of the mediating role of alexithymia and depression. Journal of Counseling Psychology. 2002;49:86–100. [Google Scholar]
- 21.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 22.Uher R, Caspi A, Houts R, Sugden K, Williams B, Poulton R, et al. Serotonin transporter gene moderates childhood maltreatment’s effects on persistent but not single-episode depression: Replications and implications for resolving inconsistent results. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munafo MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wankerl M, Wust S, Otte C. Current developments and controversies: does the serotonin transporter gene-linked polymorphic region (5-HTTLPR) modulate the association between stress and depression? Curr Opin Psychiatry. 2010;23:582–587. doi: 10.1097/YCO.0b013e32833f0e3a. [DOI] [PubMed] [Google Scholar]
- 28.Antypa N, Cerit H, Kruijt AW, Verhoeven FE, Van der Does AJ. Relationships among 5-HTT genotype, life events and gender in the recognition of facial emotions. Neuroscience. 2011;172:303–313. doi: 10.1016/j.neuroscience.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 29.Lesch KP. When the serotonin transporter gene meets adversity: the contribution of animal models to understanding epigenetic mechanisms in affective disorders and resilience. Curr Top Behav Neurosci. 2011;7:251–280. doi: 10.1007/7854_2010_109. [DOI] [PubMed] [Google Scholar]
- 30.Richardson J, Steiger H, Schmitz N, Joober R, Bruce KR, Israel M, et al. Relevance of the 5-HTTLPR polymorphism and childhood abuse to increased psychiatric comorbidity in women with bulimia-spectrum disorders. J Clin Psychiatry. 2008;69:981–990. doi: 10.4088/jcp.v69n0615. [DOI] [PubMed] [Google Scholar]
- 31.Steiger H, Richardson J, Joober R, Gauvin L, Israel M, Bruce KR, et al. The 5HTTLPR polymorphism, prior maltreatment and dramatic-erratic personality manifestations in women with bulimic syndromes. J Psychiatry Neurosci. 2007;32:354–362. [PMC free article] [PubMed] [Google Scholar]
- 32.Steiger H, Richardson J, Joober R, Israel M, Bruce KR, Ng Ying Kin NM, et al. Dissocial behavior, the 5HTTLPR polymorphism, and maltreatment in women with bulimic syndromes. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:128–130. doi: 10.1002/ajmg.b.30579. [DOI] [PubMed] [Google Scholar]
- 33.Abbott A. Psychiatric genetics: The brains of the family. Nature. 2008;454:154–157. doi: 10.1038/454154a. [DOI] [PubMed] [Google Scholar]
- 34.Stoltenberg SF, Lehmann MK, Anderson C, Nag P, Anagnopoulos C. Serotonin transporter (5-HTTLPR) genotype and childhood trauma are associated with individual differences in decision making. Frontiers in Genetics. 2011;2 doi: 10.3389/fgene.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoltenberg SF, Lehmann MK, Christ CC, Hersrud SL, Davies GE. Associations among types of impulsivity, substance use problems and Neurexin-3 polymorphisms. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saleptsi E, Bichescu D, Rockstroh B, Neuner F, Schauer M, Studer K, et al. Negative and positive childhood experiences across developmental periods in psychiatric patients with different diagnoses - an explorative study. BMC Psychiatry. 2004;4:40. doi: 10.1186/1471-244X-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussey JM, Chang JJ, Kotch JB. Child maltreatment in the United States: prevalence, risk factors, and adolescent health consequences. Pediatrics. 2006;118:933–942. doi: 10.1542/peds.2005-2452. [DOI] [PubMed] [Google Scholar]
- 38.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 39.Ocker LB, Lam ETC, Jensen BE, Zhang JJ. Psychometric Properties of the Eating Attitudes Test. Measurement in Physical Education and Exercise Science. 2007;11:25–48. [Google Scholar]
- 40.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 42.Polivy J, Herman CP. Causes of eating disorders. Annu Rev Psychol. 2002;53:187–213. doi: 10.1146/annurev.psych.53.100901.135103. [DOI] [PubMed] [Google Scholar]
- 43.van Strien T, van der Zwaluw CS, Engels RC. Emotional eating in adolescents: a gene (SLC6A4/5-HTT) - depressive feelings interaction analysis. J Psychiatr Res. 2010;44:1035–1042. doi: 10.1016/j.jpsychires.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Stoltenberg SF, Vandever JM. Gender moderates the association between 5-HTTLPR and decision-making under ambiguity but not under risk. Neuropharmacology. 2010;58:423–428. doi: 10.1016/j.neuropharm.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemogne C, Gorwood P, Boni C, Pessiglione M, Lehericy S, Fossati P. Cognitive appraisal and life stress moderate the effects of the 5-HTTLPR polymorphism on amygdala reactivity. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von dem Hagen EA, Passamonti L, Nutland S, Sambrook J, Calder AJ. The serotonin transporter gene polymorphism and the effect of baseline on amygdala response to emotional faces. Neuropsychologia. 2011;49:674–680. doi: 10.1016/j.neuropsychologia.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blair KS, Finger E, Marsh AA, Morton J, Mondillo K, Buzas B, et al. The role of 5-HTTLPR in choosing the lesser of two evils, the better of two goods: examining the impact of 5-HTTLPR genotype and tryptophan depletion in object choice. Psychopharmacology (Berl) 2008;196:29–38. doi: 10.1007/s00213-007-0920-y. [DOI] [PubMed] [Google Scholar]
- 48.Monteleone P, Santonastaso P, Tortorella A, Favaro A, Fabrazzo M, Castaldo E, et al. Serotonin transporter polymorphism and potential response to SSRIs in bulimia nervosa. Mol Psychiatry. 2005;10:716–718. doi: 10.1038/sj.mp.4001683. [DOI] [PubMed] [Google Scholar]
- 49.Mata J, Gotlib IH. 5-HTTLPR moderates the relation between changes in depressive and bulimic symptoms in adolescent girls: A longitudinal study. Int J Eat Disord. 2010 doi: 10.1002/eat.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]


