Abstract
Interactions between sodium intake, the renin angiotensin system and renal and cardiovascular outcomes are incompletely understood. Lambers Heerspink et al's analysis shows that angiotensin receptor blockade (ARB) improves diabetic nephropathy and cardiovascular disease more when dietary sodium intake is low and suggests possible harm when sodium intake is high. These findings highlight dietary salt as a modifiable cardiovascular and renal risk factor and emphasize the need for need for detailed mechanistic studies.
Angiotensin II (Ang II) contributes to the pathophysiology of diabetic nephropathy and other forms of progressive renal disease1, and the benefits of antagonizing Ang II activity in the treatment of nephropathy due to type 2 diabetes has been established for more than a decade2-4. The renin-angiotensin system (RAS) plays a similarly important role in the pathophysiology of cardiovascular disease5.
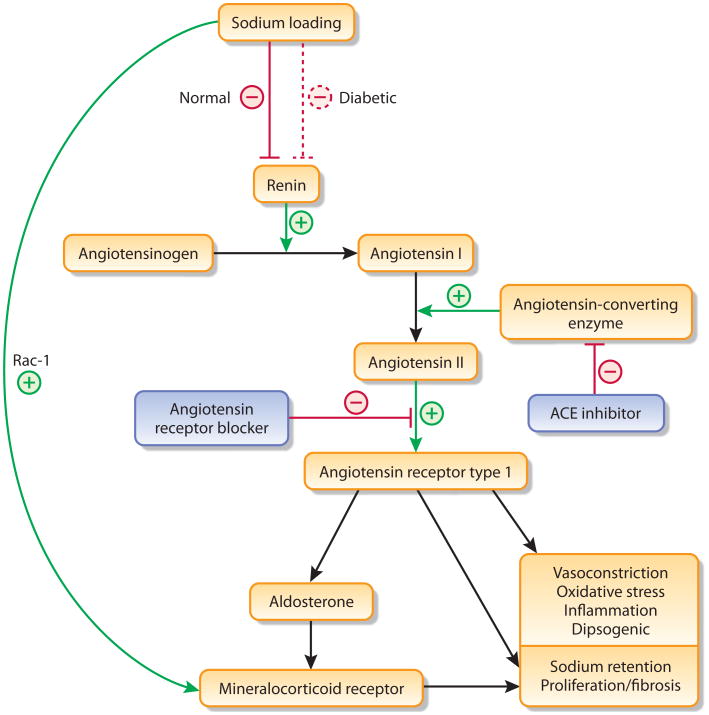
Although dietary sodium loading suppresses the systemic RAS, resulting in lower plasma renin, Ang II, and aldosterone levels, a high sodium diet is also associated with an increased risk of cardiovascular disease6. This apparent paradoxical effect of sodium (suppression of the systemic RAS but exacerbation of cardiovascular risk) may be explained, in part, by the muted effects of sodium loading in some individuals, like those with hypertension and type 2 diabetes7. Such patients have RAS activation, both systemically and at the tissue level (eg, hart, kidney), that does not suppress normally on a high sodium diet. Moreover, short-term studies in both experimental animals and humans demonstrate that a high sodium diet blunts the beneficial effects of therapy with angiotensin converting enzyme (ACE) inhibitors on proteinuria and left ventricular hypertrophy8. It was therefore unsurprising that a high sodium diet was associated with greater proteinuria and faster progression to end-stage renal disease (ESRD) in hypertensive patients with proteinuria, as was recently shown in patients treated with an ACE inhibitor in the Ramipril Efficacy in Nephropathy trial9. These data suggest that both inhibition of Ang II and control of dietary sodium intake are important factors in the treatment of CKD (Figure 1).
Figure 1.
Multifactorial role of sodium in the renin angiotensin system—Sodium loading typically suppresses renin synthesis, but this response may be attenuated in diabetes. Conversely, sodium loading can directly activate the mineralocorticoid receptor activity via up-regulation of Rac-1 signaling or via increases in angiotensin converting enzyme (ACE) activity.
Nevertheless, the long-term effects of dietary sodium on the effects of angiotensin inhibition remain poorly studied. In this context, the study by Lambers Heerspink et al. in this issue10 provides intriguing new data. The authors conducted a post-hoc analysis of 1177 (36% of those enrolled) patients with type 2 diabetic nephropathy in the RENAAL4 and IDNT 3trials who had available measurements of 24 hour urinary sodium excretion. They found that the effect of angiotensin receptor blocker (ARB) therapy on renal and cardiovascular outcomes varied according to sodium intake. Both blood pressure and albuminuria were decreased by the ARB to a greater extent in those with lower compared with higher sodium intake. More importantly, the benefits of ARB therapy on renal outcomes (defined as a doubling of serum creatinine or progression to ESRD) and cardiovascular outcomes (defined as cardiovascular death, myocardial infarction stroke, or hospitalization for heart failure or coronary revascularization) were significantly greater in those at the lowest compared with highest tertile of sodium intake. Specifically, the ARB was associated with significantly reduced risks of renal and cardiovascular events, 43% and 37% respectively, if sodium intake was low. In contrast, ARB therapy was associated with nonsignificantly higher risks when sodium intake was high. These data support the idea that renal and cardiovascular protection is maximized when both salt restriction and renin angiotensin blockade are combined.
The author's findings are intuitively compelling, and the analysis has several strengths, including the use of high-quality, randomized trial data with sufficient numbers of patients and outcomes to assess meaningful clinical endpoints. Furthermore, the collection of urine every 6 months allowed for the analysis of mean sodium intake throughout the trial rather than at baseline (or a single time point) as in most other studies of sodium intake.
However, a number of issues with the study methodology merit consideration. Although the authors analyzed data from two randomized trials, only the assignment to ARB vs. control was randomized. Sodium intake was not randomized, and there were many significant and clinically important differences in baseline characteristics at the extremes of intake. Compared with the lowest tertile of intake, for example, patients in the highest tertile were more likely to be female (46.9% vs. 20.9%), white (54.6% vs. 42.8%), and have a lower estimated GFR (42.2 vs. 45.6 ml/min/1.73m2), a lower body weight (85.4 vs 91.9 kg), and a higher urinary albumin to creatinine ratio (1905 vs. 1173 mg/g). The greater distribution of important risk factors in those with higher salt intakes suggests that the sickest patients—i.e. possibly those least able to respond to intervention—were most likely to have a high salt intake. This could be one critical factor underlying the greater benefits of ARB therapy observed in those with lower salt intakes. In fact, careful scrutiny of Lambers Heerspink's Kaplan-Meir curves raise that possibility: in ARB treated patients, the major differences in cardiovascular and renal event rates among the different sodium intake groups occur during the first year, with relatively equal event rates during subsequent years. In addition, the authors did not control for most of the potential confounding factors. Although adjusting for one factor (specifically, baseline estimated GFR or urea excretion) did not change the interpretation of the main findings, fully-adjusted multivariable models (controlling for age, sex, race, weight, baseline estimated GFR, blood pressure, urinary protein excretion and whether patients came from IDNT or RENAAL) should have been performed. Such data would have provided greater confidence that the results are valid and do not simply reflect confounding by an asymmetric distribution of comorbidity.
An additional concern is that the significant differences in benefit derived from ARB therapy observed at different levels of sodium intake was largely driven by a higher risk of cardiovascular (hazard ratio 1.25, 95% CI: 0.89-1.75) and renal events (hazard ratio 1.37, 95% CI: 0.96-1.96) associated with ARBs compared with control therapy in those in the highest tertile of sodium intake. As above, these findings could simply reflect confounding due to baseline imbalances. For example, the well known hemodynamic effect of ARB initiation (resulting in a decline in GFR) may have been more clinically dramatic in patients with a lower baseline eGFR, resulting in a greater frequency of renal events. However, if the apparent adverse effects of ARB therapy in patients consuming a high sodium diet are not spurious observations resulting from uncontrolled confounding, then this finding suggests the intriguing possibility that ARBs are helpful in individuals with a low sodium intake, but potentially harmful when sodium intake is high. The mechanism for such an effect is uncertain. Although the authors provide rationale for a blunted effect of ARBs in patients who consume a high sodium diet, they do not provide an explanation for the worse outcomes, albeit not statistically significant, that were observed.
Although flawed, the study by Lambers Heerspink and colleagues confirms prior work suggesting that both sodium restriction and blockade of the renin angiotensin system are beneficial, and it should serve as a reminder to clinicians not to ignore diet as a modifiable cardiovascular and renal risk factor. In the context of decades of research into the important roles that sodium intake and Ang II play in pathogenesis of renal and cardiovascular disease, this study should increase clinician's comfort in recommending dietary sodium restriction for their patients with diabetic nephropathy on ARB, lest a high sodium intake abrogate the protective effects. The study also raises intriguing new questions about the mechanisms of the interaction between sodium intake and ARB, and raises some concern that ARB may be harmful in some patients. Additional mechanistic studies and randomized trials are clearly warranted. In short, this study reminds us of how much further there is to go in understanding the full potential of our therapeutic armamentarium in diabetic kidney disease.
Acknowledgments
Disclosure: Dr Charytan is supported by the a Carl S. Gottschalk award from the American Society of Nephrology and NIDDK 5 R21 DK089368-02. He has no other disclosures. Dr Forman is supported by NHLBI 5 R01 HL105440-03 and 5 R01 HL101122-02. He is also a deputy editor of nephrology at UpToDate, Inc. He has no other disclosures
References
- 1.Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol. 2006;17:2985–2991. doi: 10.1681/ASN.2006040356. [DOI] [PubMed] [Google Scholar]
- 2.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 4.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 5.Probstfield JL, O'Brien KD. Progression of cardiovascular damage: the role of renin-angiotensin system blockade. Am J Cardiol. 2010;105:10A–20A. doi: 10.1016/j.amjcard.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378:380–382. doi: 10.1016/S0140-6736(11)61174-4. [DOI] [PubMed] [Google Scholar]
- 7.Price DA, De'Oliveira JM, Fisher ND, Williams GH, et al. The state and responsiveness of the renin-angiotensin-aldosterone system in patients with type II diabetes mellitus. Am J Hypertens. 1999;12:348–355. [PubMed] [Google Scholar]
- 8.Buter H, Hemmelder MH, Navis G, de Jong PE, et al. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide. Nephrol Dial Transplant. 1998;13:1682–1685. doi: 10.1093/ndt/13.7.1682. [DOI] [PubMed] [Google Scholar]
- 9.Vegter S, Perna A, Postma MJ, Navis G, et al. Sodium Intake, ACE Inhibition, and Progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambers Heerspink HJL, Holtkamp FA, Parving HH, Navis GJ, et al. Moderate sodium diet potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers: A post-hoc analysis of the RENAAL and IDNT Trials. Kidney Int. 2012 doi: 10.1038/ki.2012.74. [DOI] [PubMed] [Google Scholar]