Abstract
Several neuroinvasive viruses can be used to study the mammalian nervous system. In particular, infection by pseudorabies virus (PRV), an α-herpesvirus with broad host range, reveals chains of functionally connected neurons in the nervous systems of a variety of mammals. The specificity of PRV trans-neuronal spread has been established in several systems. One attenuated strain, PRV-Bartha, causes a reduced inflammatory response and also spreads only from infected post- to pre-synaptic neurons. We review the basics of PRV tracing and then discuss new developments and novel approaches that have enabled a more detailed understanding of the architecture of the nervous system. As questions and techniques evolve in the field of neuroscience, advances in PRV tracing will certainly follow.
Pseudorabies virus as a trans-neuronal tracer
A time-honored protocol for tracing neural connections is to inject an enzyme or dye into a small area of the brain, from which it is taken up by nerve terminals and then transported along axons. However, most of these tracers are retained within neurons and do not diffuse to connected neurons. Those that can spread to connected neurons dilute rapidly, an obvious impediment to following multisynaptic circuits. Viruses provide a noteworthy alternative to these chemical tracers. The most useful are those neuroinvasive viruses that infect the central nervous system (CNS) after peripheral inoculation. Well known examples of neuroinvasive viruses are rabies virus (an RNA virus from the family Rhabdoviridae) and the α-herpesviruses (DNA viruses from the subfamily Herpesvirinae) [1].
When infection spreads between functionally connected neurons, it is said to be trans-neuronal. The actual mechanism of trans-neuronal spread is understood poorly. The important point is that, unlike chemical tracers, as the infection spreads, viral-encoded proteins mark each infected neuron. Consequently, neuroinvasive viruses can be self-amplifying tracers of neuronal circuitry.
In recent years, pseudorabies virus (PRV) has become the most popular α-herpesvirus for circuit-tracing studies. Despite its name, PRV has absolutely no relationship to rabies virus. The natural hosts of PRV are swine, although the virus has a broad host range; it is neuroinvasive in essentially all mammals, except higher primates. PRV can be used in a wide range of animal models but remains safe for laboratory workers. It is amenable to genetic manipulation and the insertion of large segments of foreign DNA. The 142 kb double-stranded PRV genome has been cloned into a bacterial artificial chromosome (BAC) [2], which enables easy genetic modification of the genome in bacteria using standard DNA recombination technology [3].
PRV as a molecular pathfinder
PRV infection is confined to functionally connected neurons
α-herpesviruses are pantropic; that is, they infect a variety of cell types, including neurons. Specificity of infection is mediated by a single viral protein called gD that binds to one of several receptors [4,5]. This pantropic property manifests itself in the characteristic lesions of infected tissues, such as the skin and mucosa, as well as the nervous system. Despite the capacity to infect a wide variety of cell types, PRV spreads faithfully among functionally connected neurons by direct cell–cell contact, rather than by diffusion of released virus particles through the extracellular space. These cell–cell contacts are often posited to be synapses, although evidence for this supposition is circumstantial. Despite this uncertainty, evidence that PRV spreads through defined neural circuits is strong and comes from carefully designed studies of virus transport within the nervous system (reviewed in [6]). Four main points have been established: (i) PRV infection reproduces tracing studies faithfully by well characterized, nonviral tracers; (ii) infection does not spread interaxonally to non-synaptically connected, physically adjacent circuitry; (iii) spread of PRV in the nervous system requires an intact circuit and synaptic connections; and (iv) PRV appears to spread in any neuronal circuit studied to date – that is, infection is not confined to a circuit that uses a particular neurotransmitter nor is spread confined to circuits defined by anatomy or function, such as sensory or motor circuitry. Accordingly, neurobiologists have embraced the fact that the neuron-to-neuron spread of PRV infection reports functional neuronal circuitry.
Role of local host defense in trans-neuronal spread
PRV neuronal infection is constrained to neurons by action of glia cells surrounding the infected neurons as well as by the local cytoarchitecture. Astrocytes and microglia respond within a few hours of neuronal infection, perhaps by detecting cytokines released by the neurons [7,8]. These activated glia can be infected, probably through glia–neuron contacts, and suppress viral replication. Although PRV infects astrocytes and brain macrophages that are in close apposition to infected neurons, infectious virions are not produced and there is no spread of PRV from infected glial cells to nearby axons outside the circuitry being traced [9,10]. These non-permissive support cells appear to form a barrier around the infected neuron [11].
The evolution of PRV tracing as a laboratory technique
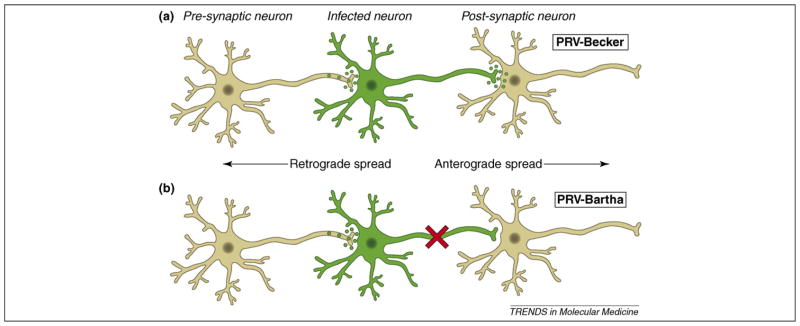
Virulent strains of PRV, such as PRV-Becker, PRV-Kaplan and NIA3, are not good tracers in general. When injected at peripheral sites, virulent PRV strains induce a powerful inflammatory response that kills animals within 2–3 days of infection [12]. Although spread of the virulent virus in the nervous system can occur, injected animals die before much of the circuitry is infected. Accordingly, most PRV-tracing viruses are based on attenuated strains, such as PRV-Bartha, that replicate well but induce a substantially reduced inflammatory response. As a result, infected animals live longer, enabling deep penetration of PRV-Bartha into the nervous system. An additional attribute of PRV-Bartha is that, unlike wild-type PRV [12–15], it spreads only from post- to pre-synaptic cells in a circuit (retrograde spread only) (Figure 1). The retrograde-only spread of Bartha clearly identifies the impulse direction of neuronal circuits, facilitating circuitry analysis. This unidirectional spread phenotype of PRV-Bartha results from a deletion that removes the gE, gI and Us9 genes [16–18]. Interestingly, if the virulence of wild-type PRV is reduced without affecting the gE, gI or Us9 proteins, it is possible to achieve both anterograde and delayed retrograde spread in neuronal circuits [19].
Figure 1.
Trans-synaptic spread of PRV infection in a neural circuit. (a) After infecting primary neurons (shown in green), wild-type strains of PRV, such as PRV-Becker or PRV-Kaplan, promote infection that spreads from the cell body to axon terminals of pre-synaptic neurons (retrograde spread). Virions of wild-type strains might also be transported down the axon to infect the cell bodies of post-synaptic neurons (anterograde spread). (b) PRV-Bartha replicates well in neurons, but infected animals have reduced symptoms compared to wild-type virus infections. In addition, PRV-Bartha infection spreads only from post- to pre-synaptic neurons in a circuit. Infection by PRV-Bartha cannot spread from pre- to post-synaptic neurons because structural components of virions cannot be sorted into axons, a process that requires the actions of viral proteins gE, gI and US9. The genes encoding these proteins are deleted in the PRV-Bartha genome.
PRV-Bartha was first exploited for defining CNS circuits that modulate the autonomic and somatic peripheral outflows [20]. Early tracing studies depended on immunohistochemistry using antibodies directed against structural PRV proteins (reviewed in [21,22]), however, recombinant viruses that expressed reporter molecules soon proved to be useful tracers. The first generation of improved PRV-Bartha recombinants expressed β-galacto-sidase or green- or red-fluorescent proteins (GFP, RFP) [23–27]. All have been used in mapping efferent neural pathways through injections of peripheral tissues (reviewed in [20]).
An important finding was that a single neuron could be infected by two different PRV strains. This dual-labeling paradigm enables neuroscientists to identify those neurons that project to two different peripheral tissues and are thus involved in orchestrating the synchronized activity of two separate afferent pathways [25,28–31]. Another important finding was that PRV-Bartha-infected neurons retain relatively normal electrophysiological properties for some time after infection [24,32–35]. Boldogkoi et al. have reported additional attenuated PRV strains that are useful for tracing [36].
PRV can map CNS circuitry directly after intracerebral injections (reviewed in [37]). Such stereotactic injections provide precise, dose-dependent infection of axon terminals. PRV virions are large, being approximately 200 nm in diameter, and have a high affinity for extracellular matrix proteins, such as heparan sulfate proteoglycan. As a result, virions do not diffuse from the site of injection. Although cell bodies at the injection site can be infected, there is more robust labeling of cell bodies that send axonal projections to the site of injection. The number of infected projecting cell bodies depends on the density of the projections at the site of injection as well as the number of infectious virions injected [38]. In all CNS injections, care must be taken to avoid the cerebral ventricles, which can lead to widespread infection of distant areas of the brain.
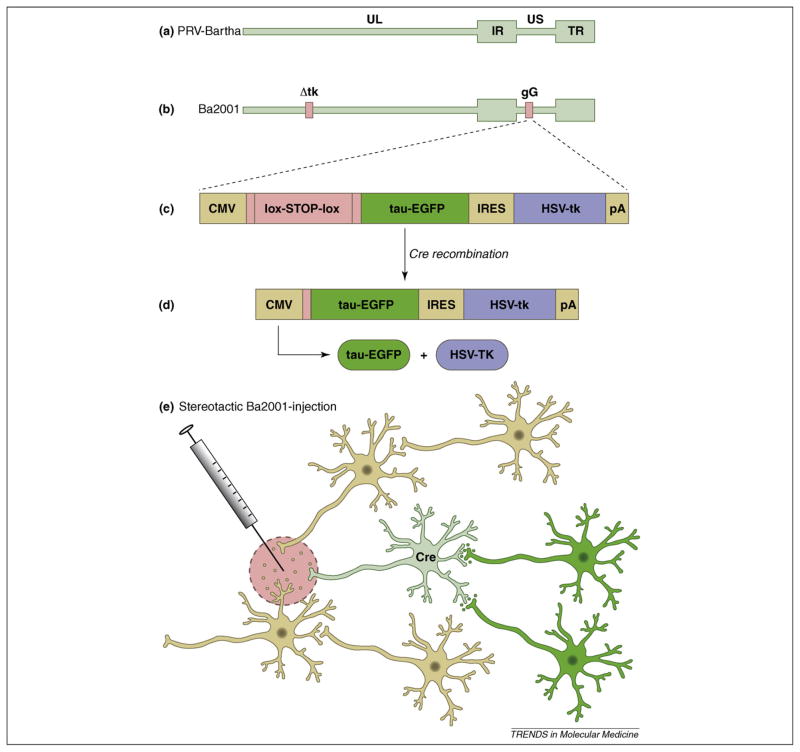
Precise mapping of CNS circuits requires better control of infection than can be obtained by simple stereotactic procedures. To that end, DeFalco and colleagues constructed a derivative of PRV-Bartha that replicates only when it infects a cell expressing the Cre-recombinase protein. This strain, Ba2001, does not express the viral thymidine kinase (TK) gene and therefore cannot replicate in non-mitotic cells [39] (Figure 2). When a cell expresses Cre, TK expression is activated and the virus replicates and spreads to all synaptically connected neurons. [40].
Figure 2.
Retrograde tracing from molecularly defined neurons. (a) The PRV genome includes a unique long (UL) and unique short (US) segment and internal (IR) and terminal (TR) repeat regions. (b) Ba2001 has a deletion in the gene encoding the viral thymidine kinase (Δtk). Without TK, PRV cannot replicate in non-dividing cells, such as neurons. Glycoprotein G (gG) is a non-essential protein and the gG locus is a common location for the insertion of reporter genes. (c) The gG locus in Ba2001 has a lox–stop–lox cassette between the CMV immediate-early promoter (CMV) and the genes for the tau–EGFP marker and TK gene from HSV. Tau–EGFP and HSV–tk are separated by an internal ribosome entry signal (IRES). (d) Recombination between the lox sites occurs only in cells expressing Cre. This enables the CMV promoter to drive expression of the tau–EGFP and HSV–tk bicistronic message. The tau–EGFP acts as a fluorescent marker in infected cells. The HSV–tk enables Ba2001 to replicate in neurons. (e) In a transgenic mouse engineered to express Cre from a specific promoter, PRV-Bartha will infect all neurons within range of the injection site (pink circle). Ba2001 will initiate replication and trace retrograde circuitry only from neurons that express Cre-recombinase
Recent advances in deciphering the complexities of the nervous system
Detection of transgenic marker genes in PRV-infected neurons
Mice lacking the melanocortin-4 receptor (MC4R) are obesity-prone when placed on a moderate-fat diet, partly owing to a defect in brown-adipose tissue (BAT) thermogenesis. BAT thermogenesis is under the control of sympathetic outflow from the brain and regulates energy expenditure and body weight. Voss-Andreae et al. used PRV-BaBlue (a PRV-Bartha expressing β-galactosidase) to trace CNS circuits from interscapular BAT in a transgenic mouse expressing enhanced green-fluorescence protein (EGFP) in MC4R neurons. Neurons expressing both β-galactosidase and EGFP were detected in several brain nuclei known to be involved in sympathetic outflow [41].
Dual tracing of sympathetic and somatomotor pathways
Presympathetic-premotor neurons (PSPMNs) participate in the coordination of sympathetic and somatomotor output from the brain. Many of these PSPMNs are located in the lateral hypothalamus, an area containing both melanin-concentrating hormone (MCH)-producing neurons and orexin-producing neurons. Both neurons are implicated in behaviors requiring synchronization between sympathetic and somatomotor activity. Kerman and colleagues injected sympathectomized gastrocnemius muscles and adrenal glands with either PRV-152 (EGFP-expressing) or PRV-BaBlue (β-galactosidase-expressing) tracing strains. Both sympathetic (to adrenals) and somatomotor (to muscles) brain-output pathways were labeled. Two distinct and spatially separate groups of double-labeled PSPMNs were identified in the lateral hypothalamus. These two groups also differed in the sense that they co-expressed MCH or orexin predominately [42].
Dual-tracing studies of organ innervation
PRV tracing was used to study the central innervation of apparently symmetrical ovaries and adrenal glands. The right or left organ was injected with a particular PRV-Bartha strain. In addition, the left and right organs were injected simultaneously with isogenic strains of PRV-Bartha that expressed different marker genes. The results indicated that the left ovary [43] and adrenal gland [44] received more central input than the corresponding right organ.
Tracing retinal circuitry controlling circadian rhythm
When PRV-Bartha is injected into the vitreous humor of one eye, infection will spread ultimately to retinal ganglion cells in the contralateral retina. In this infection paradigm, PRV-Bartha spreads into the CNS only through routes of parasympathetic and sympathetic innervation of the orbital muscles and the ciliary body, respectively. Retrograde infection by sympathetic routes leads to infection of the suprachiasmatic nuclei (SCN) and, from there, to retinal ganglion cells in the contralateral eye [14,45,46] (and reviewed in [47]). In a recent study, Viney and colleagues used this paradigm to study the local circuitry of intrinsically photosensitive retinal ganglion cells (ipRGCs). ipRGCs constitute only approximately 1–2% of all retinal ganglion cells but are of crucial importance in the control of circadian rhythm. Interestingly, the contralateral retinal ganglion cells labeled are almost exclusively ipRGCs. By injecting a low concentration of virions, the authors could identify a small number of spatially well separated ipRGCs. Through the use of a sophisticated combination of immunohistochemistry, two-photon microscopy and electrophysiology, they identified classes of ipRGCs based on their respective dendritic projections. By timing the infections carefully, spread of infection from ipRGCs to surrounding inhibitory amacrine cells could be visualized. In one experiment, they dissected the retina 3.5–4.0 days post infection and imaged the cells with two-photon microscopy. They then further incubated this retina in Ringers solution to enable the infection to progress an additional day. In this way, two time points of infection in the same tissue specimen were analyzed to demonstrate that the amacrine cells were indeed connected to the ipRGCs infected earlier. The labeled ganglion cells were functional, as evidenced by electro-physiological recordings of their response to pulses of light [34].
Tracing of inputs to neurons expressing luteinizing hormone involved in fertility and reproduction
PRV tracing has provided insight into the neuronal inputs to luteinizing hormone-releasing hormone [LHRH; also known as gonadotropin-releasing hormone (GNRH)]-producing neurons. LHRH-neurons, a small and scattered population of cells, are an important part of a key integratory circuit that controls fertility and reproduction in mammals. In addition to its role as a neurohormone, LHRH is also believed to work as a classic neurotransmitter in brain areas known to have a role in sexual behavior. In rodents, chemosensory cues are important for the onset of puberty as well as sexual behavior and, furthermore, modulate LHRH release and neuronal activity. Classic tracing studies have identified the vomeronasal pathway consistently as a main afferent to brain areas containing LHRH-neurons and it was believed consequently that this pathway communicated the chemosensory cues to LHRH-neurons. However, Yoon et al. used Ba2001 to show that LHRH-neurons, specifically, receive input from the main olfactory epithelium (MOE) but not from the vomeronasal pathway. PRV-infected cells were found readily in the MOE, as well as in all downstream structures of the main olfactory pathway, but not in areas corresponding to the vomeronasal pathway. In a crucial control experiment, the authors performed similar injections with a non-conditional PRV-152-tracing strain to show that PRV was indeed able to infect and label both pathways [48].
The same technical approach was used to study the positive feedback of estrogen on the brain that results in luteinizing hormone release. It was not clear if estrogen acts directly on LHRH-neurons or indirectly through other neurons, nor was it clear which subtype of estrogen receptor (ER) was involved (ERα versus ERβ). Detailed investigation of LHRH-neurons is hampered by their low numbers and scattered distribution. Wintermantel et al. used genetic tools to demonstrate that ERα but not ERβ is necessary and sufficient for the positive feedback of estrogenon LHRH-neurons. LHRH-neurons only produce ERβ and thus estrogen needs to modulate their activity through other cells that produce ERα. Importantly, the authors used Ba2001 tracing to identify a group of ERα-producing neurons that send axonal projections to Cre-expressing LHRH-neurons [49].
Finally, Campbell et al. used Ba2001 and LHRH-Cre expressing mice to identify brainstem inputs to LHRH-neurons [50], further showing the utility of PRV-tracing strains in dissecting CNS circuitry.
New applications of PRV tracing
Evaluation of synaptic plasticity in development, neurodegeneration and repair
PRV-tracing technology can quantify the dynamics of neuronal connectivity. The formation of synaptic connections in the developing brain can be observed by measuring the extent of PRV penetration into the cortex at progressive times after birth [51]. Recently, the effects of maternal separation and handling on development of the CNS were evaluated by comparing pups left with their dam [non-handled (NH)/non-separated (NS) group] with pups separated from their dam for either 15 min or 3 h [handled (H)/separated (S) groups] daily from postnatal day 1 (P1) to P8 [52]. P8 pups were injected with PRV-Bartha in the ventral wall of the stomach and spread of infection to the brainstem and cortex was analyzed 63–66 h post infection. The striatum, central amygdala and insular cortex in H/S pups had markedly less infection than did similarly infected NH/NS pups. The 15 min and 3 h H/S groups were in distinguishable. The differences in PRV spread are attributable presumably to differences in synaptic formation that occurred during the 8-day treatment period. The results also could indicate differences in either the number of synaptically connected neurons or the strength or number of synaptic connections between individual neurons.
PRV tracing provides a means of quantifying synaptic plasticity in the rat CNS [53] and peripheral nervous system (PNS) [54] in response to traumatic injury. Card et al. injected PRV into the entorhinal cortex (ERC) 30 days after traumatic injury was delivered to the ERC by controlled cortical impact (CCI). There was surprisingly little difference in the location of PRV tracing after CCI: in both sham and injured animals, PRV traced to the same brain regions. The major difference between injured and sham brains was that the total number of infected neocortical neurons was significantly greater in the injured brains. The authors suggest that time-course studies with PRV could determine whether the differences in neuronal labeling were owing to increased neuronal incorporation into the ERC circuitry or a decrease in neuronal apoptosis following traumatic injury.
Similarly, PRV tracing can be used to determine the innervation of transplanted tissue. As a potential treatment for retinal disease, Seiler et al. transplanted retinal sheets into the eyes of rats with different models of retinal degeneration [9]. To determine whether synaptic connections had formed between host and transplant tissue, PRV-Bartha was injected into the superior colliculus (SC) 2.2–9.9 months after surgery, in a region of the SC believed to receive projections from the site of the transplant. Based on spatial location of tissue and bromodeoxyuridine (BrdU) staining of host neurons, the authors identified PRV-infected neurons in the transplanted retinas of some of the rats, suggesting that PRV spread through new synaptic connections that formed between host and transplant.
Emerging techniques
Anterograde tracing with α-herpesviruses
PRV-Bartha spreads only from post-synaptic to pre-synaptic neurons (retrograde spread), whereas Herpes Simplex virus (HSV)-1 strain H129 appears to spread only from pre-to post-synaptic neurons (anterograde spread) [47,55]. For example, after injection into the ventral stomach wall of rats, PRV-Bartha infects projections originating from neurons in the dorsal-motor nucleus of the vagus (DMV) and then moves in a retrograde manner to the medial nucleus of the solitary tract (mNST) and to the area postrema (AP). Although HSV-H129 injected into the ventral stomach can be detected in these same CNS structures, vagal deafferentation shows that HSV-H129 reaches the mNST and AP by anterograde transport through sensory neurons projecting from the nodose ganglion. At this time, the reason for the anterograde-only spread of H129 is not understood. All attempts to date to engineer an antero-grade-only PRV strain have been unsuccessful.
Monosynaptic tracing
A particularly useful tool in CNS mapping would be technology to identify first-order neurons that synapse directly on a molecularly defined population of neurons. Currently, monosynaptic tracing is possible using engineered rabies virus [56]. Several strategies are possible to produce similar PRV mutants. First, an essential viral gene would be deleted from PRV-Bartha. Second, transgenic mice would be constructed to express this same gene from defined promoters. Virus lacking this essential gene would replicate only in cells that supplied the viral protein. Neurons that projected to this complementing neuronal population would be infected but subsequent replication of the virus would halt because these first-order neurons lack the essential viral protein.
New models for tracing defined circuits
There is substantial interest in mapping connections to genetically defined neurons by combining conditionally replicating PRV with transgenic animals. The combination of Cre-expressing animals and Cre-conditional replicating PRV described above could soon be considered the first generation of molecularly defined tracing. Second generation molecularly defined tracing strategies will employ transgenic mice expressing a transcriptional transactivator (such as the tet-On protein) under the control of a neuron-specific promoter and a tracing virus whose replication and marker expression (such as GFP) are under the control of a tet-responsive promoter. Such a virus could initiate tracing only from a tet-On-expressing neuron. Additionally, only the tet-On-expressing neuron would have a GFP label, indicating the location of the first order neuron. Currently, tetracycline responsive systems are available for HSV and are designed for inducible lytic replication [57,58]. It remains to be seen if such systems will be developed for PRV-tracing strains.
Concluding remarks
Many investigators are now using neuroinvasive viruses as tools for tracing neural circuits. However, much remains to be done to understand the mechanisms used by α-herpes-viruses for axonal, somal and dendritic uptake; targeting to specific neurons; long distance axonal transport; and assembly and release (see Box 1). With advances in imaging, proteomics and RNA interference technology, our understanding of herpesvirus infections of the nervous system and subsequent pathogenesis will improve. These studies will provide crucial information for the construction of new genetically engineered virus strains for neural tracing, imaging and analysis of neuronal physiology. Such improved viruses would be powerful tools to elucidate brain microcircuitry, thus providing a better understanding of nervous system functions.
Box 1. Outstanding questions in PRV circuit tracing.
Does PRV spread directly through or near synapses?
What are the molecular requirements for the spread of PRV between functionally connected neurons?
Are there any neurons that PRV will not infect?
Does neuronal activity influence PRV spread in neuronal circuitry?
What are the mutations in HSV-1 strain H129 that result in anterograde-only spread?
How can PRV-Bartha-infected neurons maintain relatively normal electrophysiology in the face of a cytopathic infection?
Why do PRV-Bartha-infected animals die?
Is it possible to attenuate PRV further and still maintain rapid trans-neuronal spread?
Acknowledgments
In this brief overview of a complex field, it was impossible to cite all the publications on α-herpesvirus neuroinvasion, neuronal infection and circuit tracing. We have resorted to selected reviews and papers to illustrate key findings as much as possible. We apologize to those colleagues whose work we did not cite. L.W.E. thanks his long time collaborator Pat Card for his continuing help, advice and encouragement. M.I.E. and L.E.P. wish to thank Jeffrey M. Friedman for his continued support. M.I.E. and L.E.P. are funded by NIH RO1 DA018799–03, with additional funding for M.I.E. from The Howard Hughes Medical Institute. This work is also supported by NIH R01 33506 and NCRR P40 RR01 18604 to L.W.E. and the Center for Behavioral Neuroscience Viral Tract Tracing Core at Georgia State University through the STC Program of the National Science Foundation under agreement No. IBN-9876754 to L.W.E. and Tim Bartness.
References
- 1.Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex Transm Infect. 2005;81:103–107. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith GA, Enquist LW. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc Natl Acad Sci U S A. 2000;97:4873–4878. doi: 10.1073/pnas.080502497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warming S, et al. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mettenleiter TC. Pathogenesis of neurotropic herpesviruses: role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 2003;92:197–206. doi: 10.1016/s0168-1702(02)00352-0. [DOI] [PubMed] [Google Scholar]
- 5.Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 6.Enquist LW, Card JP. Recent advances in the use of neurotropic viruses for circuit analysis. Curr Opin Neurobiol. 2003;13:603–606. doi: 10.1016/j.conb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Rinaman L, et al. Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci. 1993;13:685–702. doi: 10.1523/JNEUROSCI.13-02-00685.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomishima MJ, Enquist LW. In vivo egress of an alphaherpesvirus from axons. J Virol. 2002;76:8310–8317. doi: 10.1128/JVI.76.16.8310-8317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seiler MJ, et al. Transsynaptic virus tracing from host brain to subretinal transplants. Eur J Neurosci. 2005;21:161–172. doi: 10.1111/j.1460-9568.2004.03851.x. [DOI] [PubMed] [Google Scholar]
- 10.Denes A, et al. Attenuated pseudorabies virus-evoked rapid innate immune response in the rat brain. J Neuroimmunol. 2006;180:88–103. doi: 10.1016/j.jneuroim.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Card JP, et al. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brittle EE, et al. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol. 2004;78:12951–12963. doi: 10.1128/JVI.78.23.12951-12963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Card JP, et al. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeraski CA, et al. Suprachiasmatic nucleus input to autonomic circuits identified by retrograde transsynaptic transport of pseudorabies virus from the eye. J Comp Neurol. 2004;471:298–313. doi: 10.1002/cne.20030. [DOI] [PubMed] [Google Scholar]
- 15.Card JP, et al. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J Virol. 1998;72:4434–4441. doi: 10.1128/jvi.72.5.4434-4441.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lomniczi B, et al. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J Virol. 1987;61:796–801. doi: 10.1128/jvi.61.3.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mettenleiter TC, et al. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985;56:307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrovskis EA, et al. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J Virol. 1986;60:1166–1169. doi: 10.1128/jvi.60.3.1166-1169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen LM, et al. Role of pseudorabies virus Us3 protein kinase during neuronal infection. J Virol. 2006;80:6387–6398. doi: 10.1128/JVI.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song CK, et al. New developments in tracing neural circuits with herpesviruses. Virus Res. 2005;111:235–249. doi: 10.1016/j.virusres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Loewy AD. Viruses as transneuronal tracers for defining neural circuits. Neurosci Biobehav Rev. 1998;22:679–684. doi: 10.1016/s0149-7634(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 22.Enquist LW, et al. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- 23.Jons A, Mettenleiter TC. Green fluorescent protein expressed by recombinant pseudorabies virus as an in vivo marker for viral replication. J Virol Methods. 1997;66:283–292. doi: 10.1016/s0166-0934(97)00065-7. [DOI] [PubMed] [Google Scholar]
- 24.Smith BN, et al. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci U S A. 2000;97:9264–9269. doi: 10.1073/pnas.97.16.9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banfield BW, et al. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J Virol. 2003;77:10106–10112. doi: 10.1128/JVI.77.18.10106-10112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loewy AD, et al. β-Galactosidase expressing recombinant pseudorabies virus for light and electron microscopic study of transneuronally labeled CNS neurons. Brain Res. 1991;555:346–352. doi: 10.1016/0006-8993(91)90364-2. [DOI] [PubMed] [Google Scholar]
- 27.Standish A, et al. Dendritic morphology of cardiac related medullary neurons defined by circuit-specific infection by a recombinant pseudorabies virus expressing beta-galactosidase. J Neurovirol. 1995;1:359–368. doi: 10.3109/13550289509111025. [DOI] [PubMed] [Google Scholar]
- 28.Jansen AS, et al. Central command neurons of the sympathetic nervoussystem: basisofthe fight-or-flightresponse. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 29.Kim JS, et al. Circuit-specific coinfection of neurons in the rat central nervous system with two pseudorabies virus recombinants. J Virol. 1999;73:9521–9531. doi: 10.1128/jvi.73.11.9521-9531.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billig I, et al. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci. 2000;20:7446–7454. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boldogkoi Z, et al. Novel tracing paradigms – genetically engineered herpesviruses as tools for mapping functional circuits within the CNS: present status and future prospects. Prog Neurobiol. 2004;72:417–445. doi: 10.1016/j.pneurobio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Irnaten M, et al. Activity of cardiorespiratory networks revealed by transsynaptic virus expressing GFP. J Neurophysiol. 2001;85:435–438. doi: 10.1152/jn.2001.85.1.435. [DOI] [PubMed] [Google Scholar]
- 33.Damann N, et al. Chemosensory properties of murine nasal and cutaneous trigeminal neurons identified by viral tracing. BMC Neurosci. 2006;7:46. doi: 10.1186/1471-2202-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viney TJ, et al. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 35.Williams KW, et al. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology. 2007;148:1868–1881. doi: 10.1210/en.2006-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boldogkoi Z, et al. Construction of recombinant pseudorabies viruses optimized for labeling and neurochemical characterization of neural circuitry. Brain Res Mol Brain Res. 2002;109:105–118. doi: 10.1016/s0169-328x(02)00546-6. [DOI] [PubMed] [Google Scholar]
- 37.Aston-Jones G, Card JP. Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations. J Neurosci Methods. 2000;103:51–61. doi: 10.1016/s0165-0270(00)00295-8. [DOI] [PubMed] [Google Scholar]
- 38.Card JP, et al. Neuroinvasiveness of pseudorabies virus injected intracerebrally is dependent on viral concentration and terminal field density. J Comp Neurol. 1999;407:438–452. doi: 10.1002/(sici)1096-9861(19990510)407:3<438::aid-cne11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Gordon Y, et al. A low thymidine kinase-producing mutant of herpes simplex virus type 1 causes latent trigeminal ganglia infections in mice. Arch Virol. 1983;76:39–49. doi: 10.1007/BF01315702. [DOI] [PubMed] [Google Scholar]
- 40.DeFalco J, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 41.Voss-Andreae A, et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- 42.Kerman IA, et al. Distinct populations of presympathetic-premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J Comp Neurol. 2007;505:586–601. doi: 10.1002/cne.21511. [DOI] [PubMed] [Google Scholar]
- 43.Toth IE, et al. Predominance of supraspinal innervation of the left ovary. Microsc Res Tech. 2007;70:710–718. doi: 10.1002/jemt.20456. [DOI] [PubMed] [Google Scholar]
- 44.Gerendai I, et al. The supraspinal innervation of the left adrenal is more intense than that of the right one. Ideggyogy Sz. 2007;60:159–161. [PubMed] [Google Scholar]
- 45.Card JP, et al. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 46.Pickard GE, et al. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J Neurosci. 2002;22:2701–2710. doi: 10.1523/JNEUROSCI.22-07-02701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pomeranz LE, et al. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev. 2005;69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoon H, et al. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 49.Wintermantel TM, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell RE, Herbison AE. Definition of brainstem afferents to gonadotropin-releasing hormone (GnRH) neurons in the mouse using conditional viral tract tracing. Endocrinology. 2007;148:5884–5890. doi: 10.1210/en.2007-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinaman L, et al. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. J Neurosci. 2000;20:2731–2741. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Card JP, et al. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Card JP, et al. Plastic reorganization of hippocampal and neocortical circuitry in experimental traumatic brain injury in the immature rat. J Neurotrauma. 2005;22:989–1002. doi: 10.1089/neu.2005.22.989. [DOI] [PubMed] [Google Scholar]
- 54.Horvath S, et al. Use of a recombinant pseudorabies virus to analyze motor cortical reorganization after unilateral facial denervation. Cereb Cortex. 2005;15:378–384. doi: 10.1093/cercor/bhh140. [DOI] [PubMed] [Google Scholar]
- 55.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wickersham IR, et al. Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods. 2007;4:47–49. doi: 10.1038/NMETH999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao F, et al. Highly efficient regulation of gene expression by tetracycline in a replication-defective herpes simplex viral vector. Mol Ther. 2006;13:1133–1141. doi: 10.1016/j.ymthe.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Schmeisser F, et al. Tetracycline-regulated gene expression in replication-incompetent herpes simplex virus vectors. Hum Gene Ther. 2002;13:2113–2124. doi: 10.1089/104303402320987815. [DOI] [PubMed] [Google Scholar]