Abstract
Objective
Oxytocin is a hypothalamic neuropeptide that plays a key role in mammalian female reproductive function. Animal research indicates that central oxytocin facilitates adaptive social attachments and modulates stress and anxiety responses. Major depression is prevalent among postpubertal females, and is associated with perturbations in social attachments, dysregulation of the hypothalamic-pituitary-adrenal stress axis, and elevated levels of anxiety. Thus, depressed women may be at risk to display oxytocin dysregulation. The current study was developed to compare patterns of peripheral oxytocin release exhibited by depressed and nondepressed women.
Methods
Currently depressed (N = 17) and never-depressed (N = 17) women participated in a laboratory protocol designed to stimulate, measure, and compare peripheral oxytocin release in response to two tasks: an affiliation-focused Guided Imagery task and a Speech Stress task. Intermittent blood samples were drawn over the course of two, 1-hour sessions including 20-minute baseline, 10-minute task, and 30-minute recovery periods.
Results
The 10-minute laboratory tasks did not induce identifiable, acute changes in peripheral oxytocin. However, as compared with nondepressed controls, depressed women displayed greater variability in pulsatile oxytocin release over the course of both 1-hour sessions, and greater oxytocin concentrations during the 1-hour affiliation-focused imagery session. Oxytocin concentrations obtained during the imagery session were also associated with greater symptoms of depression, anxiety, and interpersonal dysfunction.
Conclusions
Depressed women are more likely than controls to display a dysregulated pattern of peripheral oxytocin release. Further research is warranted to elucidate the clinical significance of peripheral oxytocin release in both depressed and nondepressed women.
Keywords: major depressive disorder, oxytocin
INTRODUCTION
Oxytocin is a hypothalamic neuropeptide that has been implicated in the regulation of a variety of reproductive, social, and stress-related functions among mammalian females. Oxytocin has long been known to stimulate milk ejection during lactation and uterine contraction at parturition in humans and other mammals (1), and is critically regulated by the female reproductive hormones estrogen and progesterone. A burgeoning animal literature suggests that oxytocin also plays a key role in the regulation of affiliative behaviors, social attachments, and stress responses among mammalian females (2). Centrally released oxytocin has been shown to stimulate social motivation and approach behaviors, and to facilitate the formation of selective mother-infant attachments (3–5) and adult pair-bonds (6,7). Oxytocin is also released centrally and peripherally with stress (8,9). Acute oxytocin administration has been shown to have an anxiolytic effect on endocrine and behavioral systems (10), and the anxiolytic actions of oxytocin are most pronounced within estrogen dominated environments (11). Notably, the absence of oxytocin within genetically-engineered female oxytocin knock-out mice has been shown to result in deficits in maternal caregiving behavior (12), as well as dysregulated stress responses to psychological stimuli (13) and enhanced anxiety-like behaviors (14).
We postulate that depressed women may be at risk to display oxytocin dysregulation. Women are twice as likely as men to experience a lifetime episode of major depression (15,16). The female predominance in depression first emerges at puberty (17,18), persists throughout women’s reproductive lives (19), and is associated with elevated levels of preexisting anxiety (20). Depression is, moreover, associated with perturbations in social attachments (21), elevated levels of social distress (22), and dysregulation of the hypothalamic-pituitary-adrenal stress axis (23). Indeed, we have theorized that postpubertal females’ sensitivity to interpersonal life stress may be mediated, in part, by the neurohormone oxytocin (24).
Few well-controlled studies have investigated directly central or peripheral markers of oxytocin release or regulation among depressed humans. In one postmortem human brain tissue analysis, Purba et al. reported evidence of an increased number of oxytocin expressing neurons in the paraventricular nucleus (PVN) of depressed patients, as compared with nondepressed controls (25). Although many have theorized regarding the potential role of perturbations in oxytocin within mood disorder patients (e.g., see Ref. 26), human oxytocin findings in both the cerebrospinal fluid and plasma of depressed patients have been mixed (27–29). Inconsistencies in published reports likely reflect the heterogeneity of patient populations studied, which has included both males and females of widely different ages, inpatient and outpatient populations, and both medicated and nonmedicated patients. In one of the largest and best-controlled studies to date, van Londen et al. (29) examined levels of plasma oxytocin in 56 nonmedicated depressed patients and 48 nondepressed controls with single blood draws obtained at 8 AM, 4 PM, and 11 PM. These researchers found that as compared with nondepressed controls, depressed patients displayed greater variability in mean daily oxytocin levels, and a trend toward higher oxytocin levels, particularly in plasma samples obtained at the 8 AM and 4 PM time points.
Interpretation of available data in depressed humans is hampered, however, by several methodological issues. As noted above, few previous studies have controlled for such factors as gender, age, reproductive status, menstrual cycle phase, or antidepressant medication use. Moreover, oxytocin is known to be released into peripheral circulation in a pulsatile fashion, where it is assumed to have a relatively brief half-life (30). Thus, the use of venipuncture for single blood draw assessments almost certainly provides an incomplete picture of peripheral variability or concentrations of this hormone.
The present study was designed to compare patterns of peripheral oxytocin release and concentrations observed in depressed and nondepressed women. Normal menstrual cycling females between the ages of 20 and 40 were recruited to participate in a laboratory protocol designed to stimulate, measure and compare peripheral oxytocin release. Depressed women were antidepressant-free at time of assessment, and a repeated blood draw paradigm was used to optimize accuracy of hormone assessment. The primary study aim was to compare the variability and concentrations of peripheral oxytocin observed between depressed and nondepressed females. Secondary study aims were two-fold: 1) to evaluate whether laboratory tasks would stimulate acute oxytocin release and 2) to explore associations between peripheral oxytocin concentrations and measures of depression, anxiety, and interpersonal function.
METHODS AND MATERIALS
Participants
Women aged 20 to 40 were recruited to participate. Depressed women were recruited from on-going treatment protocols being conducted within the Depression and Manic-Depression Prevention Clinic. All depressed subjects were free of antidepressant medication treatment for a minimum of 2 weeks at time of laboratory assessment. Never-depressed women were recruited via local advertisements. Interested women were screened over the phone for basic study eligibility. Subjects were recruited for participation from January 2003 through October 2005. Informed consent was obtained from all study participants before in-clinic screening procedures and study participation. All study procedures were reviewed and approved by the University of Pittsburgh institutional review board.
Procedures
Screening Procedures and Eligibility
Eligible depressed subjects met full criteria for current major depressive disorder (MDD) of sufficient severity, documented by a Structured Clinical Interview for Axis I DSM-IV Disorders (31) diagnosis of current MDD and a 17-item Hamilton Rating Scale for Depression (HRSD-17) (32) score ≥14 at the time of initial evaluation. Women with a history of manic episodes, organic affective syndrome, or psychosis were excluded from participation. Nondepressed participants did not meet current or lifetime criteria for any mood disorder.
All subjects provided blood samples for complete blood count and serum pregnancy screening within 1 month before study participation. Subjects who were anemic or pregnant were excluded, as were those who reported drug or alcohol abuse or dependence in the past 3 months or any chronic, unstable medical illness.
Women who had had a hysterectomy/oophorectomy, took estrogen replacement therapy, were lactating or less than 6 months postpartum, or who reported gynecological problems or irregular menstrual cycles were also excluded from participation.
Laboratory Testing Procedures
Participants underwent laboratory testing procedures in the Clinical Neuroscience Research Center at the Western Psychiatric Institute and Clinic. Testing sessions were scheduled during the follicular phase of the menstrual cycle, based on self-reported bleeding patterns and cycle length. All laboratory sessions began at approximately 2:00 PM. Participants were asked to abstain from alcohol for 48 hours before participation, and to abstain from eating, drinking caffeinated beverages, and smoking after 12:30 PM on the day of testing.
Participants were situated in a comfortable chair, and an intravenous catheter was inserted into a vein in the antecubital fossa of the nondominant arm for collection of venous blood samples. The catheter was connected to sterile extension tubing, which was flushed with saline between blood draws. After catheterization, participants engaged in a 25-minute habituation period, during which time they completed study questionnaires. Next, intermittent blood samples were drawn before, during and after two laboratory tasks: a guided imagery task developed to elicit feelings of love or infatuation, and a speech stress task. The Guided Imagery Task represented a modified version of Ekman’s Relived Emotions Task (33) in which subjects were asked to imagine and relive in their memory a past experience in which they felt strong feelings of love or infatuation. This attachment-relevant mood induction procedure has been used previously to induce oxytocin release in healthy, nondepressed women (34). For the speech stress task, participants were given 2 minutes to prepare a speech defending themselves against a hypothetical shoplifting charge, followed by 4 minutes of videotaped speech delivery. This task has been widely used to induce acute cardiovascular and immune reactivity to stress in previous research1.
Each laboratory session took 1 hour to administer and included three assessment periods: a 20-minute resting baseline, a 10-minute task (which included the Guided Imagery or Stress task procedure), and a 30-minute recovery. Participants sat quietly without distraction during resting baseline and recovery periods, and were given a 20-minute break between the 2 hour-long laboratory sessions. The order in which the sessions were completed was counterbalanced across participants. Blood was sampled at approximately 5-minute intervals throughout the 2 hour-long sessions to allow for accurate calculation of hormone concentrations in the presence of changing oxytocin levels.
Biological Assessment
Blood samples were collected in chilled heparin-treated tubes and centrifuged at 3000g at 4°C. Plasma was then aspirated and stored at −80°C until assayed. Radioimmunoassay of oxytocin in plasma was performed on acetone-ether extracted material using methods previously published from this Pittsburgh laboratory (35). The minimum detectable concentration of oxytocin for this assay is 0.5 pg/ml, and the interassay and intra-assay coefficients of variation are each <10%. In the current study, 11.4% of samples fell at or below the minimum detection level, and were thus set at 0.5 pg/ml. Given the decreased reliability of the oxytocin radioimmunoassay at extreme levels, three outlying samples assessed as greater than 10 pg/ml were truncated at 10 pg/ml.
Psychosocial Assessment
Depression and Anxiety
Levels of depression and anxiety experienced over the past week were assessed using the 17-item observer-rated Hamilton Rating Scale for Depression, and the self-rated 21-item Beck Depression (BDI) (36) and 21-item Beck Anxiety (BAI) (37) Inventories. HRSD-17 total scores range from 0 to 52 and indicate no depression (score = 0–7), mild depression (score = 8–13), moderate depression (score = 14–19), severe depression (score = 20–25), and very severe depression (score = 26+). The BDI and BAI represent widely used self-report scales that assess the affective, cognitive and vegetative symptoms of depression (BDI) and the cognitive and somatic symptoms of anxiety (BAI) experienced by subjects over the past week. BDI and BAI scores range from 0 to 63, with higher scores indicating greater levels of depression or anxiety severity, respectively.
Interpersonal Function
To assess interpersonal function, subjects completed a shortened version of the Inventory for Interpersonal Problems (IIP) (38,39). The IIP is a well-validated instrument that assesses distress experienced from a variety of interpersonal difficulties. For the purpose of the current study, we evaluated the IIP circumplex scales (40) and the original Horowitz et al. Hard to be Sociable scale (38,39).
Analysis Plan
Total concentrations of oxytocin released during each of the 2 hour-long laboratory sessions (which included baseline, task, and recovery periods) were analyzed by examining the integrated area under the curve (AUC) for each session using a trapezoidal approximation. AUC values were log transformed before analyses in order to satisfy assumptions regarding normality and homogeneity of variance. A 2 (Group) ×2 (Task Order) analysis of covariance (ANCOVA) was then conducted for each session, controlling for subject age.
In order to examine potential group differences in the within-subject variability of oxytocin release during each laboratory session, standard deviations (SD) were calculated based on oxytocin levels obtained for each subject within each hour-long session. Initial evaluation of the data indicated that a bimodal distribution of SDs was obtained for each laboratory session, with three-quarters of the subjects displaying a within-session SD < 1.5, whereas another group displayed elevated levels of oxytocin variability (i.e., SDs ≥ 1.5). Given this distribution, subjects falling in the upper quartile in terms of within-session oxytocin variability (SD ≥ 1.5) were compared with subjects displaying low oxytocin variability (SD < 1.5). Logistic regression models were run to compare the proportion of depressed and nondepressed subjects categorized as high versus low on oxytocin variability for each laboratory session, controlling for subject age.
To examine acute changes in oxytocin release associated with the specific, 10-minute laboratory tasks, a 2 (Group) ×2 (Task Order) ×3 (Period) repeated measures ANCOVA with Period (baseline versus task versus recovery) as the within-subjects factor was used to examine changes in oxytocin concentrations across each 1-hour session. For these models, AUCs for each period (i.e., baseline, task, and recovery) were calculated, standardized to a 3-minute time frame, and log transformed before analysis.
Finally, tests were conducted to explore associations between total oxytocin concentrations during each 1-hour laboratory session and measures of depression, anxiety, and interpersonal function. For these analyses, partial correlations controlling for Task Order were used to examine the association of relevant psychosocial variables with log-transformed, integrated AUC values for oxytocin concentrations obtained during each 1-hour laboratory session. Given the number of associations examined and the exploratory nature of these analyses, a p < .01 value was used to identify significant associations.
RESULTS
Participants
Of the 39 women who were eligible and consented to participate in the laboratory paradigm, data from five subjects (three controls and two depressed) were lost because of problems drawing sufficient blood for the assessment of oxytocin release. This left a sample of 34 participants (17 depressed and 17 controls) for the current analyses. Mean age of participants was 29 years (SD = 6.19). Participants were mostly white (82.4%), unmarried (73.5%) but in a current romantic relationship (79.4%), nulliparous (85.2%), well-educated (73.5% with a college degree or above), and reported a median household income of 30,000 to 39,000 US$. Of the sample, 44.1% reported current oral contraceptive use.
Demographic and clinical characteristics among the depressed and never-depressed groups are provided in Table 1. Although the study groups did not differ significantly on any of the demographic characteristics, the depressed participants were, on average, 4 years older than the controls—a finding that approached significance [F(1,32) = 3.84, p = .059]. Thus, subsequent tests of group effects controlled for participant age. As would be expected, the depressed group displayed moderate to high levels of depressive (HRSD-17 and BDI) and anxiety (BAI) symptoms that were significantly greater than those reported by the nondepressed control group [for HRSD-17, F(1,32) = 79.36; for BDI, F(1,31) = 86.05; for BAI, F(1,32)=28.79; p values < .001].
TABLE 1.
Demographic and Clinical Characteristics of Subject Groups
| Never-Depressed Controls (N = 17) |
Depressed Participants (N = 17) |
|||
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (mean, SD) | 27.0 | (4.95) | 31.0 | (6.80) |
| Racial identity | ||||
| White (N, %) | 14 | (82.4) | 14 | (82.4) |
| Black (N, %) | 3 | (17.6) | 3 | (17.6) |
| Marital status (%) | ||||
| Never married | 12 | (70.5) | 10 | (58.8) |
| Married | 4 | (23.5) | 5 | (29.4) |
| Divorced or separated | 1 | (5.8) | 2 | (11.7) |
| Current romantic partner (N, %) | 13 | (76.4) | 14 | (82.3) |
| Education (%) | ||||
| HS/some college | 3 | (17.6) | 6 | (35.3) |
| College degree | 9 | (52.9) | 7 | (47.1) |
| Advanced degree | 5 | (29.4) | 4 | (23.5) |
| Childbearing status (N, % nulliparas) | 16 | (94.1) | 13 | (76.5) |
| Oral contraceptive use (N) | 9 | (52.9) | 6 | (35.3) |
| Clinical characteristics | ||||
| HRSD-17 (mean, SD) | 3.88 (2.76) | 17.0 (5.41) | ||
| BDI (mean, SD) | 2.29 (2.71) | 21.19 (7.93) | ||
| BAI (mean, SD) | 2.82 (4.19) | 17.47 (10.45) | ||
Depressed Versus Control Group Differences in Oxytocin
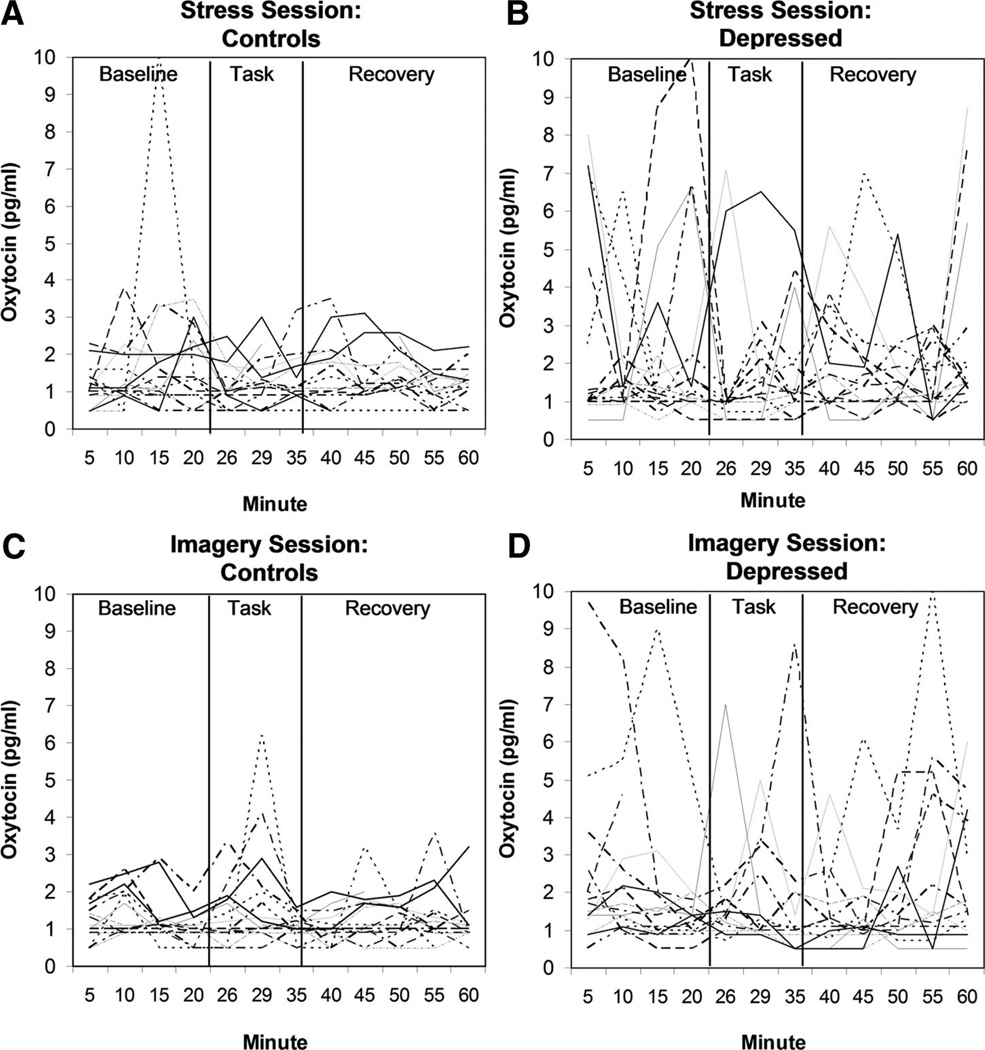
The raw data representing oxytocin levels obtained from individual subjects over the course of the 1-hour imagery session and the 1-hour stress session are displayed in Figure 1, separated by depression group. Separate ANCOVAs were performed to examine log-transformed oxytocin AUC observed during the imagery and stress sessions, controlling for subject age. Results of the 2 (Group) ×2 (Task Order) ANCOVA indicated a significant Group effect for oxytocin concentrations obtained during the 1-hour imagery session [F(1,22) = 7.9, p = .01]. Specifically, depressed women showed greater oxytocin concentrations over the course of the 1-hour imagery session, as compared with never-depressed controls, with mean log-transformed AUC = 4.46 (SD = 0.42) and 4.19 (SD = 0.28), respectively. We note that results were similar using nontransformed AUC data [mean AUC = 93.85 pg/ml (SD = 50.07) and 73.69 pg/ml (SD = 24.22), respectively]. In contrast, neither the main effect for Task Order [F(1,22) = 2.1, p = 0.16] nor the Group by Task Order interaction effect [F(1,22) = 2.26, p = .15] were significant. Results of the 2 (Group) ×2 (Task Order) ANCOVA for oxytocin concentration during the stress session did not show a significant Group effect [F(1,23) = 2.66, p = .12], although there were similar trends toward higher oxytocin concentrations among the depressed group relative to controls, with mean log-transformed AUC = 4.58 (SD = 0.49) versus 4.33 (SD = 0.33). Again, results were similar using nontransformed AUC data [mean AUC = 108.70 pg/ml (SD = 51.78) versus 79.52 pg/ml (SD = 24.68)]. Neither the main effect of Task Order [F(1,23) = 0.00, p = .96] nor the Group by Task Order interaction effect [F(1,23) = 0.01, p = .92] approached significance in this model.
Figure 1.
Individual oxytocin data obtained over the course of the 1-hour imagery and stress sessions, separated by group.
Visual inspection of Figure 1 suggests that while many of the depressed subjects displayed variable and extreme oxytocin pulses, others exhibited relatively little oxytocin variability. In order to examine differential patterns of oxytocin variability across individuals, SDs were calculated for each subject’s oxytocin samples obtained within each 1-hour laboratory session. Logistic regression analyses controlling for subject age indicated that depressed patients were generally more likely to be categorized as high (SD ≥ 1.5) versus low (SD < 1.5) in oxytocin variability for both laboratory sessions. For the Guided Imagery session, 1 of 16 control subjects (6.3%) displayed high oxytocin variability, as compared with 6 of 16 depressed subjects (37.5%) (Wald statistic = 3.51, df = 1, p = .06). For the Stress session, 1 of 17 control subjects (5.9%) displayed high oxytocin variability, as compared with 7 of 17 depressed subjects (41.2%) (Wald statistic = 4.46, df = 1, p = .03).
Results of the repeated measures ANCOVA examining changes in oxytocin concentrations that occurred across the specific baseline, task, and recovery periods of the imagery session indicated that there was no effect of study Period. Mean log-transformed AUCs for each Period (standardized to a 3-minute time frame) were as follows: baseline = 1.44 (SD = 0.42), task = 1.45 (SD = 0.51), and recovery = 1.35 (SD = 0.42), and similar results were obtained in models using nontransformed AUC data [baseline = 4.67 pg/ml (SD = 2.69); task = 4.87 pg/ml (SD = 2.70); recovery = 4.25 pg/ml (SD = 2.0)]. In line with the previous finding, a significant main effect for Group [F(1,22) = 7.21, p = .01] was obtained, with the depressed group displaying higher oxytocin concentrations across study periods. No other within-or between-subject effects or interactions approached significance in this model.
Notably, results of the repeated measures ANCOVA for the stress session did indicate a Period by Task Order interaction [Huynh-Feldt corrected F(1.84) = 3.35, p = .048]. Specifically, subjects who engaged in the stress session first displayed a decline in oxytocin concentrations from baseline to task levels; whereas this decline in oxytocin concentration was not evident in subjects who engaged in the stress session only after the imagery session2. The main effect for Group did not reach statistical significance in this model [F(1,23) = 2.04, p = .17], and no other within- or between-subject effects approached significance.
Exploring Psychological, Interpersonal, and Reproductive Associations With Oxytocin
Depressive and Anxiety Symptoms
Greater oxytocin concentrations obtained during the 1-hour imagery session were associated with both clinician-observed (HRSD-17) and self-reported (BDI) depressive symptoms, as well as self-reported anxiety symptoms (BAI). See Table 2. In contrast, oxytocin concentrations obtained during the 1-hour stress session were not associated with depressive or anxiety symptoms (p values > .22).
TABLE 2.
Partial Correlations Between Oxytocin Concentrations Obtained During the 1-Hour Imagery Session and Relevant Psychiatric and Interpersonal Variables
| Variable | Partial Correlation Controlling for Task Order |
Partial Correlation Controlling for Task Order, Age, and Oral Contraceptive Use |
|---|---|---|
| Depression and anxiety | ||
| HRSD-17 | ||
| r | 0.528 | 0.646 |
| p | .006 | .001 |
| df | 24 | 22 |
| BDI | ||
| r | 0.540 | 0.638 |
| p | .005 | .001 |
| df | 23 | 21 |
| BAI | ||
| r | 0.528 | 0.611 |
| p | .006 | .002 |
| df | 24 | 22 |
| Interpersonal function | ||
| IIP domineering | ||
| r | 0.525 | 0.567 |
| p | .006 | .004 |
| df | 24 | 22 |
| IIP socially avoidant | ||
| r | 0.500 | 0.576 |
| p | .009 | .003 |
| df | 24 | 22 |
| IIP hard to be social | ||
| r | 0.533 | 0.628 |
| p | .006 | .001 |
| df | 23 | 21 |
| IIP nonassertive | ||
| r | 0.438 | 0.628 |
| p | .028 | .001 |
| df | 23 | 21 |
Interpersonal Function
Correlational analyses indicated a pattern of associations between oxytocin concentrations obtained during the 1-hour imagery session and interpersonal problem areas tapped by the IIP. Specifically, greater oxytocin concentrations obtained during the imagery session were associated with greater difficulties being sociable, and with seeing oneself as being domineering, socially avoidant, and nonassertive. See Table 2. Oxytocin concentrations observed during the stress session were not associated with any of the interpersonal variables.
Oral Contraceptive Use
Women taking oral contraceptives displayed lower oxytocin concentrations over the course of the 1-hour imagery session as compared with women not taking oral contraceptives [F(1,25) = 4.92, p = .04], with mean log-transformed AUC = 4.16 (SD = 0.27) versus 4.45 (SD = 0.40). We note that results using nontransformed AUC data were similar [mean AUC = 66.13 pg/ml (SD = 19.12) versus 93.17 pg/ml (SD = 42.77)]. Women taking contraceptives did not, however, differ from nonusers in oxytocin concentrations observed over the course of the 1-hour stress session [F(1,26) = 0.38, p = .54], with mean log-transformed AUC = 4.40 (SD = 0.44) versus 4.51 (SD = 0.43). Again, results using nontransformed AUC data were similar [89.58 pg/ml (SD = 41.41) versus 99.33 pg/ml (SD = 45.66), respectively].
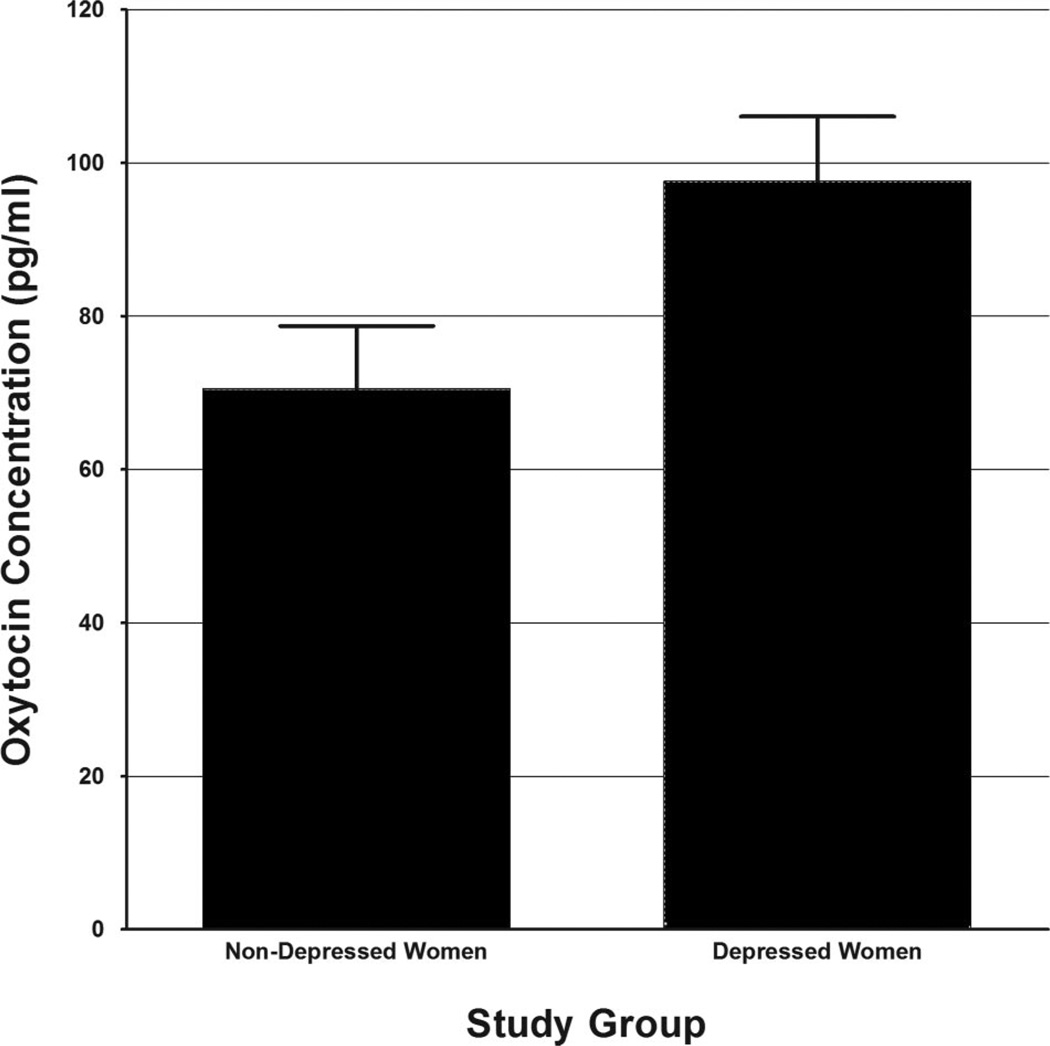
Given the obtained effect of contraceptive use on oxytocin concentrations obtained during the 1-hour imagery session, we re-examined results of the 2 (Group) ×2 (Task Order) ANCOVA controlling for both subject age and oral contraceptive use. Results of this analysis indicated independent and significant effects of Group status, age, and oral contraceptive use on oxytocin concentrations obtained during the 1-hour imagery session, with younger subjects, subjects not taking oral contraceptives, and depressed subjects displaying higher oxytocin concentrations (Table 3). Adjusted, nontransformed mean oxytocin concentrations observed across depressed and nondepressed women are depicted graphically in Figure 2. Similarly, associations between oxytocin concentrations obtained during the imagery session and measures of depression, anxiety, and interpersonal function remained significant following control for age, task order, and oral contraceptive use (see Table 2, column 2).
TABLE 3.
Results of 2 (Group) ×2 (Task Order) ANCOVA Examining Oxytocin Concentrations During the 1-Hour Imagery Session, Controlling for Age and Oral Contraceptive Use
| Variable | F Statistic | df | p |
|---|---|---|---|
| Age | 5.60 | (1,21) | .028 |
| Contraceptive use | 5.99 | (1,21) | .023 |
| Group | 4.43 | (1,21) | .047 |
| Task order | 2.32 | (1,21) | .143 |
| Group X Task order | 2.22 | (1,21) | .151 |
Figure 2.
Oxytocin concentrations observed in depressed and nondepressed women over the course of the one-hour imagery session. * Note: Figures represent estimated marginal means of nontransformed AUC values, collapsed across task order and adjusted for age and oral contraceptive use.
DISCUSSION
The current study evaluated patterns of peripheral oxytocin release in depressed and nondepressed women using a rigorous, repeated blood sampling paradigm in which peripheral oxytocin levels were evaluated at approximately 5 minute intervals over the course of two, 1-hour laboratory sessions. AUC analyses indicated that, on the whole, the depressed group displayed significantly greater concentrations of peripheral oxytocin during the hour-long imagery session (which included a 20-minute baseline, 10-minute task, and 30-minute recovery period), and a trend toward greater oxytocin concentrations during the hour-long stress session.
Visual inspection of Figure 1, however, indicates that this finding is not the result of chronically elevated oxytocin levels, but instead is the result of a potentially dysregulated pattern of peripheral oxytocin release among depressed women relative to controls. Specifically, about 40% of the depressed women displayed a pattern of elevated oxytocin variability, characterized by multiple pulses of peripheral oxytocin release. In contrast, only 6% of the never-depressed controls displayed a similar pattern of oxytocin variability. Notably, the current findings extend those reported earlier by van Londen et al. (29), in which depressed patients similarly displayed greater levels of peripheral oxytocin variability. These results, moreover, support the pulsatile nature of endogenous oxytocin release into peripheral circulation, and highlight the brief half-life that this hormone displays in peripheral blood.
Recently, Taylor et al. observed that postmenopausal women reporting gaps or difficulties in their social relationships display elevated levels of plasma oxytocin, as compared with their less socially distressed counterparts (41). Indeed, Taylor et al. have argued that elevations in peripheral oxytocin may represent a biomarker of social separation or distress, and that release of oxytocin may serve as an impetus for female affiliative behaviors under conditions of stress—a process they have termed the “tend and befriend” response (41–43). In line with this hypothesis, Grippo et al. showed that female prairie voles exposed to extended periods of social isolation displayed elevated levels of plasma oxytocin and an increased number of oxytocin-immunoreactive cells in the hypothalamic PVN (44). Moreover, these same prairie voles displayed a pattern of depression-like behavioral responses, characterized by an anhedonic reduction in sucrose intake and sucrose preference.
Depression has long been associated with elevated levels of interpersonal dysfunction and distress. Thus, it is particularly noteworthy that oxytocin concentrations were positively associated with an array of interpersonal difficulties as assessed with the Inventory of Interpersonal Problems (IIP). Specifically, greater concentrations of peripheral oxytocin observed during the relationship-focused imagery session were associated with self-reports of greater difficulties being sociable, greater social avoidance, greater difficulties being assertive, and the tendency to view oneself as being domineering within relationships. On the surface, viewing oneself as being domineering as well as nonassertive and socially avoidant may seem contradictory. However, items included within the IIP Domineering subscale—such as trying to control, to change, or to manipulate other people to get what one needs—may represent dysfunctional strategies aimed at achieving closeness in relationships. Taken as a whole, this constellation of interpersonal difficulties may reflect a type of insecure or “fearful-avoidant” style of adult attachment, in which individuals desire close relationships yet fear interpersonal rejection or abandonment. Indeed, we have previously shown that recurrently depressed women display elevated endorsements of this fearful-avoidant attachment style. In one study, for example, 43% of 162 recurrently-depressed women described this relationship pattern as most typical of themselves (45).
Numerous lines of evidence support a link between oxytocin dysregulation and MDD in women. Women are twice as likely as men to experience a lifetime episode of depression. This gender gap first emerges at puberty, and data suggest that puberty may sensitize females to the depressogenic effects of interpersonal life stress (46). Likewise, animal models suggest that oxytocin plays a key role in female reproductive functions, affiliative behaviors, and the modulation of stress.
We have theorized that after pubertal maturation, at-risk females may face a depressogenic diatheses that includes a social and biological intensification in affiliative need that—when experienced in conjunction with an insecure attachment style, anxious temperament, and ruminative (rather than instrumental) coping style—increases the likelihood that negative life events will trigger the onset of depressive episodes (see 24). For females, pubertal increases in oxytocin, estrogen, and progesterone may serve to prime the “tend-and-befriend” stress response described by Taylor et al. (42,43). When properly regulated within a securely-attached female, oxytocin release in response to stress may serve to modulate physiologic stress responses by promoting positive affiliation with supportive attachment figures and adaptive interpersonal problem-solving. In contrast, oxytocin-driven increases in affiliative need may have very different consequences among depressed females, who tend to exhibit insecure attachment styles, a propensity toward maladaptive levels of anxiety and rumination, and distressed social relationships. In this scenario, oxytocin-related elevations in affiliative need may interact with insecure attachment styles, maladaptive interpersonal coping responses, and unsupportive relationships to generate even greater levels of social distress. This may, in turn, lead to even greater oxytocin release and elevated or protracted stress responses (see 42). In this way, oxytocin dysregulation may represent a biological factor that contributes to the recurring cycle of interpersonal stress and depression articulated as part of Hammen’s interpersonal stress generation model of depression in women (22).
Neither the 10-minute Guided Imagery Task nor the 10-minute Stress Task produced obvious, task-specific increases in peripheral oxytocin concentration from baseline to task period assessment. Although an early report by Turner et al. (34) showed preliminary evidence of an acute peripheral oxytocin response in women undergoing massage or positive versus negative emotion induction procedures, results of a subsequent report by the same research team using a more intensive blood draw paradigm were inconsistent with initial results (47). Similarly, the report by Taylor et al. (41) showed no change in peripheral oxytocin levels obtained from blood draws occurring before versus after a sample of postmenopausal women participated in the Trier Social Stress Task. Thus, the ability to induce peripheral oxytocin release in humans using acute, psychosocial laboratory induction paradigms remains in question.
It is noteworthy, however, that we found initial evidence of a Period by Task Order interaction for changes in oxytocin concentrations assessed over the course of the stress session. This finding, while preliminary, raises the possibility that the interpersonal context in which mild stress induction procedures occur has an impact on the pattern of oxytocin release. Clearly, future studies will be needed to examine potential contextual interactions within larger study samples.
The current study also found initial evidence of associations between oxytocin and such variables as subject age and oral contraceptive use. The fact that younger women appeared to display higher oxytocin concentrations is in line with previous research. The association between oral contraceptive use and lower oxytocin concentrations during the imagery session is, however, in need of further investigation. Previously, Taylor et al. (41) found that hormone replacement therapy was associated with higher oxytocin levels in a sample of postmenopausal women. However, this finding was specific to women taking unopposed estrogen. We note that all of the oral contraceptives used by women in the current study included estrogen/progesterone combinations. In addition, a recent report by Salonia et al. (48) suggested relatively similar follicular-phase levels of peripheral oxytocin obtained among young oral contraceptive users versus nonusers. It is important to note, however, that none of the above studies included hormone use as a randomly assigned variable, leaving open the possibility that hormone users differ from nonusers in other significant ways. Finally, we would note that the primary finding of greater oxytocin concentrations in the depressed women during the hour-long imagery session remained significant after statistically controlling for both age and oral contraceptive use (see Figure 2).
It is important to consider limitations to the current study. First, the current study included assessment of peripheral, rather than central, levels of oxytocin. Oxytocin is synthesized primarily in the paraventricular and supraoptic nuclei (PVN and SON) of the hypothalamus. Oxytocin that is synthesized in magnocellular neurons of these nuclei is routed to the posterior lobe of the pituitary gland for storage before its release into peripheral circulation. In contrast, oxytocin that is released within the brain derives from parvocellular neurons of the PVN and other brain regions. Because oxytocin released centrally and peripherally are derived from separate neuronal populations, peripheral oxytocin may or may not reflect centrally released oxytocin.
It is also important to consider that the current study was limited to women between the ages of 20 and 40. Finally, the small sample size may have limited the power of the current study to test potential interaction effects. Clearly, future research with larger study groups will be needed to replicate and extend the current study findings.
The current study represents one of the most rigorous examinations of peripheral oxytocin release in depressed and nondepressed women to date. The repeated blood draw paradigm allowed for the characterization of differential patterns of peripheral oxytocin release between depressed and nondepressed women. Current findings suggest that depressed women are more likely to display a highly variable pattern of peripheral oxytocin release. Both the pathophysiology and clinical implications of this pattern remain to be fully elucidated.
Acknowledgments
The authors would like to thank the nursing staff of the WPIC Clinical Neuroscience Research Center, the clinical staff of the WPIC Depression and Manic-Depression Prevention Program, Melissa Behanna at the Department of Pharmaceutical Science at the University of Pittsburgh, and all of the women who participated in the current research. Thanks also go to Dr. Anna Marsland for comments on earlier drafts of this manuscript.
Supported by National Institute of Health (NIH) Grants MH64144 (to J.M.C.), the WPIC Mental Health Intervention Research Center (MH30915), the WPIC Clinical Neuroscience Research Center (RR0000056), and the Pittsburgh Mind Body Center (NHLBI 076852/076858); NIH (to H.S., J.J.A.); NIH and Forest Research Institute and royalties from Guilford Press, and having served on an Advisory Board for Servier (to E.F.).
Glossary
- HPA
hypothalamic-pituitary-adrenal
- MDD
major depressive disorder
- SCID-IV
Structured Clinical Interview for Axis I DSM-IV disorders
- HRSD-17
17-item Hamilton Rating Scale for Depression
- BDI
Beck Depression Inventory
- BAI
Beck Anxiety Inventory
- IIP
Inventory of Interpersonal Problems
- AUC
area under the curve
- pg/ml
picograms per milliliter
- CSF
cerebrospinal fluid
- PVN
paraventricular nucleus
- SON
supraoptic nucleus
Footnotes
Ms. Hofkens and Dr. Cai report no biomedical financial interests or potential conflicts of interest.
Secondary data from the current study support the effectiveness of this laboratory stressor. Specifically, study participants displayed significant increases in heart rate (mean increase = 12.47 bpm), systolic and diastolic blood pressure (mean increases of 18.52 and 13.64 mm Hg, respectively), and POMS tension scores (mean increase of 7.16 points on a 0–24 scale) from baseline to stress task assessment.
Results using nontransformed AUC data were similar. Mean, nontransformed AUC values (standardized to a 3-minute period) for study Periods during each Task Order condition were as follows: When the stress session was completed first, baseline = 6.84 pg/ml (SD = 4.36); task = 3.82 pg/ml (SD = 3.05); recovery = 4.79 pg/ml (SD = 2.47). When the stress session followed the imagery session, baseline = 4.88 pg/ml (SD = 2.27); task = 4.91 pg/ml (SD = 4.12); recovery = 5.18 pg/ml (SD = 1.92).
REFERENCES
- 1.Burbach JP, Young LJ, Russell J. Oxytocin: synthesis, secretion and reproductive functions. In: Neill JD, editor. Knobil and Neill’s physiology of reproduction. New York: Elsevier; 2006. pp. 3055–3128. [Google Scholar]
- 2.Lim M, Young L. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Keverne E, Kendrick K. Oxytocin facilitation of maternal behavior. Ann N Y Acad Sci. 1992;652:83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen C. Oxytocin control of maternal behavior: regulation by sex steroids and offspring stimuli. Ann N Y Acad Sci. 1997;807:126–145. doi: 10.1111/j.1749-6632.1997.tb51916.x. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen C, Ascher J, Monroe Y, Prange A. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–649. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 6.Carter C, DeVries A, Taymans S, Roberts R, Williams J, Getz L. Peptides, steroids, and pair bonding. Ann N Y Acad Sci. 1997;807:260–271. doi: 10.1111/j.1749-6632.1997.tb51925.x. [DOI] [PubMed] [Google Scholar]
- 7.Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- 8.Nishioka T, Anselmo-Franci J, Li P, Callahan M, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:57–61. doi: 10.1016/s0006-8993(97)01159-1. [DOI] [PubMed] [Google Scholar]
- 9.Wotjak C, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 10.Windle R, Shanks N, Lightman S, Ingram C. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy M, McDonald C, Brooks P, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen C, Vadlamudi S, Boccia M, Amico J. Maternal behavior deficits in nulliparous oxyotcin knockout mice. Genes Brain Behav. 2006;5:274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 13.Mantella R, Rinaman L, Vollmer R, Li X, Amico J. Enhanced plasma corticosterone concentrations and diminished Fos expression in the medial amygdala of female oxytocin deficient mice exposed to psychogenic stress. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1494–R1504. doi: 10.1152/ajpregu.00387.2004. [DOI] [PubMed] [Google Scholar]
- 14.Mantella R, Vollmer R, Li X, Amico J. Female oxytocin deficient mice display enhanced anxiety like behavior. Endocrinology. 2003;144:2291–2296. doi: 10.1210/en.2002-0197. [DOI] [PubMed] [Google Scholar]
- 15.Kessler R, McGonagle K, Swartz M, Blazer D, Nelson C. Sex and depression in the National Comorbidity Survey I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 16.Wolk SI, Weissman MM. Women and depression: an update. In: Oldham J, Riba M, editors. American psychiatric press review of psychiatry. Vol 14. Washington, DC: American Psychiatric Press; 1995. pp. 227–259. [Google Scholar]
- 17.Angold A, Costello E, Worthman C. Puberty and depression: the roles of age pubertal status and pubertal timing. Psychol Med. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- 18.Cohen P, Cohen J, Kasen S, Velez C, Hartmark C, Johnson J, Rojas M, Brook J, Streuning E. An epidemiological study of disorders in late childhood and adolescence: I age- and gender-specific prevalence. J Child Psychol Psychiatry. 1993;34:851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- 19.Kessler R, McGonagle K, Nelson C, Hughes M, Swartz M, Blazer D. Sex and depression in the National Comorbidity Survey II: cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 20.Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatric Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 21.Mickelson K, Kessler R, Shaver P. Adult attachment in a nationally representative sample. J Pers Soc Psychol. 1997;73:1092–1106. doi: 10.1037//0022-3514.73.5.1092. [DOI] [PubMed] [Google Scholar]
- 22.Hammen C. Interpersonal stress and depression in women. J Affect Disord. 2003;74:49–57. doi: 10.1016/s0165-0327(02)00430-5. [DOI] [PubMed] [Google Scholar]
- 23.Gold P, Goodwin F, Chrousos G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. N Engl J Med. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- 24.Cyranowski J, Frank E, Young E, Shear M. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- 25.Purba J, Hoogendijk W, Hofman M, Swaab D. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 26.Panksepp J. Oxytocin effects on emotional processes: separation distress, social bonding, and relationships to psychiatric disorders. Ann N Y Acad Sci. 1992;652:243–252. doi: 10.1111/j.1749-6632.1992.tb34359.x. [DOI] [PubMed] [Google Scholar]
- 27.Pitts A, Samuelson S, Meller W, Bissette G, Nemeroff C, Kathol R. Cerebrospinal fluid corticotropin-releasing hormone, vasopressin, and oxytocin concentrations in treated patients with major depression and controls. Biol Psychiatry. 1995;38:330–335. doi: 10.1016/0006-3223(95)00229-A. [DOI] [PubMed] [Google Scholar]
- 28.Frasch A, Zetzsche T, Steiger A, Jirikowski G. Reduction of plasma oxytocin levels in patients suffering from major depression. Adv Exp Med Biol. 1995;395:257–258. [PubMed] [Google Scholar]
- 29.van Londen L, Goekoop J, van Kempen G, Frankhuijzen-Sierevogel A, Wiegant V, van der Velde E, De Wied D. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 30.Amico J, Ulbrecht J, Robinson A. Clearance studies of oxytocin in humans using radioimmunoassay measurements of the hormone in plasma and urine. J Clin Endocrinol Metab. 1987;64:340–345. doi: 10.1210/jcem-64-2-340. [DOI] [PubMed] [Google Scholar]
- 31.First MB, Spitzer RL, Gibbon M, Williams SD. Structured clinical interview for DSM-IV axis I disorders. New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekman P, Levenson R, Friesen W. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- 34.Turner R, Altemus M, Enos T, Cooper B, McGuinness T. Preliminary research on plasma oxytocin in normal cycling women: investigating emotion and interpersonal distress. Psychiatry. 1999;62:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- 35.Amico J, Ervin M, Leake R, Fisher D, Finn F, Robinson A. A novel oxytocin-like and vasotocin-like peptide in human plasma after administration of estrogen. J Clin Endocrinol Metab. 1985;60:5–12. doi: 10.1210/jcem-60-1-5. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Steer RA. Manual for the revised Beck Depression Inventory. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- 37.Beck AT, Steer RA. Manual for the revised Beck Anxiety Inventory. San Antonio: Psychological Corporation; 1990. [Google Scholar]
- 38.Horowitz L, Rosenberg S, Baer B, Ureno G, Villasenor V. Inventory of Interpersonal Problems: psychodynamic properties and clinical applications. J Consult Clin Psychol. 1988;56:885–892. doi: 10.1037//0022-006x.56.6.885. [DOI] [PubMed] [Google Scholar]
- 39.Horowitz L, Rosenberg S, Ureno G, Kalehzan B, O’Halloran P. Psychodynamic formulation, consensual response method, and interpersonal problems. J Consult Clin Psychol. 1989;57:599–606. doi: 10.1037//0022-006x.57.5.599. [DOI] [PubMed] [Google Scholar]
- 40.Alden L, Wiggins J, Pincus A. Construction of circumplex scales for the Inventory of Interpersonal Problems. J Pers Assess. 1990;55:521–536. doi: 10.1080/00223891.1990.9674088. [DOI] [PubMed] [Google Scholar]
- 41.Taylor S, Gonzaga G, Klein L, Hu P, Greendale G, Seeman T. Relation of oxytocin to psychosocial stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosom Med. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- 42.Taylor S. Tend and befriend: biobehavioral bases of affiliation under stress. Curr Dir Psychol Sci. 2006;15:273–277. [Google Scholar]
- 43.Taylor S, Klein L, Lewis B, Gruenwald T, Gurung R, Updegraff J. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 44.Grippo A, Cushing B, Carter C. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cyranowski J, Bookwalla J, Feske U, Houck P, Pilkonis P, Kostelnik B, Frank E. Adult attachment profiles, interpersonal difficulties, and response to interpersonal psychotherapy in women with recurrent major depression. J Soc Clin Psychol. 2002;21:191–217. [Google Scholar]
- 46.Ge X, Lorenz F, Conger R, Elder G, Simons R. Trajectories of stressful life events and depressive symptoms during adolescence. Dev Psychol. 1994;30:467–483. [Google Scholar]
- 47.Turner R, Altemus M, Yip D, Kupferman E, Fletcher D, Bostrom A, Lyons D, Amico J. Effects of emotion on oxytocin, prolactin, and ACTH in women. Stress. 2002;5:269–276. doi: 10.1080/1025389021000037586-1. [DOI] [PubMed] [Google Scholar]
- 48.Salonia A, Nappi R, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]