Abstract
Human corneal endothelial cells (HCEC) have become increasingly important for a range of eye disease treatment therapies. Accordingly, a more detailed understanding of the processing and preservation associated stresses experienced by corneal cells might contribute to improved therapeutic outcomes. To this end, the unfolded protein response (UPR) pathway was investigated as a potential mediator of corneal cell death in response to hypothermic storage. Once preservation-induced failure had begun in HCECs stored at 4°C, it was noted that necrosis accounted for the majority of cell death but with significant apoptotic involvement, peaking at several hours post-storage (4-8 hours). Western blot analysis demonstrated changes associated with apoptotic activation (caspase 9, caspase 3, and PARP cleavage). Further, the activation of the UPR pathway was observed through increased and sustained levels of ER folding and chaperone proteins (Bip, PDI, and ERO1-Lα) in samples experiencing significant cell death. Modulation of the UPR pathway using the specific inhibitor, salubrinal, resulted in a 2-fold increase in cell survival in samples experiencing profound cold-induced failure. Furthermore, this increased cell survival was associated with increased membrane integrity, cell attachment, and decreased necrotic cell death populations. Conversely, addition of the UPR inducer, tunicamycin, during cold exposure resulted in a significant decrease in HCEC survival during the recovery period. These data implicate for the first time that this novel cell stress pathway may be activated in HCEC as a result of the complex stresses associated with hypothermic exposure. The data suggest that the targeted control of the UPR pathway during both processing and preservation protocols may improve cell survival and function of HCEC thus improving the clinical utility of these cells as well as whole human corneas.
Keywords: Apoptosis, Cornea, Unfolded Protein Response, Hypothermia, Endoplasmic Reticulum Stress
Introduction
Recent advances in cell therapy and transplantation technology have increased uses, and in turn driven demand, for corneal tissue and cells[1,14,18,19]. In particular, the optimal processing and preservation of human corneal endothelial cells (HCEC) has become an area of focus because of their importance to the development of new cell based technologies[20,24,25,29]. HCECs are progressively being considered for a range of uses, including corneal bioengineering for replacement therapies to treat diseases of the eye[62]. Reports have also shown the importance of endothelial maintenance for retention of whole corneal function[3,21,31,57]. Further, HCECs do not divide in vivo so the preservation of these cells in particular is of great importance for clinical application of corneal tissue [37,58]. The role of corneal endothelial cells is to regulate the osmotic balance and nutrient exchange to maintain proper optical clarity for correct vision, given the avascular nature of the cornea.
There has been considerable research examining the whole cornea and isolated endothelial cells in relation to their biology, preservation and transplantation, including investigations into the role that molecular alterations have in different corneal models[3,5,11,13,27,39-41,44,46,49,54,56]. Some studies have focused on the role that storage temperature has on endothelial survival, as storage at normothermic temperatures (organ culture) can have some benefits over hypothermic storage [38,41-43,47,49]. There have been numerous reports on apoptotic involvement, particularly in endothelial cells related to transplantation, that implicate reactive oxygen species formation, inflammation, and chemical exposure as molecular-based response triggers that ultimately result in decreased endothelial functionality[9,45,48,50-52,60,63,64]. Other studies have examined additional molecular-related events, detailing how disease states, media supplementations, genetic changes, and transcription factors have profound effects on corneal biology at a molecular level[10,15,17,19,22,26,37,44,45,52,59]. Despite this increased molecular focus, there remains a void in our understanding of the complex molecular responses of corneal cells in response to hypothermic exposure. An understanding of cold induced changes is critical given that hypothermic conditions are often employed to maintain these biologics prior to utilization. Knowledge of the molecular responses could result in not only improved processing strategies but also improved therapeutic outcomes through targeted modulation of stress pathways.
Numerous reports have demonstrated that a molecular based cell death response, apoptosis, is initiated in cells in response to cold exposure[8]. Studies have shown that changes associated with cold exposure, such as decreased membrane fluidity, pH change, osmotic imbalances, mitochondrial permeability transition pore opening, and oxidative stress can trigger a cell death response in a number of different cell systems[6,7,55]. Furthermore, studies have demonstrated the beneficial effects of targeting these cold-induced molecular responses through solution formulation changes as well as the addition of specific chemical modulators (i.e. anti-oxidants, protease inhibitors, ion chelators)[33-36]. While this research has led to the identification of specific molecular events, a void remains in our understanding of cold stress pathway activation, particularly in corneal endothelial cells.
The unfolded protein response (UPR) is the process in which a cell responds to the accumulation of misfolded proteins in the endoplasmic reticulum (ER). The UPR pathway has several functions, including correction of this accumulation through inhibiting translation of new proteins and up-regulating ER chaperone and folding proteins in an effort to clear the ER of these proteins[30]. Another function of the UPR pathway is to initiate an apoptotic response if the ER stress remains too severe or prolonged[30]. The UPR pathway, while identified relatively recently in human cell systems, has become a major area of study with various reports detailing its involvement in response to various cellular stress events[2,4,16,23,28,32,61]. ER stress and subsequent UPR activation has been implicated in response to disease states, chemical exposure, cancer proliferation, aging, cell death, inflammation, autophagy, among others. A recent study by Engler et. al.[15] demonstrated the activation of the UPR pathway in the corneal endothelium of patients suffering from Fuchs endothelial dystrophy. Further, this report suggests that the UPR may be a central pathogenic pathway with its activation triggering endothelial cell apoptosis through a mitochondrial based, caspase 9 mediated response. However, review of the previous investigations questioned whether this pathway is activated in response to complex changes and stresses associated with cold exposure. To this end, we investigated the role of the UPR to determine its potential involvement in cellular demise associated with cold exposure.
Methods and Materials
Cell Culture
HCEC were obtained from Dr. Nancy Joyce at Schepens Eye Research Institute (Boston, MA) and were maintained under standard culture conditions (37°C, 5% CO2/95% air) in OptiMEM (Invitrogen, Grand Island, NY) supplemented with 8% Fetal Bovine Serum (Atlanta Biologicals, Lawrenceville, GA), bovine pulmonary extract (Biomedical Technology Inc., Stoughton, MA), gentamicin sulfate (Invitrogen, Carlsbad, CA), epidermal growth factor (Millipore, Billerica, MA), nerve growth factor (Biomedical Technology Inc.), Anti-biotic/mycotic (Sigma-Aldrich, St. Louis, MO), ascorbic acid (Sigma-Aldrich), calcium chloride (Sigma-Aldrich), and chondroitin sulfate (Sigma-Aldrich). Cells were propagated in Falcon T-75 flasks and media was replenished every two days of cell culture.
Hypothermic Solutions
Four different storage media were utilized for hypothermic storage: complete growth media (CGM), Hank’s Balance Salt Solution with calcium and magnesium(HBSS) (Mediatech, Inc., Manassas, VA ), ViaSpan (commercially available University of Wisconsin solution), and OptiSol (commercial whole cornea storage solution) (Bausch and Lomb, Rochester, NY)
Hypothermic Storage
Cells were seeded into 96-well tissue culture plates (13,000 cells per well) and cultured for 24 hours into a monolayer. Culture media was decanted from experimental plates and replaced with 100 μl/well of the pre-cooled (4°C) solution (complete growth media, HBSS with calcium and magnesium, ViaSpan, or OptiSol). Cultures were maintained at 4°C for 18 hours to 9 days. Following cold storage, the media were decanted, replaced with 100 μl/well of room temperature (~25°C) complete culture media and then placed into standard culture conditions (37°C, 5% CO2) for recovery and assessment.
Cell Viability Assay
To assess cell viability the metabolic activity assay, alamarBlue™ (Invitrogen) was utilized. Cell culture medium was decanted from the 96-well plates and 100 μl/well of the working alamarBlue™ solution (1:20 dilution in HBSS) was applied. Samples were then incubated for 60 minutes (± 1 min) at 37°C in the dark. The fluorescence levels were analyzed using a Tecan SPECTRAFluorPlus plate reader (TECAN, Austria GmbH). Relative fluorescence units were converted to a percentage compared to normothermic controls set at 100%. Readings were taken immediately following removal from hypothermic storage as well as 24 and 48 hours of recovery.
Chemical Additions
Modulation of the UPR was accomplished through the use of salubrinal (UPR-specific inhibitor) and tunicamycin (UPR-specific inducer). Salubrinal (EMD Chemicals Inc., Gibbstown, NJ) was added to hypothermic media at working concentrations of 10 and 25 μM immediately before utilization. Tunicamycin (EMD Chemicals Inc.) was added to ViaSpan at a working concentration of 2ug/mL immediately prior to use. All chemicals were diluted in Me2SO prior to utilization and Me2SO controls were conducted to ensure no effect of the dilution vehicle.
Fluorescence Microscopy
Samples in 96-well plates were assessed for the presence of live, necrotic or apoptotic cells through triple labeling using Hoechst [81μM], propidium iodide [9μM], and YoPro-1 [.8 μM] (Molecular Probes, Eugene, OR), respectively. Probes were added to samples and incubated in the dark for 20 minutes prior to imaging. To assess membrane integrity a dual label of calcein-AM [4μM] and propidium iodide [9μM] was utilized. Dual label probes were added to the samples and incubated for 30 minutes in the dark. All fluorescence images of labeled cells were obtained at 1, 4, 8 and 24 hours post-storage using a Zeiss Axiovert 200 fluorescent microscope with the AxioVision 4 software (Zeiss, Germany).
Flow Cytometric Analysis
Counts of the unlabled (live), necrotic (PI [1.5μM]) and apoptotic (YOPRO-1 [0.1μM]) labeled cells were obtained using microfluidic flow cytometry (Millipore). Probes were added to each sample and incubated in the dark for 20 minutes prior to cell collection. Counts of cells with polarized and depolarized mitochondria (JC-1 [7.7μM]) were also obtained via microfluidic flow cytometry. Samples were labeled, collected and analyzed at 1, 4, 8 and 24 hours post-storage. Analysis was performed using the CytoSoft 5.2 software for the Guava PCA-96 system.
Western Blot Analysis
Cells were cultured in 60mm Petri dishes to form a monolayer. Cell culture media was removed and replaced with 4mL of pre-cooled (4°C) solution and dishes were placed at 4°C for 18 hours. Following cold storage, solutions were decanted and replaced with 4mL of room temperature culture media and placed into standard culture conditions (37°C, 5% CO2) for recovery. Cell lysates were collected 1, 4, 8 and 24 hours post-storage using ice-cold radio-immunoprecipitation assay cell lysis buffer with protease inhibitors. Samples were homogenized by vortex mixing and centrifuged at 15,000 rpm for 15 minutes at 4°C. Protein concentrations were quantified using the bicinchonic acid protein assay (Thermo Fisher Scientific, Rockford, IL) and a Tecan SPECTRAFluorPlus plate reader. Equal amounts of protein (30μg) for each sample were loaded and separated on a 10% SDS-PAGE gel (Bio-Rad, Hercules, CA). Proteins were transferred to PVDF membranes (Bio-Rad) and blocked with a 1:1 mixture of NAP™-Blocker (G-Biosciences, Maryland Heights, MO) with 0.05% Tween-20 in PBS for 2 hours at room temperature. Membranes were incubated at 4°C overnight in the presence of each antibody: anti-human caspase 9, anti-human caspase 3, anti-human PARP, anti-human Bip, anti-human calnexin, anti-human ERO1-Lα, anti-human PDI and anti-human β-Tubulin (Cell Signaling Technology, Danvers, MA). Membranes were then washed three times with 0.05% Tween-20 in PBS and exposed with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. Membranes were again washed three times with 0.05% Tween-20 in PBS before detection with the LumiGLO®/Peroxide chemiluminescent detection system (Cell Signaling Technology). Membranes were visualized using a Fujifilm LAS-3000 luminescent image analyzer. Equal protein loading was achieved through initial quantification of all samples and confirmed by Ponceau S staining of PVDF membranes prior to blocking as well as probing for β-Tubulin levels.
Data Analysis
Viability experiments were repeated a minimum of three times with an intra-experiment repeat of seven replicates. Western blots, flow cytometry and fluorescence microscopy were all conducted on a minimum of three separate experiments. Standard errors were calculated for viability values and single-factor analysis of variance (ANOVA) was utilized to determine statistical significance.
Results
Effect of Hypothermic Exposure on Human Corneal Endothelial Cell Survival
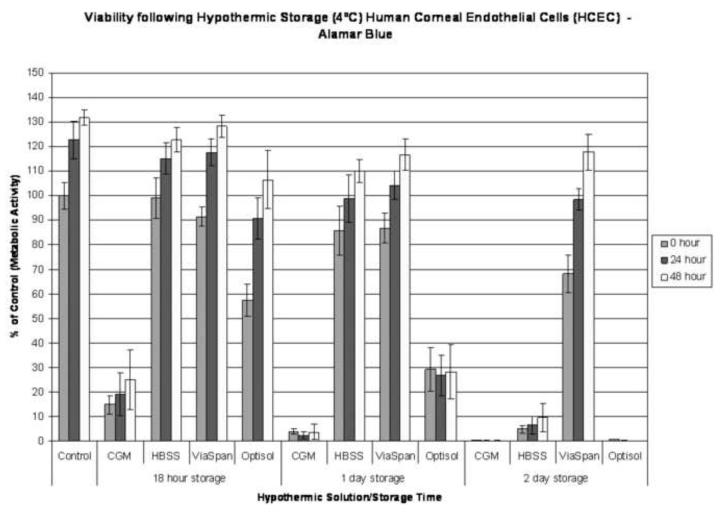
HCECs displayed a variable level of cold sensitivity in vitro that was dependent upon the media and duration of hypothermic storage. This was characterized by a decrease in viability immediately following 18 hours of cold storage in all solutions utilized: CGM, HBSS, ViaSpan and OptiSol (Figure 1). Storage in CGM resulted in 14.9% (±3.8) viability, while samples stored in OptiSol retained 57.4% (±6.5) viability and storage in HBSS and ViaSpan yielded retention of 99.0% (±8.2) and 91.5% (±4.0) viability, respectively. Despite the levels of cell death observed, samples were able to recover and repopulate in culture following 18 hours of cold exposure regardless of the media utilized. As the storage interval was increased, however, corresponding decreases in sample viability were evident across all conditions (Figure 1). Following 24 hours of cold exposure samples stored in CGM yielded few remaining viable cells (3.9% (±1.0)) and no replicative capacity. HCECs stored in OptiSol demonstrated a marked decrease in viability, compared to 18 hour exposure, retaining only 29.3% (±8.9) metabolic activity and an inability to repopulate to normothermic control levels by 48 hours post-storage. Storage in HBSS and ViaSpan demonstrated lower reductions in viability retaining 85.8% (±9.9) and 86.8% (±6.1) respectively, immediately following 24 hour exposure. Further increasing the cold exposure interval to two days resulted in more pronounced cell loss with a complete loss of viability in the CGM and OptiSol stored HCEC samples. HBSS samples decreased to 4.9% (±1.5) viability while the ViaSpan samples retained a high level of metabolic activity (68.2% (±7.7)) as well as an ability to repopulate to normothermic control levels during the recovery period (Figure 1).
Figure 1. Viability Assessment of Hypothermically Stored Human Corneal Endothelial Cells.
HCEC’s were placed at 4°C in either: Complete Media (CGM), HBSS with Ca++ and Mg++, ViaSpan, or OptiSol for 18 hours, 1 day, or 2 days. Resultant cell viability following storage was determined 0, 24 and 48 hours post-storage. The data illustrate profound differences between storage media and sensitivity of corneal endothelial cells to cold exposure in vitro. Immediate following 18 hours of storage viabilities of 14.9% (±3.8), 99.0% (±8.2), 91.5% (±4.0), and 57.4% (±6.5) were noted for CGM, HBSS, ViaSpan and OptiSol storage, respectively. As the interval was increased to 2 days, only ViaSpan samples remained viable (68.2% ±7.7).
Post-cold exposure sample viability data were confirmed via fluorescence microscopy probing for viable, necrotic and apoptotic cells using hoechst, propidium iodide (PI) and Yo-Pro-1, respectively. Micrographic assessment demonstrated a similar level of viable cells remained following the various hypothermic exposure intervals in the four solutions utilized as measured by alamarBlue (data not shown, representative micrographs presented in Figure 5A). These analyses also allowed for the assessment of the modes of cell death contributing to sample demise following hypothermic storage of HCECs. Fluorescence microscopy revealed that the primary mode of cell death involved in HCEC preservation-induced cellular demise was necrosis as indicated by the high incidence of PI-labeled (red) cells. Along with necrosis it was noted that a considerable amount of apoptotic cells (green) were detected throughout the 24 hours post-storage, with a peak occurrence of this population at 4-8 hours following removal from the cold. These data were consistent with studies on other cells systems following cold exposure, where a delayed apoptotic peak and large necrotic population have been reported [6-8,12,35].
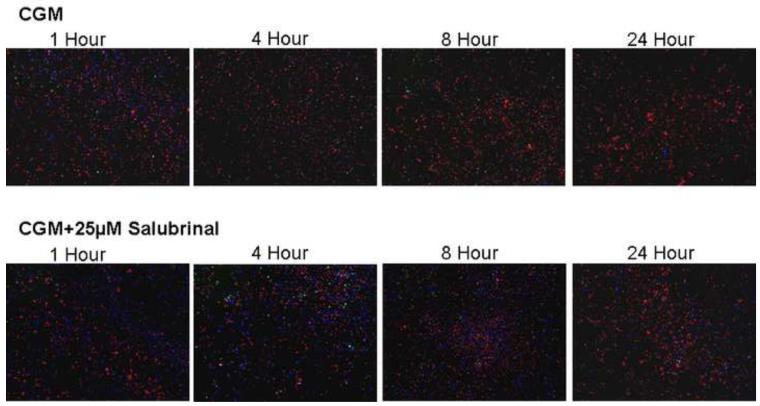
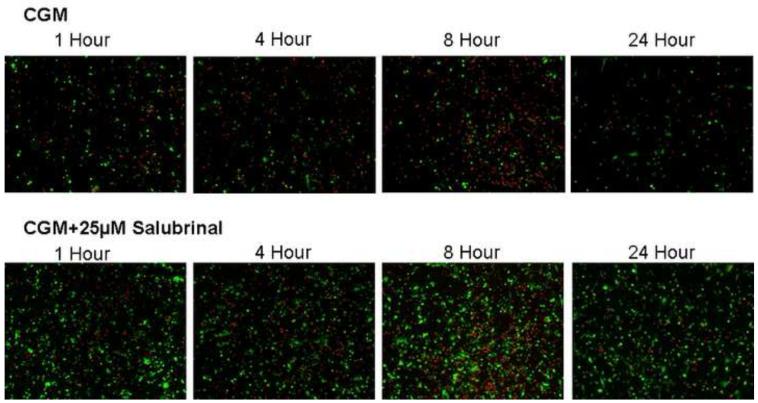
Figure 5. Time Course Fluorescent Microscopy Assessment of UPR Inhibition on HCEC Cells Following Hypothermic Storage.
Fluorescent images were taken 1, 4, 8 and 24 hours post-storage following 24 hours of cold storage in Complete Media (CGM) with either 0μM or 25 μM salubrinal. (A) Blue labeled (Hoechst) cells denote living cells, red (propidium iodide) are necrotic and green (Yo-Pro-1) indicates apoptotic cells. The micrographs corroborated the trends in cell death as determined with flow cytometric analysis with salubrinal addition resulting in increased viable cell and total cell retention populations. (B) Green labeled (calcein-AM) cells indicate viable cells and red (propidium iodide) cells denote necrotic cells. The micrographs demonstrate that salubrinal addition resulted in increased cellular membrane integrity, cell attachment and overall cell viability throughout the initial 24 hours post-expsoure.
Activation of Apoptosis Following Hypothermic Exposure
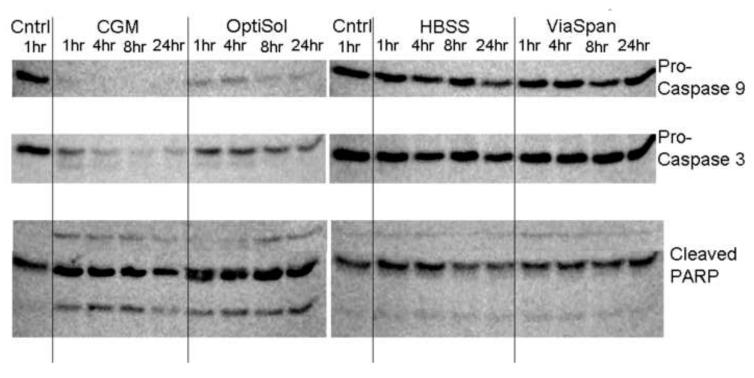
Following the establishment of viability profiles for HCECs stored for various intervals coupled with the observation of a molecular-based component to cell death, western blot analysis was conducted in an effort to decipher the activation of specific cold-induced cell stress pathways. Samples were collected after 1, 4, 8 and 24 hours of recovery following 18 hours of cold storage in either CGM, HBSS, ViaSpan or OptiSol and total protein was extracted. The assessment of proteins associated with apoptotic activation revealed a correlation between the loss of viability and activation of the apoptotic cascade (Figure 2A). Targeted assessment of caspase 9, a specific mediator of mitochondrial (intrinsic) based apoptotic activation, revealed cleavage (activation) of the pro-form intermediate at 1 hour post-storage in the CGM stored samples (21.5% as compared to control levels) with a subsequent complete loss (2.5%) by 24 hours post-exposure while OptiSol storage demonstrated a reduction in pro-caspase 9 without a complete loss throughout the time course (19.2% at 24 hours of recovery). Pro-caspase 9 levels in HBSS and ViaSpan stored HCECs maintained higher and more consistent levels throughout the initial 24 hours post-cold exposure recovery period (~60-70% as compared to controls). This maintenance in pro-caspase 9 levels was consistent with the elevated viability levels observed under these conditions. Assessment of the downstream apoptotic mediator, caspase 3, revealed a similar pattern of cleavage as observed with pro-caspase 9. Decreases in pro-caspase 3 levels were detected in both the CGM and OptiSol stored HCECs (13.2% and 42.3% at 24 hours post-storage, respectively), with more pronounced cleavage noted in the CGM samples and to a lesser extent in OptiSol storage as compared to normothermic control levels. As with caspase 9, caspase 3 activation correlated with the viability data. Likewise, HBSS and ViaSpan stored cells demonstrated a pattern of sustained pro-caspase 3 levels throughout the post-storage time course indicating a lower level of apoptotic activity and overall cell death. With the observed caspase 3 activation, assessment of the active caspase 3 target, Poly (ADP-ribose) polymerase (PARP), was conducted to further support the evidence of apoptotic signal progression following cold storage (Figure 2A). PARP is a DNA repair enzyme whose cleavage is indicative of late stage apoptosis and cells’ commitment to completing cell death. Western blots revealed an increase in PARP cleavage in the CGM and OptiSol samples as compared to the HBSS and ViaSpan samples and normothermic controls. Examination of PARP cleavage revealed a similar pattern of apoptotic activation in the CGM and OptiSol samples with noticeably less activation in the HBSS and ViaSpan samples. These data further confirmed apoptotic activation in HCECs leading to preservation-induced viability loss.
Figure 2. Assessment of Apoptosis and UPR Activation Following Hypothermic Exposure.
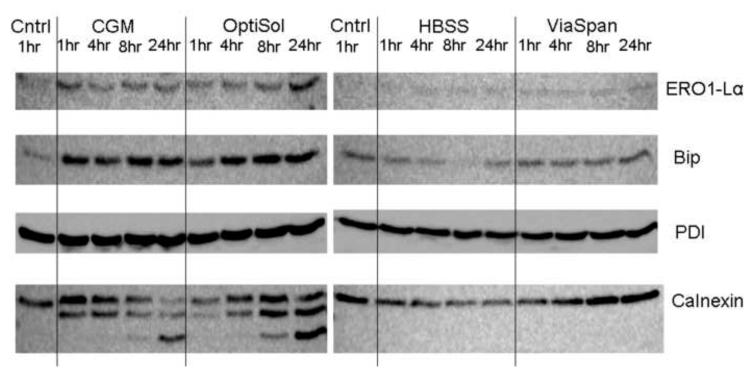
HCEC’s were placed at 4°C in either: Complete Media (CGM), HBSS with Ca++ and Mg++, ViaSpan, or OptiSol for 18 hours. Protein isolates were collected at 1, 4, 8 and 24 hours post-storage and western blot analysis was performed on these samples. (A) Analysis of apoptotic associated proteins revealed increased activation (increased caspase and PARP cleavage)in CGM and OptiSol stored samples that correlated with decreased viability. (B) Analysis of ER folding maintenance proteins demonstrated an increase in UPR specific protein activation in samples which were shown to have decreased viability. Specifically, there was a marked increase in ERO1-Lα and Bip levels for CGM and OptiSol stored samples as compared to controls as well as HBSS and ViaSpan stored counter-parts. Furthermore, a sustain level of PDI in addition to cleavage of calnexin was noted in these samples illustrating the further evidence of UPR specific activation.
Involvement of the Unfolded Protein Response Following Hypothermic Exposure
Following the identification of apoptosis through both fluorescence microscopy and western blot analysis, an investigation of the involvement of ER stress and the UPR pathway in response to hypothermic exposure was conducted. Western blots were performed examining the levels of ER folding and chaperone proteins, including Bip, ERO1-Lα, PDI and calnexin, in HCEC isolates at 1, 4, 8 and 24 hours recovery post 18 hours cold storage. Similar to apoptotic-associated protein changes, a correlation was noted between a loss of sample viability and changes in the ER stress protein levels and the subsequent UPR activation. In the CGM and OptiSol samples, increases in Bip and ERO1-Lα were observed as compared to normothermic controls (Figure 2B). A time dependent increase in Bip was seen in CGM and OptiSol stored samples as both peaked at 24 hours post-storage (359.7% and 455.8%, respectively). Analysis of ERO1-Lα levels demonstrated an immediate peak 1 hour post-exposure for CGM samples (123.0%) and a delayed peak for OptiSol samples (172.9% at 24 hours of recovery). Comparatively, HBSS and ViaSpan stored samples demonstrated lower overall levels of these proteins with no time dependent up-regulation. Examination of PDI revealed that levels remained relatively consistent throughout the initial 24 hours following hypothermic exposure for all conditions tested. Probing for calnexin also revealed a time related cleavage in CGM and OptiSol samples, an event reported in response to apoptotic stimuli. Conversely, HBSS and ViaSpan samples exhibited no calnexin cleavage throughout the 24 hour recovery interval. These data suggest that changes in proteins associated with ER stress and UPR activation occur in a manner that correlates with the activation of the apoptotic cascade in response to cold exposure.
Effect of UPR Specific Chemical Modulation on Human Corneal Endothelial Cell Survival
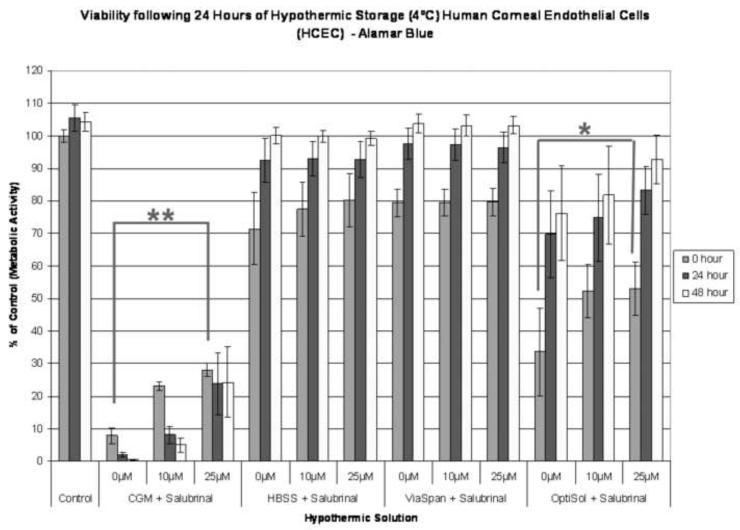
Identification of ER stress and UPR involvement in HCEC response to cold exposure led us to investigate the effect of targeted, specific modulation of the UPR pathway. UPR inhibition and UPR induction studies were conducted through the addition of salubrinal (inhibition) and tunicamycin (induction) during hypothermic storage to confirm the role of the UPR pathway in cold-induced cell death. To examine the effect of UPR specific inhibition, HCECs were stored at 4°C in the presence of salubrinal for 18 hours, 24 hours and 2 days, allowed to recover and sample viability assessed. Cold storage for 24 hours in the presence of salubrinal resulted in a pattern of improved HCEC survival during the 48 hours recovery period (Figure 3A). HBSS and ViaSpan samples remained highly viable (70-80%) and demonstrated no differences with salubrinal addition at this storage interval (p = 0.28 and p = 0.98, respectively). CGM and OptiSol stored HCECs demonstrated considerable improvements following salubrinal addition as these systems began to undergo hypothermic stress dependent failure. Storage in CGM alone resulted in 7.9% (±2.4) viability immediately following storage with a subsequent decrease during recovery resulting in complete loss of viability. Addition of 10μM and 25μM salubrinal, however, yielded improved post-storage viabilities of 23.1% (±1.4) and 28.0% (±2.1), respectively (p < 0.001). Interestingly, the 25μM condition allowed for the subsequent maintenance of HCECs as viability was retained over the 48 hour recovery period. Similarly, HCEC storage in OptiSol with and without salubrinal addition for 24 hours resulted in similar patterns as the aforementioned CGM storage with post storage viabilities of 33.5% (±13.3), 52.3% (±8.2) and 53.9% (±8.2) (OptiSol with 0μM, 10μM and 25μM salubrinal, respectively) (p < 0.01). These UPR inhibition data demonstrated a concentration dependent increase in HCEC viability that translated to improved survival throughout the 48 hour recovery period.
Figure 3. UPR Specific Chemical Modulation on Human Corneal Endothelial Cell Survival.
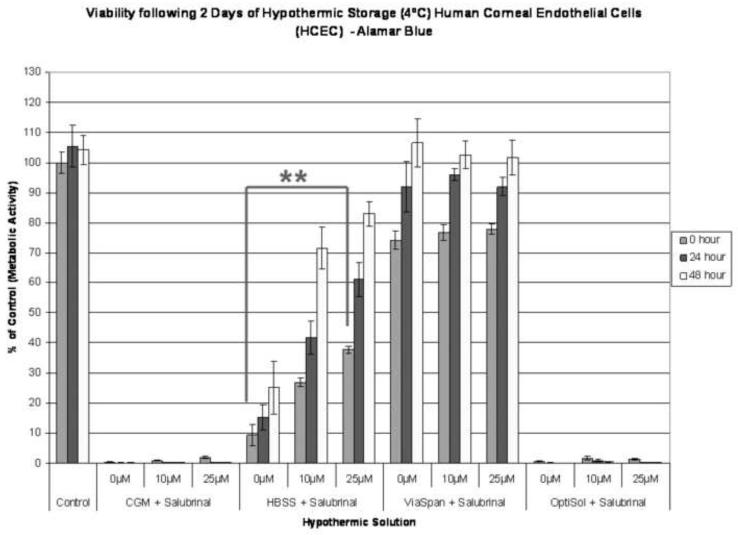
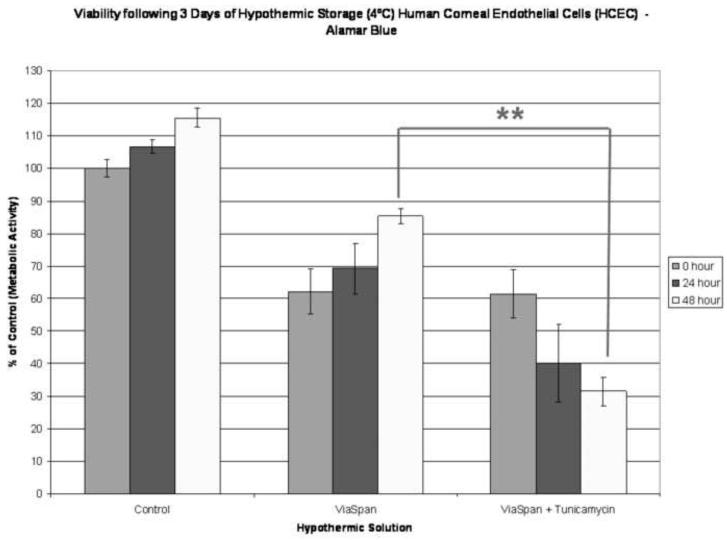
(A) HCEC samples were stored at 4°C for 24 hours in Complete Media (CGM), HBSS with Ca++ and Mg++, ViaSpan, or OptiSol been supplemented with either 0μM, 10 μM, or 25 μM salubrinal (UPR inhibitor). Increased survival with salubrinal addition was seen in the CGM and OptiSol samples as post-thaw viabilities of 7.9%, 23.1% and 28.0% for CGM storage and 33.5%, 52.3% and 53.0% for OptiSol storage with 0μM, 10 μM, and 25 μM salubrinal, respectively. (** p<0.001, * p<0.01) (B) Samples were stored at 4°C for 2 days in Complete Media (CGM), HBSS with Ca++ and Mg++, ViaSpan, or OptiSol supplemented with either 0μM, 10 μM, or 25 μM salubrinal (UPR inhibitor). Increases in sample viability in the HBSS condition were observed with salubrinal additions (26.1% and 36.5% vs. 12.7% without addition). (** p<0.001) (C) HCEC’s were stored at 4°C for 3 days in ViaSpan supplemented with either 0μg/mL or 25 μg/mL tunicamycin (UPR inducer). Immediately, post storage no significant difference was observed with tunicamycin addition with viabilities of 62.2% (±6.9) without addition and 61.4% (±7.3) with tunicamycin. As samples were allowed to recover a significant difference was noted between the conditions as ViaSpan without tunicamycin re-grew to 85.4% (±2.4) viability while ViaSpan with tunicamycin addition declined to 31.5% (±4.4). (** p<0.001)
An extension of the hypothermic exposure interval from 1 to 2 days provided additional verification of the involvement of UPR and beneficial effect of its inhibition (Figure 3B). Following 2 days of cold storage there was a complete loss in viability in all CGM and OptiSol HCECs samples. Interestingly, following this storage interval, HBSS samples succumbed to preservation-induced cell death yielding sample viability of 9.3% (±3.5) immediately post-storage. Inclusion of salubrinal during cold storage provided dose-dependent increases in overall viability to 26.9% (±1.5) and 37.8% (±1.4) (10μM and 25μM, respectively) (p < 0.001). As with the 24 hour sample, 48 hour storage in ViaSpan yielded minimal cell loss and no effect of salubrinal supplementation (74.3% vs. 76.7%, p = 0.87). These data suggest that UPR specific inhibition has a beneficial effect only once HCECs begin to demonstrate substantial losses in viability (greater than 50%).
To further test UPR involvement, induction studies were conducted to determine whether UPR activation would have a negative effect on HCEC survival following hypothermic storage. As such, the UPR specific inductor, tunicamycin, was added to ViaSpan and HCEC samples were stored at 4°C for 3 days in the presence and absence of tunicamycin then assayed for viability at 0, 24 and 48 hours of recovery (Figure 3C). Interestingly, tunicamycin addition had no immediate effect on post-storage viability as both storage conditions yielded ~60% cell survival (p = 0.86). However, significant differences in the storage conditions became evident as assessment continued through the recovery period. Following 24 hours of recovery a survival difference of 29.0% was noted between the conditions. This differential further increased to 53.9% by 48 hours post-storage (p < 0.001). These findings further support the significant effect that the UPR pathway may play in cell survival following cold exposure.
Effect of UPR Specific Chemical Modulation on Cell Death Populations Following Cold Storage
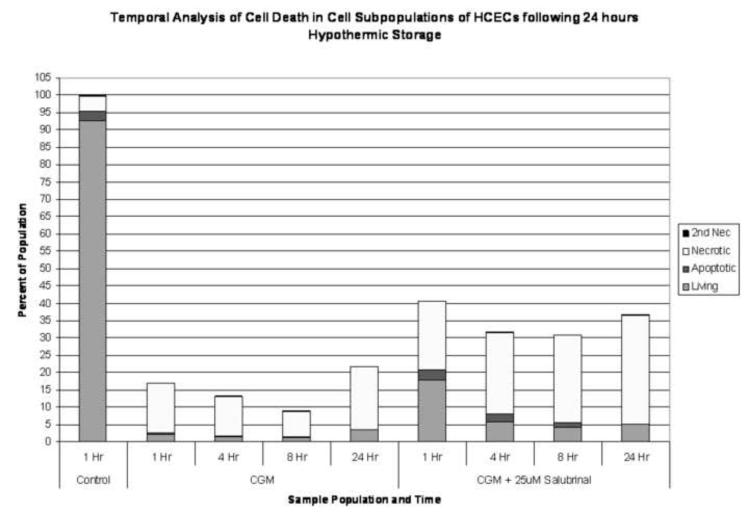
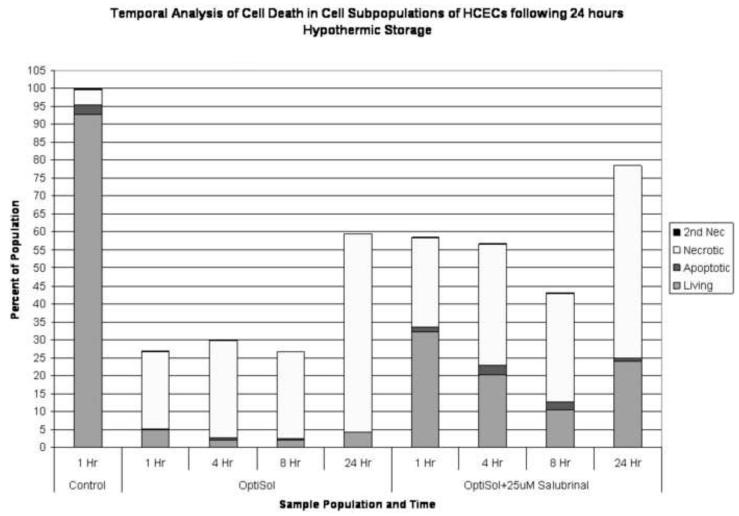
Following the observation of differences in viability through the targeted modulation of the UPR, we next analyzed what effect this targeted approach has on the level and timing of cell death following hypothermic storage. HCECs were held at 4°C for 24 hours in various solutions (CGM, HBSS, ViaSpan and OptiSol) with and without the addition of 25μM salubrinal and assessed via microfluidic flow cytometry and fluorescence microscopy. Analysis of the apoptotic and necrotic populations was conducted to examine the effect UPR inhibition (25μM salubrinal) had on cell death populations post-storage. Temporal Yo-Pro-1/PI flow cytometric analysis revealed that necrosis accounted for a larger percentage of the total population in uninhibited samples as compared to their UPR inhibited counterpart. In this regard, the CGM and OptiSol stored cells demonstrated the largest differences in levels of necrosis (data not shown). Interestingly, examination of the apoptotic populations revealed the opposite trend, with the UPR inhibited samples having a larger apoptotic population than in uninhibited samples. This suggests that UPR inhibition may have resulted in an increased prevalence of cells that survive the initial stress of cold exposure but experienced sufficient damaging effects to trigger an apoptotic response following storage, thus resulting in increased populations of both apoptotic and viable cells as compared to uninhibited samples. When samples were normalized to the normothermic control, it was observed that necrotic populations between UPR inhibited and uninhibited samples were similar. The levels of apoptosis, however, were elevated in the inhibited samples as compared to uninhibited conditions at each time point (Figures 4A and 4B). The most noticeable differences between the conditions were in the level of viable cells as well as the overall total cell retention (as noted by the total stacked population in both Figures 4A and 4B). This was particularly evident in the CGM and OptiSol HCEC samples. These data illustrate that UPR specific inhibition had a beneficial effect through an increased retention of total cells during hypothermic exposure resulting in an elevated viable cell population.
Figure 4. Effect of UPR Inhibition on Human Corneal Endothelial Cell Death Populations Following Hypothermic Exposure.
(A) HCEC’s were stored at 4°C for 24 hours in CGM supplemented with either 0μM or 25 μM salubrinal. The total numbers of viable, apoptotic, necrotic and secondary necrotic cells were assessed at 1, 4, 8 and 24 hours post-exposure using flow cytometry and the relative percentages of each population determined. An increase in both viable cell and total cell number was noted at all time points examined with the addition of salubrinal. (B) HCEC’s were stored at 4°C for 24 hours in OptiSol supplemented with either 0μM or 25 μM salubrinal. The total numbers of viable, apoptotic, necrotic and secondary necrotic cells were assessed at 1, 4, 8 and 24 hours post-exposure using flow cytometry and the relative percentages of each population determined. An increase in both viable cell and total cell number was noted at all time points with the addition of salubrinal.
These data were corroborated visually via fluorescence microscopy utilizing tri-stain (Hoechst, PI, Yo-Pro-1) to examine the levels of viable, necrotic and apoptotic and a dual-staining (calcein-AM, PI) to examine membrane integrity (live and dead cells) between UPR inhibited and uninhibited samples (Figures 5A and 5B). Specifically, CGM storage for 24 hours with and without salubrinal yielded patterns similar to those observed with flow cytometry. Increases in viable cell populations and overall cell retention in UPR inhibited samples compared with uninhibited samples were found (Figure 5A). Micrographs of calcein-AM and propidium iodide probed samples revealed that salubrinal addition resulted in a marked increase in both membrane integrity as well as cellular attachment during the recovery period (Figure 5B). These data further support the beneficial effect that the specific targeting of the UPR pathway has on HCEC tolerance to hypothermic stress.
Discussion
In this study we examined the role of UPR activation in cellular demise following cold exposure. In an effort to examine the universality of UPR involvement, four unique storage media were utilized, each of which represented a vastly different composition ranging from a balanced salt solution (HBSS) to complete growth media (CGM) to commercial cold storage media (OptiSol and ViaSpan). The motivation behind the selection of these diverse media was not to compare solution performance but to examine the activation, progression, and modulation of UPR based cell death associated in preservation failure regardless of storage time or base medium. Investigation of the differential level of survival after a given storage time due to basal medium formulation, while interesting, was beyond the scope of this study. As it is progressively recognized that all cold storage media fail to protect cells as storage time increases, we focused on those “extended” intervals specific to each media in examining UPR involvement. Examination of HCECs revealed a contrast in cold sensitivity in vitro with viability dependent on exposure duration and solution. Cell death analysis demonstrated that necrosis was the dominate mode of cell death once failure had begun regardless of the media utilized. A sizeable population of apoptotic cells was also noted in the hours following storage, illustrating the involvement of a complex molecular-based response to lethal cold exposure. Furthermore, these cell death populations displayed a temporal response to the cold with a peak in apoptosis observed several hours (4-8) into recovery in addition to a continued increase in necrosis throughout the initial 24 hour recovery period. This illustrates the fact that physical stress alone is not the only factor responsible for cellular demise but instead cold-induced stress pathways are being activated in response to cold exposure. Previous studies have reported on the involvement of caspases in response to cold stress as well as the beneficial effect of their inhibition. Western blot investigations demonstrated the involvement of caspases as well as the down-stream target PARP in HCEC preservation-induced cell death. In particular, this analysis suggested that the mitochondrial-mediated (intrinsic) pathway of apoptosis was being activated. This was an important observation as it led us to investigate UPR involvement, as reports have shown that the UPR mediates the activation of an apoptotic response, at least partially, through the mitochondria[53].
The examination of proteins specific to ER stress and the subsequent UPR activation confirmed that changes, such as the up-regulation of ER chaperones and protein folding proteins, were observed in correlation with increased losses in sample viability. This finding was significant because it provided the first implication of the UPR pathway activation in response to severe cold stress in an in vitro cell model. While there has been an increasing focus on the UPR pathway in relation to other cell stressors (i.e. ischemia, oxidative stress, disease states), there was little to no evidence that the UPR is involved with hypothermic-induced cellular demise, particularly in the cornea. Further, investigation of UPR pathway involvement through its specific modulation provided supporting evidence of its role during hypothermic storage. Comparison between UPR inhibited and non-inhibited samples revealed that modulation of the UPR resulted in increased metabolic activity, membrane integrity, cellular attachment, and overall sample viability. Additionally, the specific induction of the UPR resulted in a pronounced increase in cell death throughout the recovery period as well as the accelerated activation of preservation-induced cell death as compared to non-induced samples. These data provide additional evidence implicating the UPR’s involvement as a cold-induced stress pathway playing a role in delayed cell death.
Our data indicate a clear need for further in-depth studies on the UPR in response to cold exposure. This study was an important first step for human corneal endothelial applications given that many of these processes involve subjecting corneal tissue to cold prior to utilization. The in vitro model employed in this study was deemed most relevant for studying this specific subset of corneal cells as HCECs have little to no replicative capacity, this is in contrast to using whole cornea from an animal as the model which contain endothelial cells that have the ability to divide and repopulate following endothelial death and damage. It is also important to note that the results obtained in this study on individual HCEC populations differed from the results of studies examining whole cornea storage in terms of the length of storage times achievable (i.e. 24 hours for HCEC storage in OptiSol vs. 7 or more days for whole cornea storage in OptiSol). The difference may be attributed to a number of factors that differ between a whole cornea and HCEC cell culture. The activation of the UPR was observed in all solutions tested at these “extended” storage times demonstrating that this pathway may serve as an important target particularly for cell-based therapies and potentially for future studies on whole cornea.
This study represents an important first step in linking UPR activation and HCEC storage failure, however there remains a need for understanding the direct causal relationship. While the identification and modulation of caspases represents a fundamental advance, it does not account for the entire preservation-induced cell death story. As such, there remains a definitive need for further stress pathway identification for improved control. The identification of novel cold stress pathways, such as the UPR, may allow for a more specific molecular control of cell responses to improve survival and function. This in turn may translate into improved outcomes for down-stream corneal utilizations (i.e. transplants, engineered tissues, etc.). In summary, the UPR appears to be an important pathway for future studies and holds potential for manipulation as technologies continue to more forward into more molecular based approaches.
Acknowledgement
Funding provided through NIH Grant: R44EY015575-02A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmad S, Kolli S, Lako M, Figueiredo F, Daniels JT. Stem cell therapies for ocular surface disease. Drug Discov Today. 2010;15:306–13. doi: 10.1016/j.drudis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Al-Rawashdeh FY, Scriven P, Cameron IC, Vergani PV, Wyld L. Unfolded protein response activation contributes to chemoresistance in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2010;22:1099–105. doi: 10.1097/MEG.0b013e3283378405. [DOI] [PubMed] [Google Scholar]
- 3.Albon J, Tullo AB, Aktar S, Boulton ME. Apoptosis in the endothelium of human corneas for transplantation. Invest Ophthalmol Vis Sci. 2000;41:2887–93. [PubMed] [Google Scholar]
- 4.Austin RC. The Unfolded Protein Response in Health and Disease. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2686. [DOI] [PubMed] [Google Scholar]
- 5.Barcia RN, Dana MR, Kazlauskas A. Corneal graft rejection is accompanied by apoptosis of the endothelium and is prevented by gene therapy with bcl-xL. Am J Transplant. 2007;7:2082–9. doi: 10.1111/j.1600-6143.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- 6.Baust JG. Concepts in Biopreservation. In: Baust JG, Baust JM, editors. Advances in Biopreservation. CRC Press; Boca Raton: 2007. pp. 1–14. [Google Scholar]
- 7.Baust JM. Properties of Cells and Tissues Influencing Preservation Outcome: Molecular Basis of Preservation-Induced Cell Death. In: Baust JG, Baust JM, editors. Advances in Biopreservation. CRC Press; Boca Raton: 2007. pp. 63–87. [Google Scholar]
- 8.Baust JM, Snyder KK, Van Buskirk RG, Baust JG. Changing Paradigms in Biopreservation. Biopreservation and Biobanking. 2009;7:3–12. doi: 10.1089/bio.2009.0701.jmb. [DOI] [PubMed] [Google Scholar]
- 9.Borazan M, Karalezli A, Oto S, Akova YA, Karabay G, Kocbiyik A, Celasun B, Demirhan B. Induction of apoptosis of rabbit corneal endothelial cells by preservative-free lidocaine hydrochloride 2%, ropivacaine 1%, or levobupivacaine 0.75% J Cataract Refract Surg. 2009;35:753–8. doi: 10.1016/j.jcrs.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Borderie VM, Baudrimont M, Vallee A, Ereau TL, Gray F, Laroche L. Corneal endothelial cell apoptosis in patients with Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 2000;41:2501–5. [PubMed] [Google Scholar]
- 11.Borderie VM, Laroche L. Microbiologic study of organ-cultured donor corneas. Transplantation. 1998;66:120–3. doi: 10.1097/00007890-199807150-00020. [DOI] [PubMed] [Google Scholar]
- 12.Brockbank KGM, Taylor MJ. Tissue Preservation. In: Baust JG, Baust JM, editors. Advances in Biopreservation. Taylor & Francis; NY: 2007. pp. 157–196. [Google Scholar]
- 13.Camposampiero D, Tiso R, Zanetti E, Ruzza A, Bruni A, Ponzin D. Improvement of human corneal endothelium in culture after prolonged hypothermic storage. Eur J Ophthalmol. 2003;13:745–51. doi: 10.1177/1120672103013009-1002. [DOI] [PubMed] [Google Scholar]
- 14.Deng C, Li F, Hackett JM, Chaudhry SH, Toll FN, Toye B, Hodge W, Griffith M. Collagen and glycopolymer based hydrogel for potential corneal application. Acta Biomater. 2010;6:187–94. doi: 10.1016/j.actbio.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Engler C, Kelliher C, Spitze AR, Speck CL, Eberhart CG, Jun AS. Unfolded protein response in fuchs endothelial corneal dystrophy: a unifying pathogenic pathway? Am J Ophthalmol. 2010;149:194–202.e2. doi: 10.1016/j.ajo.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fribley A, Zhang K, Kaufman RJ. Regulation of apoptosis by the unfolded protein response. Methods Mol Biol. 2009;559:191–204. doi: 10.1007/978-1-60327-017-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong N, Ecke I, Mergler S, Yang J, Metzner S, Schu S, Volk HD, Pleyer U, Ritter T. Gene transfer of cyto-protective molecules in corneal endothelial cells and cultured corneas: analysis of protective effects in vitro and in vivo. Biochem Biophys Res Commun. 2007;357:302–7. doi: 10.1016/j.bbrc.2007.03.146. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi R, Yamato M, Takayanagi H, Oie Y, Kubota A, Hori Y, Okano T, Nishida K. Validation system of tissue-engineered epithelial cell sheets for corneal regenerative medicine. Tissue Eng Part C Methods. 2010;16:553–60. doi: 10.1089/ten.TEC.2009.0277. [DOI] [PubMed] [Google Scholar]
- 19.He Z, Pipparelli A, Manissolle C, Acquart S, Garraud O, Gain P, Thuret G. Ex vivo gene electrotransfer to the endothelium of organ cultured human corneas. Ophthalmic Res. 2010;43:43–55. doi: 10.1159/000246577. [DOI] [PubMed] [Google Scholar]
- 20.Honda N, Mimura T, Usui T, Amano S. Descemet stripping automated endothelial keratoplasty using cultured corneal endothelial cells in a rabbit model. Arch Ophthalmol. 2009;127:1321–6. doi: 10.1001/archophthalmol.2009.253. [DOI] [PubMed] [Google Scholar]
- 21.Hsu JK, Cavanagh HD, Jester JV, Ma L, Petroll WM. Changes in corneal endothelial apical junctional protein organization after corneal cold storage. Cornea. 1999;18:712–20. doi: 10.1097/00003226-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Joyce NC, Harris DL, Mc Alister JC, Ali RR, Larkin DF. Effect of overexpressing the transcription factor E2F2 on cell cycle progression in rabbit corneal endothelial cells. Invest Ophthalmol Vis Sci. 2004;45:1340–8. doi: 10.1167/iovs.03-0335. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakakis E, Philippova M, Joshi MB, Pfaff D, Bochkov V, Afonyushkin T, Erne P, Resink TJ. T-cadherin attenuates the PERK branch of the unfolded protein response and protects vascular endothelial cells from endoplasmic reticulum stress-induced apoptosis. Cell Signal. 2010;22:1308–16. doi: 10.1016/j.cellsig.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Lai JY, Li YT. Functional assessment of cross-linked porous gelatin hydrogels for bioengineered cell sheet carriers. Biomacromolecules. 2010;11:1387–97. doi: 10.1021/bm100213f. [DOI] [PubMed] [Google Scholar]
- 25.Lai JY, Lu PL, Chen KH, Tabata Y, Hsiue GH. Effect of charge and molecular weight on the functionality of gelatin carriers for corneal endothelial cell therapy. Biomacromolecules. 2006;7:1836–44. doi: 10.1021/bm0601575. [DOI] [PubMed] [Google Scholar]
- 26.Li QJ, Ashraf MF, Shen DF, Green WR, Stark WJ, Chan CC, O’Brien TP. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119:1597–604. doi: 10.1001/archopht.119.11.1597. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Sabater AL, Chen YT, Hayashida Y, Chen SY, He H, Tseng SC. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007;48:614–20. doi: 10.1167/iovs.06-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lisbona F, Hetz C. Turning off the unfolded protein response: an interplay between the apoptosis machinery and ER stress signaling. Cell Cycle. 2009;8:1643–4. [PubMed] [Google Scholar]
- 29.Lu PL, Lai JY, Ma DH, Hsiue GH. Carbodiimide cross-linked hyaluronic acid hydrogels as cell sheet delivery vehicles: characterization and interaction with corneal endothelial cells. J Biomater Sci Polym Ed. 2008;19:1–18. doi: 10.1163/156856208783227695. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 31.Mannis MJ, Miller RB, Carlson EC, Hinds D, May DR. Effect of hypothermic perfusion on corneal endothelial morphology. Br J Ophthalmol. 1983;67:804–7. doi: 10.1136/bjo.67.12.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao XR, Crowder CM. Protein misfolding induces hypoxic preconditioning via a subset of the unfolded protein response machinery. Mol Cell Biol. 2010 doi: 10.1128/MCB.00922-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathew A, Baust JG, Van Buskirk RG. Optimization of HypoThermosol® for the hypothermic storage of cardiomyocytes--Addition of EDTA. In Vitro Toxicology. 1997;10:407–415. [Google Scholar]
- 34.Mathew AJ, Baust JM, Van Buskirk RG, Baust JG. Cell preservation in reparative and regenerative medicine: evolution of individualized solution composition. Tissue Eng. 2004;10:1662–1671. doi: 10.1089/ten.2004.10.1662. [DOI] [PubMed] [Google Scholar]
- 35.Mathew AJ, Hollister WR, Addona T, Baust JG, Van Buskirk RG. Vitamin E and EDTA Improve the Efficacy of Hypothermosol-Implication of Apoptosis. In Vitr Mol Toxicol. 1999;12:163–172. [PubMed] [Google Scholar]
- 36.Mathew AJ, Van Buskirk RG, Baust JG. Improved Hypothermic Preservation of Human Renal Cells Through Suppression of Both Apoptosis and Necrosis. Cell Preservation Technology. 2002;1:239–253. [Google Scholar]
- 37.McAlister JC, Joyce NC, Harris DL, Ali RR, Larkin DF. Induction of replication in human corneal endothelial cells by E2F2 transcription factor cDNA transfer. Invest Ophthalmol Vis Sci. 2005;46:3597–603. doi: 10.1167/iovs.04-0551. [DOI] [PubMed] [Google Scholar]
- 38.Nejepinska J, Juklova K, Jirsova K. Organ culture, but not hypothermic storage, facilitates the repair of the corneal endothelium following mechanical damage. Acta Ophthalmol. 2010;88:413–9. doi: 10.1111/j.1755-3768.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 39.Nelson LR, Hodge DO, Bourne WM. In vitro comparison of Chen medium and Optisol-GS medium for human corneal storage. Cornea. 2000;19:782–7. doi: 10.1097/00003226-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Ohno K, Nelson LR, Mitooka K, Bourne WM. Transplantation of cryopreserved human corneas in a xenograft model. Cryobiology. 2002;44:142–9. doi: 10.1016/s0011-2240(02)00016-0. [DOI] [PubMed] [Google Scholar]
- 41.Pels E, Beele H, Claerhout I. Eye bank issues: II. Preservation techniques: warm versus cold storage. Int Ophthalmol. 2008;28:155–63. doi: 10.1007/s10792-007-9086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pels E, Rijneveld WJ. Organ culture preservation for corneal tissue. Technical and quality aspects. Dev Ophthalmol. 2009;43:31–46. doi: 10.1159/000223837. [DOI] [PubMed] [Google Scholar]
- 43.Raeder S, Utheim TP, Utheim OA, Nicolaissen B, Roald B, Cai Y, Haug K, Kvalheim A, Messelt EB, Drolsum L, Reed JC, Lyberg T. Effects of organ culture and Optisol-GS storage on structural integrity, phenotypes, and apoptosis in cultured corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:5484–93. doi: 10.1167/iovs.07-0494. [DOI] [PubMed] [Google Scholar]
- 44.Rauen U, Kerkweg U, Wusteman MC, de Groot H. Cold-induced injury to porcine corneal endothelial cells and its mediation by chelatable iron: implications for corneal preservation. Cornea. 2006;25:68–77. doi: 10.1097/01.ico.0000167880.96439.c6. [DOI] [PubMed] [Google Scholar]
- 45.Roh DS, Cook AL, Rhee SS, Joshi A, Kowalski R, Dhaliwal DK, Funderburgh JL. DNA cross-linking, double-strand breaks, and apoptosis in corneal endothelial cells after a single exposure to mitomycin C. Invest Ophthalmol Vis Sci. 2008;49:4837–43. doi: 10.1167/iovs.08-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruusuvaara P. The fate of preserved and transplanted human corneal entothelium. Acta Ophthalmol (Copenh) 1980;58:440–53. doi: 10.1111/j.1755-3768.1980.tb05745.x. [DOI] [PubMed] [Google Scholar]
- 47.Sachs U, Goldman K, Valenti J, Kaufman HE. Corneal storage at room temperature. Arch Ophthalmol. 1978;96:1075–7. doi: 10.1001/archopht.1978.03910050595022. [DOI] [PubMed] [Google Scholar]
- 48.Sagoo P, Chan G, Larkin DF, George AJ. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Invest Ophthalmol Vis Sci. 2004;45:3964–73. doi: 10.1167/iovs.04-0439. [DOI] [PubMed] [Google Scholar]
- 49.Schroeter J, Meltendorf C, Ohrloff C, Rieck P. Influence of temporary hypothermia on corneal endothelial cell density during organ culture preservation. Graefes Arch Clin Exp Ophthalmol. 2008;246:369–72. doi: 10.1007/s00417-007-0711-5. [DOI] [PubMed] [Google Scholar]
- 50.Serbecic N, Beutelspacher SC. Anti-oxidative vitamins prevent lipid-peroxidation and apoptosis in corneal endothelial cells. Cell Tissue Res. 2005;320:465–75. doi: 10.1007/s00441-004-1030-3. [DOI] [PubMed] [Google Scholar]
- 51.Serbecic N, Beutelspacher SC. Vitamins inhibit oxidant-induced apoptosis of corneal endothelial cells. Jpn J Ophthalmol. 2005;49:355–62. doi: 10.1007/s10384-005-0209-9. [DOI] [PubMed] [Google Scholar]
- 52.Serbecic N, Lahdou I, Scheuerle A, Hoftberger R, Aboul-Enein F. Function of the tryptophan metabolite, L-kynurenine, in human corneal endothelial cells. Mol Vis. 2009;15:1312–24. [PMC free article] [PubMed] [Google Scholar]
- 53.Simmen T, Lynes EM, Gesson K, Thomas G. Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM) Biochim Biophys Acta. 2010;1798:1465–73. doi: 10.1016/j.bbamem.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinhardt RA, Alderton JM. Poloxamer 188 enhances endothelial cell survival in bovine corneas in cold storage. Cornea. 2006;25:839–44. doi: 10.1097/01.ico.0000224638.51224.c1. [DOI] [PubMed] [Google Scholar]
- 55.Taylor MJ. Biology of Cell Survival in the Cold: The Basis for Biopreservation of Tissues and Organs. In: Baust JG, Baust JM, editors. Advances in Biopreservation. CRC Press; Boca Raton: 2007. pp. 15–62. [Google Scholar]
- 56.Taylor MJ, Hunt CJ. Hypothermic preservation of corneas in a hyperkalaemic solution (CPTES): I. Short-term storage in the absence of colloid osmotic agents. Br J Ophthalmol. 1989;73:781–91. doi: 10.1136/bjo.73.10.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor MJ, Hunt CJ, Madden PW. Hypothermic preservation of corneas in a hyperkalaemic solution (CPTES): II. Extended storage in the presence of chondroitin sulphate. Br J Ophthalmol. 1989;73:792–802. doi: 10.1136/bjo.73.10.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whikehart DR, Parikh CH, Vaughn AV, Mishler K, Edelhauser HF. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol Vis. 2005;11:816–24. [PubMed] [Google Scholar]
- 59.Williams KA, Brereton HM, Coster DJ. Prospects for genetic modulation of corneal graft survival. Eye (Lond) 2009;23:1904–9. doi: 10.1038/eye.2008.378. [DOI] [PubMed] [Google Scholar]
- 60.Wu KY, Wang HZ, Hong SJ. Mechanism of mitomycin-induced apoptosis in cultured corneal endothelial cells. Mol Vis. 2008;14:1705–12. [PMC free article] [PubMed] [Google Scholar]
- 61.Yan Y, Gao YY, Niu XF, Liu BQ, Zhuang Y, Wang HQ. Resveratrol-induced cytotoxicity in human Burkitt’s lymphoma cells is coupled to the unfolded protein response. BMC Cancer. 2010;10:445. doi: 10.1186/1471-2407-10-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoeruek E, Saygili O, Spitzer MS, Tatar O, Bartz-Schmidt KU, Szurman P. Human anterior lens capsule as carrier matrix for cultivated human corneal endothelial cells. Cornea. 2009;28:416–20. doi: 10.1097/ICO.0b013e31818c2c36. [DOI] [PubMed] [Google Scholar]
- 63.Yoeruek E, Spitzer MS, Saygili O, Tatar O, Biedermann T, Yoeruek E, Bartz-Schmidt KU, Szurman P. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J Cataract Refract Surg. 2008;34:2139–45. doi: 10.1016/j.jcrs.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 64.Yoeruek E, Spitzer MS, Tatar O, Biedermann T, Grisanti S, Luke M, Bartz-Schmidt KU, Szurman P. Toxic effects of recombinant tissue plasminogen activator on cultured human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:1392–7. doi: 10.1167/iovs.07-1079. [DOI] [PubMed] [Google Scholar]