Abstract
Four new eunicellin-based diterpenes, simplexins P–S (1–4), and the known compound simplexin A (5), have been isolated from the soft coral Klyxum simplex. The structures of the new metabolites were determined on the basis of extensive spectroscopic analysis, particularly 1D and 2D NMR experiments. Compounds 1 and 3–5 were shown to exhibit cytotoxicity against a limited panel of cancer cell lines, 3 being the most cytotoxic.
Keywords: soft coral, eunicellin-based diterpenes, cytotoxicity, Klyxum simplex
1. Introduction
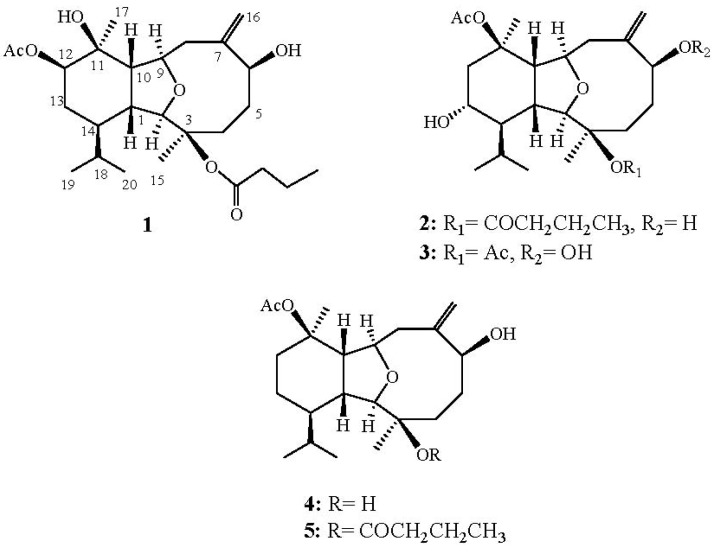
In the investigation of the bioactive metabolites from soft corals of Taiwanese waters, many bioactive eunicellin-based diterpenoids have been isolated from wild-type octocoral Pachyclavularia violacea [1,2], Cladiella australis [3], Vigularia juncea [4], Cladiella hirsute [5], Cladiella krempfi [6], Klyxum molle [7], and a cultured soft coral Klyxum simplex [8,9,10,11]. Our previous study on the secondary metabolites of a Dongsha Atoll soft coral K. simplex Thomson & Dean (Alcyonacea, Alcyoniidae) has resulted in the isolation of a series of new eunicellin-based diterpenoids, simplexins A–O [12,13]. In continuation of our search for metabolites from the Dongsha Atoll soft coral K. simplex, we have isolated another four new eunicellin-type metabolites, simplexins P–S (1–4) (Chart 1) and a known compound simplexin A (5). The structures of 1–4 were established by extensive spectroscopic analysis, including careful examination of 2D NMR (1H–1H COSY, HMQC, HMBC and NOESY) correlations. The cytotoxicity of 1–5 against human erythroleukemia (K562), human leukemia (CCRF-CEM), human breast earcinoma (T47D), and human lymphoid T (MOLT 4) cell lines was investigated. The results showed that compound 3, being the most cytotoxic, is worthy of further biomedical investigation.
Chart 1.
Structures of Metabolites 1–5.
2. Results and Discussion
Simplexin P (1) was obtained as a white powder. Its molecular formula C26H42O7 was determined by the HRESIMS (m/z 489.2827 [M + Na]+) was which deduced six degrees of unsaturation. The IR absorptions bands at νmax 3255 and 1717 cm–1 revealed the presence of hydroxy and ester carbonyl functionalities. The 13C NMR spectrum measured in CDCl3 showed signals of 26 carbons (Table 1) which were assigned as six methyls, six sp3 methylenes, one sp2 methylene, eight sp3 methines (including four oxymethines), two sp3 and three sp2 quaternary carbons (including two ester carbonyls) by DEPT. In the 13C NMR spectrum of 1, two carbonyl resonances at δ 172.6 and 170.2 ppm confirmed the presence of two ester groups. In the 1H NMR spectrum of 1 (Table 2), one acetate methyl (δ 2.12) and one n-butyryloxy [δ 0.92 (3H, t, J = 7.5 Hz), 1.60 (2H, m), and 2.13 (2H, m)] groups were observed. Moreover, two 1H NMR singlet signals at δ 5.13 and 5.46 revealed the presence of one olefinic methylene. In addition, the diagnostic signals at δ 4.17 and 3.58 implied the presence of an ether linkage between C-9 and C-2. On the basis of the above results and by the assistance of 1H–1H COSY and HMBC experiments (Figure 1), the molecular framework of 1 could be established as an eunicellin-type skeleton. Furthermore, the acetoxy group positioned at C-12 was confirmed by HMBC correlations from oxymethine [δ 4.89 (H-12)] and acetate methyl (δ 2.12) to the ester carbonyl carbon at δ 170.2 (C). Thus, the remaining one n-butyryloxy group was located at C-3, an oxygen-bearing quaternary carbon resonating at δ 84.4 ppm. On the basis of the above analysis, the planar structure of 1 was established unambiguously.
Table 1.
13C NMR data for compounds 1–4a.
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 41.7 (CH) b | 43.0 (CH) | 43.1 (CH) | 41.5 (CH) |
| 2 | 89.8 (CH) | 91.6 (CH) | 91.4 (CH) | 91.4 (CH) |
| 3 | 84.4 (C) | 84.5 (C) | 84.6 (C) | 74.1 (C) |
| 4 | 28.7 (CH2) | 29.4 (CH2) | 30.0 (CH2) | 35.2 (CH2) |
| 5 | 35.3 (CH2) | 35.4 (CH2) | 30.0 (CH2) | 35.0 (CH2) |
| 6 | 73.0 (CH) | 73.5 (CH) | 87.3 (CH) | 74.1 (CH) |
| 7 | 150.3 (C) | 150.3 (C) | 145.6 (C) | 152.0 (C) |
| 8 | 41.0 (CH2) | 41.2 (CH2) | 41.9 (CH2) | 41.5 (CH2) |
| 9 | 78.4 (CH) | 79.2 (CH) | 78.9 (CH) | 78.2 (CH) |
| 10 | 50.2 (CH) | 45.5 (CH) | 45.0 (CH) | 46.4 (CH) |
| 11 | 71.1 (C) | 83.5 (C) | 83.3 (C) | 82.1 (C) |
| 12 | 75.5 (CH) | 42.2 (CH2) | 42.7 (CH2) | 32.4 (CH2) |
| 13 | 24.2 (CH2) | 66.8 (CH) | 66.8 (CH) | 18.1 (CH2) |
| 14 | 43.4 (CH) | 50.3 (CH) | 49.8 (CH) | 42.7 (CH) |
| 15 | 22.2 (CH3) | 22.7 (CH3) | 22.9 (CH3) | 27.6 (CH3) |
| 16 | 116.9 (CH2) | 117.0 (CH) | 118.2 (CH) | 117.2 (CH2) |
| 17 | 26.2 (CH3) | 25.2 (CH3) | 25.3 (CH3) | 25.4 (CH3) |
| 18 | 27.4 (CH) | 28.4 (CH) | 28.5 (CH) | 28.0 (CH) |
| 19 | 21.7 (CH3) | 24.8 (CH3) | 24.8 (CH3) | 21.8 (CH3) |
| 20 | 15.5 (CH3) | 15.8 (CH3) | 15.7 (CH3) | 15.0 (CH3) |
| 3-Ac | 22.3 (CH3) | |||
| 169.9 (C) | ||||
| 11-Ac | 22.4 (CH3) | 22.4 (CH3) | 22.6 (CH3) | |
| 170.0 (C) | 170.0 (C) | 170.3 (C) | ||
| 12-Ac | 21.2 (CH3) | |||
| 170.2 (C) | ||||
| 3- n-Butyrate | 13.6 (CH3) | 13.6 (CH3) | ||
| 18.4 (CH2) | 18.6 (CH2) | |||
| 37.3 (CH2) | 37.4 (CH2) | |||
| 172.6 (C) | 172.6 (C) |
a Spectra recorded at 125 MHz in CDCl3 at 25 °C; b Attached protons were determined by DEPT experiments.
Table 2.
1H NMR Data for compounds 1–4a.
| Position | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 2.42 dd (12.0, 7.5) b | 2.24 dd (11.5, 7.0) | 2.20 dd (12.5, 7.0) | 2.27 dd (11.5, 7.5) |
| 2 | 3.58 s | 3.59 s | 3.58 s | 3.56 s |
| 4 | 2.20 m 1.80 m |
2.17 m 1.84 m |
2.10 m 1.97 m |
1.73 m |
| 5 | 2.12 m 1.71 m |
2.10 m 1.73 m |
2.13 m 1.54 m |
2.06 m 1.95 m |
| 6 | 4.33 dd (11.0, 4.0) | 4.30 br d (10.5) | 4.62 dd (10.5, 2.0) | 4.32 br d (10.5) |
| 8 | 2.84 dd (14.0, 4.5) | 2.83 dd (14.0, 5.0) | 2.83 dd (13.5, 5.0) | 2.86 dd (13.5, 5.5) |
| 2.47 d (14.0) | 2.47 d (14.0) | 2.55 d (13.5) | 2.51 d (13.5) | |
| 9 | 4.17 dd (11.0, 4.0) | 4.13 dd (10.5, 4.5) | 4.11dd (11.0, 5.0) | 4.09 dd (10.0, 5.5) |
| 10 | 2.66 dd (11.0, 7.5) | 3.08 dd (10.5,7.5) | 3.17 dd (10.5, 7.0) | 2.96 dd (10.0, 7.5) |
| 12 | 4.89 dd (11.7, 4.2) | 1.52 m | 1.54 m | 1.44 m |
| 2.41 m | 2.34 dd (13.5, 3.5) | 2.25 m | ||
| 13 | 1.61 m 1.70 m |
3.90 ddd (15.0, 13.2, 4.5) | 3.90 ddd (16.0, 11.0, 5.0) | 1.34 m |
| 1.46 m | ||||
| 14 | 1.41 m | 1.26 m | 1.26 t (11.0) | 1.19 m |
| 15 | 1.59 s | 1.56 s | 1.53 s | 1.16 s |
| 16 | 5.13 s 5.46 s |
5.22 s 5.47 s |
5.35 s 5.46 s |
5.30 s 5.61 s |
| 17 | 1.21 s | 1.57 s | 1.58 s | 1.54 s |
| 18 | 1.92 m | 1.92 m | 1.89 m | 1.79 m |
| 19 | 0.95 d (7.0) | 1.18 d (7.0) | 1.19 d (7.0) | 0.94 d (7.0) |
| 20 | 0.83 d (7.0) | 0.96 d (7.0) | 0.97 d (7.0) | 0.78 d (7.0) |
| 3-acetate | 1.95 s | |||
| 11-acetate | 2.00 s | 2.01 s | 2.01 s | |
| 12-acetate | 2.12 s | |||
| 3-n-butyrate | 0.92 t (7.5) | 0.94 t (7.5) | ||
| 1.60 m | 1.59 m | |||
| 2.13 m | 2.15 m | |||
| 6-OOH | 7.78 s |
a Spectra recorded at 500 MHz in CDCl3 at 25 °C; b J values are (in Hz) in parentheses.
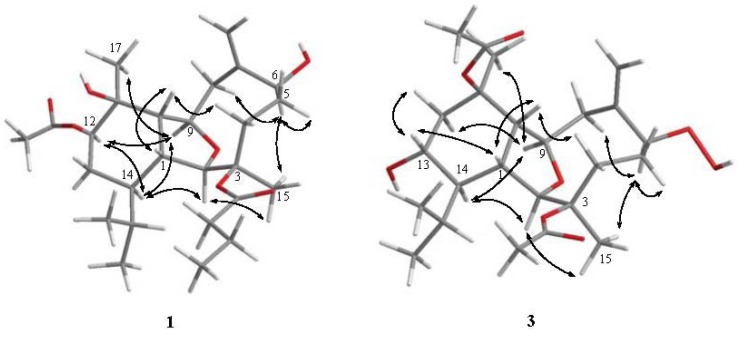
The relative configuration of 1 was determined by analysis of NOE correlations observed in the NOESY spectrum (Figure 2), which showed NOE interactions between H-1 and H-10, revealing they were both β-oriented. Also, correlations between H-2 and both H3-15 and H-14; H-14 and both H-9 and H-12; H-9 and both H-12 and H3-17; and H3-15 and H-6 suggested that of all of H-2, H-6, H-9, H-12, H-14, H3-15 and H3-17 were all α-oriented. Thus, the NOESY spectrum indicated that 1 was found to possess the (1R*, 2R*, 3R*, 6S*, 9R*, 10S*, 11S*, 12R*, 14R*)-configuration.
Figure 1.
Key 1H–1H COSY and HMBC correlations of 1 and 2.
Figure 2.
Selective NOESY correlations of 1 and 3.
Compound 2, simplexin Q, was assigned as the molecular formula C26H42O7 from its HRESIMS data, appropriate for six degrees of unsaturation. 1H and 13C NMR spectral data of 2 (Table 1 and Table 2) also showed the presence of one acetoxy group (δC 170.0, C; 22.4, CH3; δH 2.00, 3H, s) and one n-butyryloxy group (δC 172.6, C; 37.4, CH2; 18.6, CH2; 13.6, CH3; δH 2.15, 2H, m; 1.59, 2H, m; 0.94, 3H, t, J = 7.5 Hz). Comparison of the NMR data of 2 with those of the known compound simplexin A (5) [12], revealed that the only difference was the presence of an oxymethine (δH 3.90; δC 66.8) at C-13 in 2, instead of the methylene (δH 2.27 and 1.44; δC 18.1) in 5 arising from the substitution of a hydroxy moiety at C-13 in 2, instead of a methylene moiety at the same carbon in 1. Furthermore, the molecular framework was also established by 1H–1H COSY and HMBC experiments (Figure 1). The relative configuration of 2, deduced using a NOESY spectrum, is similar to that of 5. In addition, H-13 was found to exhibit a NOE correlation with H-1 but not with H-14, revealing the α-orientation of the hydroxyl group at C-13. Therefore, the structure of 2 was found to possess the (1R*, 2R*, 3R*, 6S*, 9R*, 10S*, 11R*, 13R*, 14R*)-configuration.
Simplexin R (3), isolated as a white powder, was assigned a molecular formula C24H38O8 from high resolution ESIMS analysis. The presence of the acetate groups was indicated by IR absorption at 1733 cm–1, 1H NMR signals (Table 2) at δ 1.95 (s, 3H) and 2.01 (s, 3H) and 13C NMR (Table 1) signals at δ 22.3 (CH3), 22.4 (CH3), 169.9 (C) and 170.0 (C). The NMR spectral data of 3 showed the presence of a 1,1-disubstituted carbon–carbon double bond (δC 118.2, CH2 and 145.6, C; δH 5.35, s and 5.46, s) and a hydroperoxy proton (δH 7.78, s). Comparison of the NMR data of 3 with those of 2 revealed the replacement of the n-butyryloxy moiety at C-3 and the hydroxy group at C-6 in 2 by the acetoxy and the hydroperoxy groups in 3, respectively. The relative configuration of 3 was determined mainly by the assistance of the NOESY experiment. The NOE correlations of 3 indicated that 3 possessed the same configurations for each chiral center as those of 2.
Simplexin S (4) showed the pseudomolecular ion peak [M + Na]+ atm/z 403.2463 in the HRESIMS and the molecular formula was determined as C22H36O5. NMR spectroscopic data of 4 (Table 1 and Table 2) showed the presence of one acetoxy group (δC 170.3, C; 22.6, CH3; δH 2.01, 3H, s). Comparison of the NMR data of 4 with those of 5 revealed that the only difference between both compounds arises from the replacement of the hydroxy group at C-3 in 4 by one n-butyryloxy moiety in 5. The NOESY spectrum indicated that 4 was found to possess the (1R*, 2R*, 3R*, 6S*, 9R*, 10S*, 11R*, 14R*)-configuration.
The cytotoxicity of compounds 1–5 against the proliferation of a limited panel of cancer cell lines, including K562, CCRF-CEM, T47D, and MOLT 4 was evaluated by the Alamar Blue assay, using 5-fluorouracil as a positive control. It was found that 3 showed activity against the proliferation of K-562, CCRF-CEM, T47D, and MOLT 4 cancer cells (ED50 values of 7.2 ± 2.4, 2.7 ± 0.1, 13.5 ± 2.8, and 3.8 ± 0.5 µg/mL, respectively) (Table 3).
Table 3.
Cytotoxicities of Compounds 1–5.
| Cell Lines ED50 (µg/mL) | ||||
|---|---|---|---|---|
| Compound | K-562 | CCRF-CEM | T47D | MOLT 4 |
| 1 | >20 | 12.0 ± 1.6 | >20 | 30.3 ± 3.4 |
| 2 | >20 | >20 | >20 | >20 |
| 3 | 7.2 ± 2.4 | 2.7 ± 0.1 | 13.5 ± 2.8 | 3.8 ± 0.5 |
| 4 | >20 | 13.0 ± 0.9 | >20 | 16.4 ± 3.1 |
| 5 | >20 | 17.0 ± 2.9 | >20 | 18.2 ± 2.6 |
| 5-Fluorouracil | 2.3 ± 0.2 | 1.8 ± 0.3 | 9.8 ± 1.5 | 2.3 ± 0.3 |
3. Experimental Section
3.1. General Experimental Procedures
Melting points were determined using a Fisher-Johns melting point apparatus and were uncorrected. Optical rotations were measured on a JASCO DIP-1000 digital polarimeter. IR spectra were recorded on a JASCO FT/IR-4100 infrared spectrophotometer. ESIMS were obtained with a Bruker APEX II mass spectrometer. NMR spectra were recorded on a Varian Unity INOVA 500 FT-NMR at 500 MHz for 1H and 125 MHz for 13C in CDCl3. Si gel 60 (Merck, 230–400 mesh) was used for column chromatography. Precoated silica gel plates (Merck, Kieselgel 60 F254, 0.2 mm) were used for analytical TLC. High-performance liquid chromatography was performed on a Hitachi L-7100 HPLC apparatus with a Merck Hibar Si-60 column (250 × 21 mm, 7 µm).
3.2. Animal Material
Klyxum simplex (230 g, wet wt), was collected by hand using scuba off the coast of Dongsha Atoll, in September, 2006, at a depth of 11 m, and stored in a freezer until extraction. A voucher sample (specimen No. 20060901-1) was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The frozen bodies of K. simplex (230 g, wet wt) were minced and exhaustively extracted with EtOAc (1 L × 4). The organic extract was evaporated under reduced pressure to give a residue (2.5 g) which was subjected to Si gel column chromatography and eluted with EtOAc in n-hexane (0–100%, gradient) to yield 22 fractions. Fractions 10–12 (1.05 g) eluted with EtOAc–n-hexane (1:3), were further purified over silica gel using EtOAc–n-hexane (1:3 to 1:1) to afford 46 subfractions. Subfraction 37 was also purified by normal phase HPLC using acetone–n-hexane (1:2) to afford 3 (0.9 mg, 0.036%). Fractions 13–15 (0.47 g), eluted with EtOAc–n-hexane (1:1), were further purified over silica gel using EtOAc–n-hexane (1:1) to afford 19 subfractions. Subfraction 17 was separated by normal phase HPLC using acetone–n-hexane (1:2) to yield 4 (1.6 mg, 0.064%) whilst subfraction 19 was purified by normal phase HPLC using acetone–n-hexane (1:2) to afford 2 (1.3 mg, 0.052%). Fractions 16–19 (0.51 g) eluted with EtOAc–n-hexane (2:1), were further purified over silica gel using EtOAc–n-hexane (2:1) to afford 4 subfractions. Subfraction 4 was separated by normal phase HPLC using MeOH–CH2Cl2 (1:30) to afford 1 (3.3 mg, 0.132%).
Simplexin P (1): white powder (3.3 mg); mp 179.0–180.0 °C; [α]26D = −27 (c 1.2, CHCl3); IR (neat) νmax 3255 (broad) and 1717 cm–1; 1H and 13C NMR data, see Table 1 and Table 2; ESIMS m/z 489 (100, [M + Na]+); HRESIMS m/z 489.2827 (calcd for C26H42O7Na, 489.2828).
Simplexin Q (2): colorless oil (1.3 mg); [α]26D = −11 (c 0.8, CHCl3); IR (neat) νmax 3410 (broad) and 1732 cm–1; 1H and 13C NMR data, see Table 1 and Table 2; ESIMS m/z 489 (100, [M + Na]+); HRESIMS m/z 489.2830 (calcd for C26H42O7Na, 489.2828).
Simplexin R (3): white powder (0.9 mg); mp 167–168 °C; [α]26D = −27 (c 0.4, CHCl3); IR (neat) νmax 3395 (broad) and 1733 cm–1; 1H and 13C NMR data, see Table 1 and Table 2; ESIMS m/z 477 (100, [M + Na]+); HRESIMS m/z 477.2467 (calcd for C24H38O8Na, 477.2464).
Simplexin S (4): colorless oil (1.6 mg); [α]26D = −41 (c 0.6, CHCl3); IR (neat, CHCl3) νmax 3354 (broad) and 1716 cm–1; 1H and 13C NMR data, see Table 1 and Table 2; ESIMS m/z 403 (100, [M + Na]+); HRESIMS m/z 403.2463 (calcd for C22H36O5Na, 403.2460).
3.4. Cytotoxicity Testing
Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of compounds 1–5 were performed using the Alamar Blue assay [14,15].
In previous studies, a series of new eunicellin-based diterpenoids were isolated from the cultured and wild-type soft corals Klyxum simplex. Our continued investigation on the chemical constituents of wild-type soft coral K. simplex has again led to the isolation of four new eunicellin-based diterpenoids, simplexins P–S. Simplexin R (3) exhibited significant cytotoxicity against CCRF-CEM and MOLT 4 cells, and moderate to weak cytotoxicity against K-562 and T47D cells. Also, compounds 1, 4 and 5 exhibited moderate to weak cytotoxicity toward CCRF-CEM and MOLT 4 cell lines. Besides our research, many recent studies have showed the versatile structures and bioactivities of eunicellin-type compounds [16,17,18,19,20,21]. These studies along with the new simplexins described here suggest that eunicellin-type compounds, in particular 3, are worthy of further biomedical investigation.
Acknowledgments
This work was supported by grants from the Ministry of Education (00C030205) and National Science Council of Taiwan (NSC 98-2113-M-110-002-MY3 and 100-2320-B-110-001-MY2) awarded to J.-H.S.
Supplementary Files
PDF-Document (PDF, 1673 KB)
Footnotes
Samples Availability: Not available.
References
- 1.Wang G.-H., Sheu J.-H., Chiang M.Y., Lee T.-J. Pachyclavulariaenones A–C, three novel diterpenoids from the soft coral Pachyclavularia violacea. Tetrahedron Lett. 2001;42:2333–2336. [Google Scholar]
- 2.Wang G.-H., Sheu J.-H., Duh C.-Y., Chiang M.Y. Pachyclavulariaenones D–G, new diterpenoids from the soft coral Pachyclavularia violacea. J. Nat. Prod. 2002;65:1475–1478. doi: 10.1021/np020095d. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed A.F., Wu M.-H., Wang G.-H., Wu Y.-C., Sheu J.-H. Eunicellin-based diterpenoids, australins A–D, isolated from the soft coral Cladiella australis. J. Nat. Prod. 2005;68:1051–1055. doi: 10.1021/np0500732. [DOI] [PubMed] [Google Scholar]
- 4.Su J.-H., Huang H.-C., Chao C.-H., Yan L.-Y., Wu Y.-C., Wu C.-C., Sheu J.-H. Vigulariol, a new metabolite from the sea pen Vigularia juncea. Bull. Chem. Soc. Jpn. 2005;78:877–879. doi: 10.1246/bcsj.78.877. [DOI] [Google Scholar]
- 5.Chen B.-W., Chang S.-M., Huang C.-Y., Chao C.-H., Su J.-H., Wen Z.-H., Hsu C.-H., Dai C.-F., Wu Y.-C., Sheu J.-H. Hirsutalins A–H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010;73:1785–1791. doi: 10.1021/np100401f. [DOI] [PubMed] [Google Scholar]
- 6.Tai C.-J., Su J.-H., Huang M.-S., Wen Z.-H., Dai C.-F., Sheu J.-H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs. 2011;9:2036–2045. doi: 10.3390/md9102036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu F.-J., Chen B.-W., Wen Z.-H., Huang C.-Y., Dai C.-F., Su J.-H. Klymollins A–H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle. J. Nat. Prod. 2011;74:2467–2471. doi: 10.1021/np200589n. [DOI] [PubMed] [Google Scholar]
- 8.Chen B.-W., Wu Y.-C., Chiang M.Y., Su J.-H., Wang W.-H., Fan T.-Y., Sheu J.-H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron. 2009;65:7016–7022. [Google Scholar]
- 9.Chen B.-W., Chao C.-H., Su J.-H., Wen Z.-H., Sung P.-J., Sheu J.-H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010;8:2363–2366. doi: 10.1039/b926353e. [DOI] [PubMed] [Google Scholar]
- 10.Chen B.-W., Chao C.-H., Su J.-H., Tsai C.-W., Wang W.-H., Wen Z.-H., Huang C.-Y., Sung P.-J., Wu Y.-C., Sheu J.-H. Klysimplexins I–T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2011;9:834–844. doi: 10.1039/c0ob00351d. [DOI] [PubMed] [Google Scholar]
- 11.Chen B.-W., Huang C.-Y., Wen Z.-H., Su J.-H., Wang W.-H., Sung P.-J., Wu Y.-C., Sheu J.-H. Klysimplexins U–X, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011;84:1237–1242. doi: 10.1246/bcsj.20110156. [DOI] [PubMed] [Google Scholar]
- 12.Wu S.-L., Su J.-H., Wen Z.-H., Hsu C.-H., Chen B.-W., Dai C.-F., Kuo Y.-H., Sheu J.-H. Simplexins A–I, eunicellin-based diterpenoids from the soft coral Klyxum simplex. J. Nat. Prod. 2009;72:994–1000. doi: 10.1021/np900064a. [DOI] [PubMed] [Google Scholar]
- 13.Wu S.-L., Su J.-H., Lu Y., Chen B.-W., Huang C.-Y., Wen Z.-H., Kuo Y.-H., Sheu J.-H. Simplexins J–O, eunicellin-based diterpenoids from a Dongsha Atoll soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011;84:626–632. doi: 10.1246/bcsj.20110013. [DOI] [Google Scholar]
- 14.Nakayama G.R., Caton M.C., Nova M.P., Parandoosh Z. Assessment of the Alamar Blue assayfor cellular growth and viability in vitro. J. Immunol. Methods. 1997;204:205–208. doi: 10.1016/S0022-1759(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien J., Wilson I., Orton T., Pognan F. Investigation of the Alamar Blue (resazurin)fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.-H., Hwang T.-L., Su Y.-D., Chang Y.-C., Chen Y.-H., Hong P.-H., Hu L.-C., Yen W.-H., Hsu H.-Y., Huang S.-J., et al. New 6-hydroxyeunicellins from a soft coral Cladiella sp. Chem. Pharm. Bull. 2012;60:160–163. doi: 10.1248/cpb.60.160. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.-H., Tai C.-Y., Su Y.-D., Chang Y.-C., Lu M.-C., Weng C.-F., Su J.-H., Hwang T.-L., Wu Y.-C., Sung P.-J. Discovery of New eunicellins from an Indonesian octocoral Cladiella sp. Mar. Drugs. 2011;9:934–943. doi: 10.3390/md9060934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tai C.-Y., Chen Y.-H., Hwang T.-L., Fang L.-S., Wang W.-H., Liu M.-C., Su J.-H., Wu Y.-C., Sung P.-J. Cladielloides C and D: novel eunicellin-based diterpenoids from an Indonesian octocoral Cladiella sp. Bull. Chem. Soc. Jpn. 2011;84:531–536. doi: 10.1246/bcsj.20100348. [DOI] [Google Scholar]
- 19.Chen Y.-H., Tai C.-Y., Kuo Y.-H., Kao C.-Y., Li J.-J., Hwang T.-L., Fang L.-S., Wang W.-H., Sheu J.-H., Sung P.-J. Cladieunicellins A–E, new eunicellins from an Indonesian soft coral Cladiella sp. Chem. Pharm. Bull. 2011;59:353–358. doi: 10.1248/cpb.59.353. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y.-H., Tai C.-Y., Hwang T.-L., Weng C.-F., Li J.-J., Fang L.-S., Wang W.-H., Wu Y.-C., Sung P.-J. Cladielloides A and B: new eunicellin-type diterpenoids from an Indonesian octocoral Cladiella sp. Mar. Drugs. 2010;8:2936–2945. doi: 10.3390/md8122936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan H.M., Khanfar M.A., Elnagar A.Y., Mohammed R., Shaala L.A., Youssef D.T.A., Hifnawy M.S., El Sayed K.A. Pachycladins A–E, prostate cancer invasion and migrationinhibitory eunicellin-based diterpenoids from the Red Sea soft coral Cladiella pachyclados. J. Nat. Prod. 2010;73:848–853. doi: 10.1021/np900787p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDF-Document (PDF, 1673 KB)