Abstract
To compare the chemical differences between the medicinal and cultured oyster shells, their chemical profiles were investigated. Using the ultra performance liquid chromatography-electron spraying ionization-mass spectrometry (UPLC-ESI-MS), combined with principal component analysis (PCA) and orthogonal projection to latent structures discriminant analysis (OPLS-DA), the discrimination of the chemical characteristics among the medicinal and cultured oyster shells was established. Moreover, the chemometric analysis revealed some potential key compounds. After a large-scale extraction and isolation, one target key compound was unambiguously identified as caffeine (1) based on extensive spectroscopic data analysis (1D and 2D NMR, MS, and UV) and comparison with literature data.
Keywords: oyster shell, traditional Chinese medicine, chemical profile, principal component analysis, caffeine
1. Introduction
The oyster is a traditional and popular seafood in most coastal countries and its cultivation has become an industry. Over the past twenty years, oyster cultivation has greatly developed in China. Oysters are the largest commercial molluscan group cultured in China, even in the world. According to the statistical data published by the food and agriculture organization of the United Nations (FAO), the production of oysters reached 350 metric tons in 2009 in China, accounting for 81.4% of total oyster production in the world [1]. The oyster meat is eaten, however the shells of oysters were mostly discarded. Enormous “oyster shell-mountains” can be seen in the oyster culture areas and this has become a big environmental issue. The disposal or application of the remaining shells is therefore investigated from many aspects. Actually, Ostreae concha (Pinyin: Mu-li), the shell of oysters has been widely used in traditional Chinese medicine (TCM). It was first documented in Shen Nong Ben Cao Jing and first used in prescriptions by Zhong-jing Zhang (150–219 AD). Ostreae concha is commonly used with other herbs in prescriptions to treat symptoms such as palpitations, insomnia, dizziness, tinnitus, scrofula, subcutaneous nodules, and abdominal mass. Pharmacological effects of Ostreae concha include strengthening the immune system, anti-gastric ulcer, sedation, anti-tumor, anti-virus, etc. [2,3].
The shells of three species of oyster [Crassostrea gigas Thunberg (Ostrea gigas Thunberg), Crassostrea talienwhanensis Crosse and Crassostrea rivularis Gould] are together listed in the 2010 Edition of the Chinese Pharmacopoeia (CP). The process of harvesting the medicinal materials of Ostreae concha involves the collection of oyster shells over the whole year, removal of the meat and drying the clean shell in the sun. Determination of calcium carbonate content is the only quality control measure for Ostreae concha [4]. There are no requirements for declaration of the natural source or cultured source, nor of the nuber of years of growth oysters in the CP. According to our investigation, natural perennial C. gigas is a main source for medicinal materials, while shells of edible oysters that were cultured for one to three years are seldom used in the clinic. It is still unknown whether the cultured oyster shells possess potential medicinal values or if they can replace the natural perennial ones in TCM. As different chemical compositions result in different pharmacological effects, comprehensive quality control methods for Ostreae concha should be established. In view of this situation, it is of urgent importance to clarify the active ingredients of TCM Ostreae concha and compare the ingredients between the medicinal shells and the cultured shells.
Calcium carbonate has been confirmed to be the major effective ingredient in Ostreae concha [5,6]. Its weight proportion should be above 94.0% according to the CP. However, calcium carbonate is also the most abundant component (≥95.0%) in some other marine-shell TCMs, such as Haliotidis Concha (Pinyin: Shi-Jue-Ming), Arcae concha (Pinyin: Wa-Leng-Zi), Meretricis concha (Pinyin: Ge-Ke), Cyclinae concha (Pinyin: Ge-Ke) [4]. Since the function and usage of these other marine shells are different from Ostreae concha, we therefore hypothesize that trace organic components and/or minor inorganic elements are also potential bioactive ingredients. In our research for new biologically active constituents from marine-shell TCM, Ostreae concha attracted our attention as its crude methanol extract showed good cytotoxic activity against the human hepatoma cell line BEL-7402, cervical cancer cell line HeLa and murine leukemic cell line P388 [7].
Until now, only few documents focused on the organic matrix of oyster shells [8]. The analysis of organic components of Ostreae concha remains a difficult task due to its low concentrations and high complexity. To address this problem, the ultra performance liquid chromatography-mass spectrometry (UPLC-MS) is utilized here as it is a powerful tool for analyzing complex mixtures. A typical UPLC-MS chromatogram of a complex chemical mixture contains a large amount of chemical information, provided in a relatively short time [9]. The objectives of this study were to (1) study the chemical profiles of oyster shells from different sources applying the UPLC-MS method; (2) clarify the potential key compounds differing between various sources of oyster shells based on principal component analysis (PCA) and orthogonal projection to latent structures discriminant analysis (OPLS-DA); (3) carry out a preliminary evaluation of the potential medicinal value of cultured oyster shells.
2. Results and Discussion
2.1. Chemical Profiles Analysis
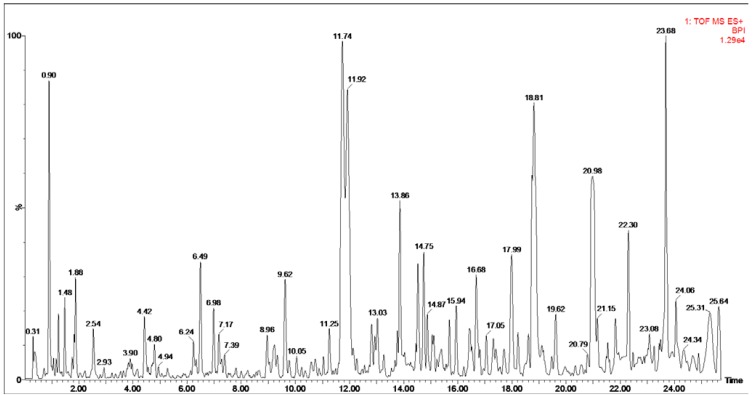
Twenty-four oyster shell samples purchased from different drugstores, aquatic product markets and farms were obtained and prepared for UPLC-MS analysis (Table 1). Both positive and negative ion modes of electrospray interface (ESI) were used in UPLC-MS analysis to result in exhaustive information. After optimization of chromatographic parameters, 459 to 562 masses in positive ion mode and 432 to 595 masses in negative ion mode were recorded in base peak intensity (BPI) chromatograms of each oyster shell. According to the extracted mass-retention time pairs (EMRT) and the intensity of peaks in the BPI chromatograms (Figure 1,others are provided as supplementary files), the 24 shell samples were dissimilar due to the chemical diversity.
Table 1.
Oyster shellsamples.
| No. | Source | Time | Site | Growth Years | Species |
|---|---|---|---|---|---|
| A1 | Medicinal materials a | August, 2008 | Anguo, Hebei | unknown b | c |
| A2 | December, 2009 | Bozhou, Anhui | perennial | ||
| A3 | September, 2010 | Bozhou, Anhui | unknown b | ||
| A4 | May, 2010 | Yishui, Shandong | perennial | ||
| A5 | October, 2010 | Heze, Shandong | perennial | ||
| A6 | August, 2009 | Kaifeng, Henan | unknownb | ||
| A7 | February, 2010 | Qingdao, Shandong | unknown b | ||
| A8 | September,2009 | Weifang, Shandong | perennial | ||
| A9 | October, 2010 | Beijing | unknown b | ||
| A10 | June, 2010 | Wuhan, Hubei | perennial | ||
| A11 | May, 2010 | Anguo, Hebei | perennial | ||
| A12 | October, 2011 | Anguo, Hebei | perennial | ||
| B1 | Cultured materials d | October, 2009 | Rongcheng, Shandong | 2 | C. gigas |
| B2 | October, 2008 | Rongcheng, Shandong | 1 | C. gigas | |
| B3 | October, 2009 | Qingdao, Shandong | 1 | C. gigas | |
| B4 | October, 2009 | Yantai, Shandong | 1 | C. gigas | |
| B5 | October, 2009 | Rushan, Shandong | 1 | C. gigas | |
| B6 | October, 2009 | Lianyungang, Jiangsu | 2 | C. gigas | |
| B7 | October, 2010 | Rongcheng, Shandong | 2 | C. talienwhanensis | |
| B8 | October, 2009 | Rongcheng, Shandong | 3 | C. talienwhanensis | |
| B9 | June, 2010 | Zhanjiang, Guangdong | 2 | C. rivularis | |
| B10 | June, 2010 | Lianyungang, Jiangsu | 3 | C.plicatula | |
| B11 | October, 2008 | Rongcheng, Shandong | 1.5 | C. gigas | |
| B12 | October, 2009 | Rongcheng, Shandong | 1.5 | C. talienwhanensis |
a Purchased from drugstores; b Obtained as crude decoction pieces; c Identification not reliable; d Purchased from aquatic product markets and farms.
Figure 1.
Base peak intensity (BPI) chromatogram of sample A11 in positive ion mode. Column: Waters ACQUITYTM UPLC BEH C18 column (50 mm×2.1 mm i.d., 1.7 μm). Flow rate: 0.4 mL·min−1. Column temperature: 35°C. Mobile phase: a linear gradient elution with 0.1% formic acid in water and 0.1% formic acid in acetonitrile. Ion source: electrospray interface (ESI).
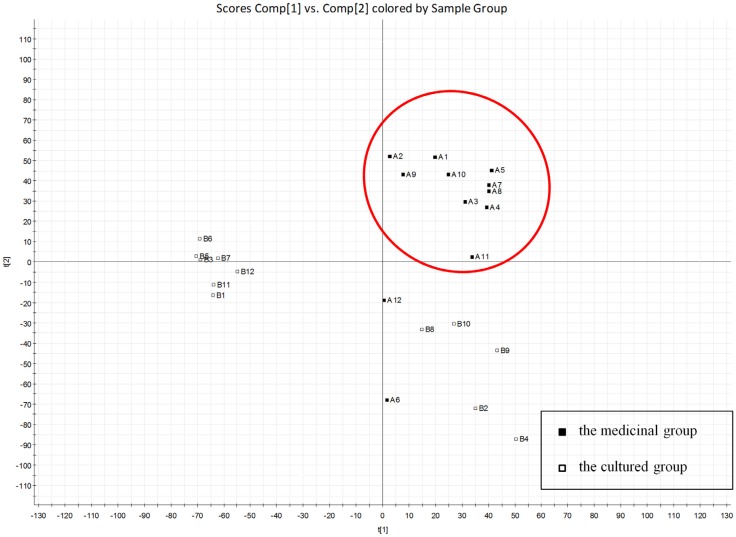
To facilitate the identification of differences or similarities among the samples, PCA was used for analyzing the chromatographic data. PCA can discriminate between sample categories by reducing the dimensions of the variables in order to simplify subsequent analysis [10]. Twenty-four samples were separated into blocks in the PCA score plots: samples A1-5, A7-11 were one group, while two different clusters of the cultured materials can be seen in the scatter plot of the positive ion mode (Figure 2). It was interesting that samples A1-5, A7-11 were medicinal shells purchased from drugstores, and samples B1-12 were cultured shells grown for 1 to 3 years. Therefore, PCA allows a clear discrimination of the chemical characteristics among perennial medicinal material shells and cultured shells based on UPLC-MS data. The scattered distribution of the cultured samples revealed that chemical differences existed within the same species even from the same geographic provenience. Ecological factors, such as number of years of growth, local environment and food chain, might contribute to the different metabolites. In addition, oyster shell is a kind of constructive material which could absorb the organic matter in sea water during its growth process. All of these elements might result in the differences of the chemical constituents observed. The deviation of samples A6 and A12 from the main trajectory in Figure 2 indicated that they might not be the perennial shells. Moreover, the results suggested that potential key compounds existed in these two groups of shells, which might be potential efficient substances and could be regarded as determination standards for the quality control of Ostreae concha.
Figure 2.
Principal component analysis (PCA) score plot of oyster shell samples in positive ion mode; for their sources refer to Table 1.
2.2. Clarification of Potential Key Compounds
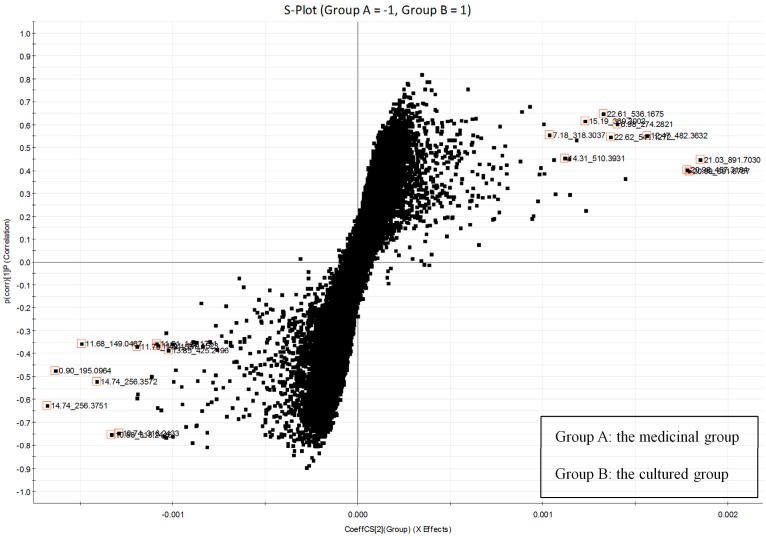
OPLS-DA was applied to the chemical profiles in order to better discriminate between perennial medicinal shells and cultured shells [11]. OPLS-DA score plots in the component P1 direction separated oyster shell samples into two blocks, and component P2 properly explained the individual variation in each group. The ions that sit at both ends of the S-plot contributed significantly to the clustering of the two groups and might be regarded as the potential key compounds (Figure 3). Furthermore, all the detected ions were arranged in descending order according to the variant weight parameters (VIP). The top twenty VIP variables were selected as potential key compounds (Table 2).
Table 2.
The top 20variant weight parameters (VIP)variables in positive ion mode.
| No. | Retention Time (min) | Mass (m/z) | VIP |
|---|---|---|---|
| 1 | 14.74 | 256.3751 | 16.22 |
| 2 | 12.47 | 482.3632 | 15.23 |
| 3 | 6.98 | 274.2821 | 13.12 |
| 4 | 14.74 | 256.3572 | 13.08 |
| 5 | 10.88 | 318.2416 | 12.49 |
| 6 | 11.73 | 149.1551 | 12.12 |
| 7 | 10.74 | 318.2433 | 12.03 |
| 8 | 21.03 | 891.703 | 11.86 |
| 9 | 15.19 | 369.3002 | 11.48 |
| 10 | 11.68 | 149.0467 | 11.41 |
| 11 | 11.91 | 149.1791 | 11.40 |
| 12 | 11.91 | 149.1523 | 11.20 |
| 13 | 22.61 | 536.1675 | 11.06 |
| 14 | 14.31 | 510.3931 | 11.02 |
| 15 | 20.99 | 891.6757 | 10.99 |
| 16 | 20.96 | 457.3184 | 10.98 |
| 17 | 0.90 | 195.0964 | 10.97 |
| 18 | 22.62 | 541.1272 | 10.83 |
| 19 | 13.85 | 425.2196 | 10.21 |
| 20 | 7.18 | 318.3037 | 10.17 |
Figure 3.
Orthogonal projection to latent structures discriminant analysis (OPLS-DA) S-plot of oyster shell samples in positive ion mode (group A: the medicinal group; group B: the cultured group). Each point represents an individual extracted mass-retention time pair (EMRT). The Y-axis denotes confidence of a marker’s contribution to the group differences, and the X-axis denotes the contribution of a particular marker to the group differences. The top 20 VIP variables were marked in the S-plots.
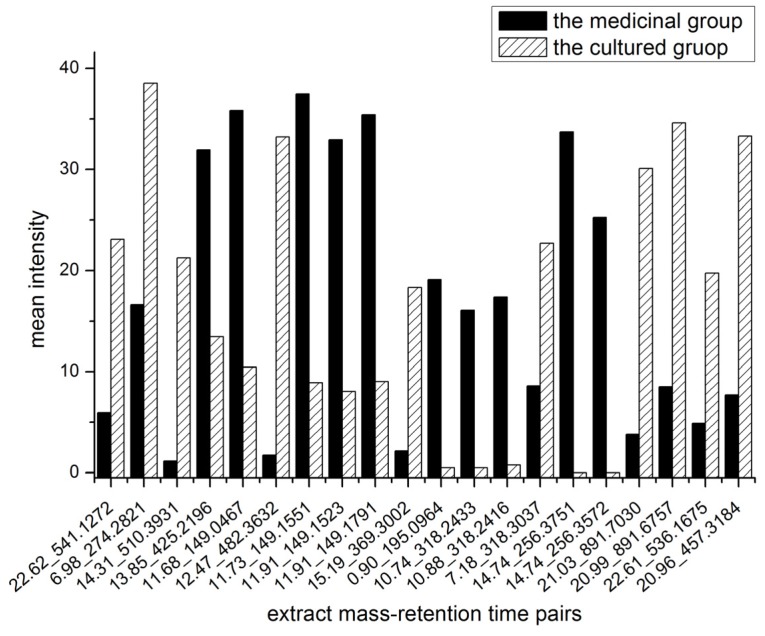
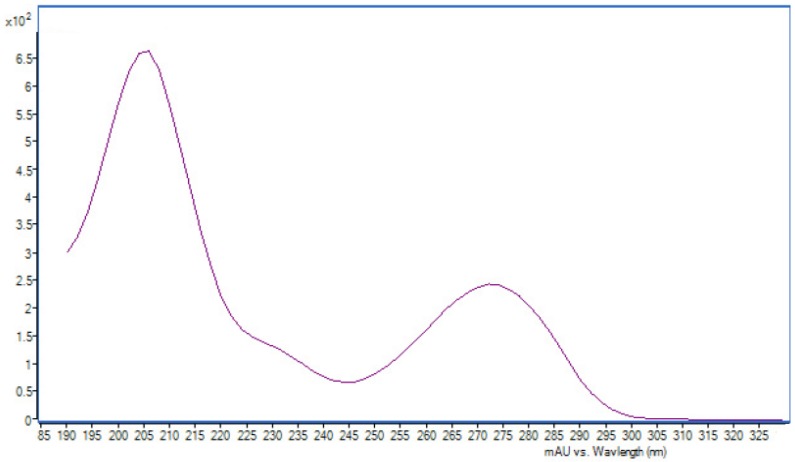
It is difficult to identify all the key compounds due to the very limited database on the chemical constituents of oyster shells. Natural product isolation is still an inefficient strategy to solve this problem, since the challenge of the low content of the chemical constituents is hard to overcome. After a detailed comparison of the mean peak areas of the potential key compounds (Figure 4), five EMRT pairs that existed mainly in perennial medicinal shells were found. They were 0.90_195.0964, 10.74_318.2433, 10.88_318.2416, 14.74_256.3572 and 14.74_256.3751 in positive mode. With comprehensive analyses of the UPLC-DAD chromatograms, the compound (0.90_195.0964) with UV absorption at 205, 274 nm (Figure 5) was suggested to be separable.
Figure 4.
Relative contents of the potential key compounds in medicinal shells and cultured shells in positive ion mode.
Figure 5.
The ultraviolet spectrum of compound 1.
2.3. Isolation and Identification of Caffeine
The target compound (0.90_195.0964, 1) was obtained through repeated chromatographic methods including semi-preparative HPLC from perennial medicinal material of oyster shells.
Compound 1 was isolated as white needles. The molecular formula C8H10N4O2 was determined from positive HR-ESI-MS (m/z 195.0882 [M + H]+, calc. 195.0886) in association with NMR data. The 1H NMR spectrum in DMSO-d6 exhibited one proton at δH 8.00 (s, H-8), three methyl singles at δH 3.87 (s, N7-CH3), 3.40 (s, N3-CH3) and 3.21 (s, N1-CH3). The 13C NMR spectrum displayed in total 8 resonances, including four carbons in 140 to 155 ppm [C-6 (δC 154.6), C-2 (δC 151.1), C-4 (δC 148.1) and C-8 (δC 142.8)], and one carbon at δC 106.6 (C-5), in addition to three methyls at δC 33.1 (N7-CH3), 29.4 (N3-CH3) and 27.5 (N1-CH3). Based on 2D NMR data analysis (Figure 6) and by comparison with literature data [12,13], compound 1 was identified as caffeine (all spectra are provided as supplementary materials).
Figure 6.
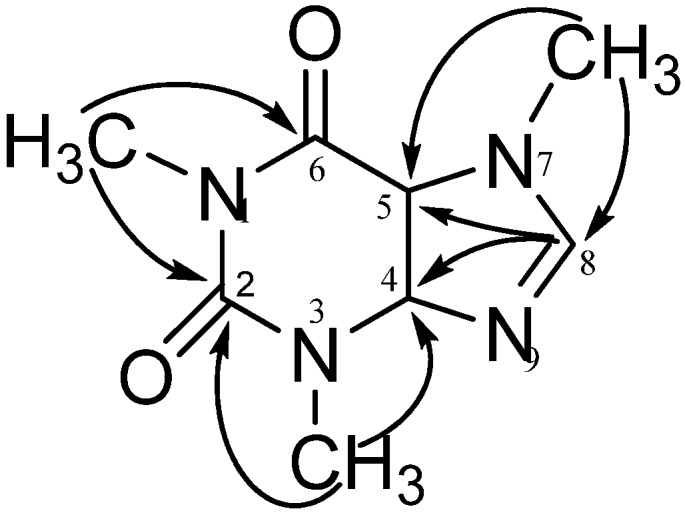
Key HMBC correlations of compound 1.
It is well known that caffeine is a plant-derived secondary metabolite and is found in coffee plants, tea trees, cocoa trees, as well as in the leaves and fruits of other plants [14,15]. Moreover, caffeine is a stimulant of the central nervous system. It is able to promote secretion of gastric acid and alleviate migraine, and plays wide-ranging roles in other systems of the body [14,16,17,18]. Our findings represent the first report of caffeine found in marine shells. Until now there is only one report that caffeine was found in marine animals and microorganisms: It was isolated from gorgonian by several research groups [19,20,21,22,23]. However, the biological origin of caffeine should be investigated. There is also a need for thorough evaluation of the action of caffeine on TCM Ostreae concha.
3. Experimental Section
3.1. General Experimental Procedures
The UPLC-MS analysis was carried out using a Waters ACQUITY™ ultra performance liquid chromatography system (Waters Corp., Milford, MA, USA) coupled with a Synapt™ Q-TOF High Definition Mass Spectrometry (Waters Corp., Milford, MA, USA). Chromatographic separations were achieved on an ACQUITY™ UPLC BEH C18 column (50 mm × 2.1 mm i.d., 1.7 μm; Waters Corp., Milford, MA, USA).
1D and 2D NMR spectra were recorded on the JEOL JNM-ECP 600 spectrometers using TMS as internal standard and chemical shifts were recorded as δ-values. ESI-MS analysis was measured on a Waters Q-TOF ULTIMA GLOBAL GAA076LC mass spectrometer. HPLC analysis was performed by using an Aglient 1100 HPLC system coupled to a photodiode array detector. Routine detection was at 210, 230, 254 and 280 nm. The separation column (250 × 4.6 mm i.d., 5 μm) was prefilled with YMC C18, and a linear gradient of 0.02% H3PO4 in H2O and MeOH was used. Semi-preparative HPLC was performed on a Shimadzu LC-6AD using a C-18 column (YMC, 250 × 10 mm i.d., 5 μm; flow rate 4.0 mL·min−1).
The reagents used for UPLC-ESI-MS measurements were of HPLC grade and were obtained from Merck Inc. (Merck KGaA, Darmstadt, Germany). All other reagents were of analytical grade (Yuwang Reagent Company, Yucheng, Shandong, China). The purified water was prepared using a Millipore water purification system (Millipore, Bedford, MA, USA). Spectral grade solvents were used for spectroscopic measurements.
3.2. Collection of Oyster Shell Samples and Preparation for UPLC-MS Analysis
Samples were purchased from different drugstores, aquatic product markets and farms in China. Macroscopic identification was applied according to CP by Dr. Hong-bing Liu (School of Medicine and Pharmacy, Ocean University of China). Voucher specimens have been deposited in the School of Medicine and Pharmacy of Ocean University of China.
After milled to 40 mesh powder, each shell sample (100 g) was refluxed with MeOH for 1.5 h (500 mL, 3×) and the combined supernatant was further concentrated to dryness under reduced pressure. Prior to UPLC analysis, the MeOH extracts of oyster shells were dissolved in acetonitrile and filtered using a 0.22 μm filter.
3.3. UPLC-MS Conditions
Chemical profiles were recorded by UPLC-MS. The column temperature was maintained at 35 °C and the injection volume was fixed at 5 μL. The binary mobile phase consisted of solvent A, composed of 0.1% formic acid in water, and solvent B, which was 0.1% formic acid in acetonitrile. Separations were performed using a linear gradient of B into A at a flow rate of 0.4 mL·min−1 as follows: 0–0.5 min, 5–25% B; 0.5–3 min, 20–30% B; 3–5 min, 30–35% B; 5–10 min, 35–49% B; 10–14 min, 49–71% B; 14–20 min, 71–88% B; 20–23 min, 88–100% B, 23–26 min, 100% B. All the samples were kept at 10 °C during the analysis.
An ESI source with a V-spray interface was used, and each sample was analyzed in both positive and negative ionization modes. The ESI-MS conditions were optimized as follows: capillary voltage, 3 kV; cone voltage, 35 kV; source temperature, 100 °C; and desolvation temperature, 350 °C. Nitrogen was used as the desolvation gas and cone gas with the flow rate of 800 and 50 L·h−1, respectively. MS data were collected in the full scan mode from m/z 100 to 1000 amu over 0 to 26 min.
In order to ensure the stability and reliability of the analysis method, each sample was analyzed in triplicate.
3.4. Data Analysis
Chemical profiles were represented by a BPI chromatogram. All the raw data were processed using the MassLynx V4.1 software with a MarkerLynx program (Waters Corp.: Milford, MA, USA, 2009), which allows the detection of the mass, retention time and intensity of the peak eluted in each chromatogram. The resulting three-dimensional data, containing retention time-extract mass pairs and normalized ion intensities were automatically exported to SIMCA-P 11.5 software (Umetrics: Umea, Sweden, 2009) for further multivariate statistical analysis by PCA and OPLS-DA.
3.5. Separation of Caffeine (1)
The large-scale of crude drug of Ostreae concha was purchased from An-guo drugmarket, Hebei province, which was identified as C. gigas according to morphological attributes. For initial analysis of the natural products, 37.5 kg milled crude drug was extracted with 80% EtOH and then desalted by CH2Cl2 extraction. The CH2Cl2 extract (1.64 g) was fractionated by Sephadex LH-20 (GE Healthcare, Sweden) eluting with CH2Cl2-MeOH (1:1) to yield 4 fractions. Fraction 2 (0.87 g) was repeatedly separated by Sephadex LH-20 eluting with MeOH and further by semi-preparative HPLC (MeOH·H2O, 25:75) to give compound 1 (7.4 mg, 1 μM per kg).
4. Conclusions
The results of this study allow a clear discrimination of the chemical characteristics among perennial medicinal shells and cultured shells based using UPLC-MS data and chemometric analysis. Considering the differences between the cultured and medicinal shells, it is considered inappropriate to replace medicinal shells with the cultured shells as an alternative resource. Further chemical works on minor inorganic elements and bioassay tests are needed to evaluate the potential medicinal values of the cultured shells. Caffeine was isolated and identified as a key compound in medicinal materials of oyster shell,but its biological origin should be investigated. Clarification of more key compounds needs further study using appropriate multi-technologies. This study preliminarily provided the basis for a needed quality standard of marine-derived TCM Ostreae concha.
Acknowledgments
This work was financially supported by a grant from the Scientific Research Foundation for the Excellent Middle-Aged and Youth Scientists of Shandong Province of China (BS2011YY065).
Supplementary Files
PDF-Document (PDF, 1147 KB)
Footnotes
Samples Availability: Available from the authors.
References
- 1.Bureau of Fisheries, Ministry of Agriculture. China Fishery Statistical Yearbook 2010. China Agriculture Press; Beijing, China: 2010. p. 26. [Google Scholar]
- 2.Guan H.S., Wang S.G. Chinese Marine Materia Medica (III) 1st Ocean Press; Beijing, China: the Science and Technology Press in Shanghai; Shanghai, China: 2009. pp. 321–348. [Google Scholar]
- 3.Zhong J.W., Chen J.W., Li X., Cai B.C., Hou K.L. Influence of oyster on the hypnotic effect of pentobarbital sodium in mice. Chin. Arch. Tradit. Chin. Med. 2009;27:499–501. [Google Scholar]
- 4.China Pharmacopoeia Committee. Chinese Pharmacopoeia (I) China Medica Science Press; Beijing, China: 2010. pp. 161–162. [Google Scholar]
- 5.Zhang H., Zhang L., Liu Y. Studies on chemical components and pharmacological activities of Os draconis (Longgu) and Ostreae concha. Chin. J. Chin. Mater. Med. 2011;36:1839–1840. [PubMed] [Google Scholar]
- 6.Chen Y.Z., Lin S. Analysis of components in Os draconis and oyster shells. J. Fujian Med. Univ. 1999;33:432–434. [Google Scholar]
- 7.Guan H.S., Liu H.B., Yang X. A method of preparation and application of the Ostreae concha extract. 201010280361.8. China Patent. 2011 Jan 26;
- 8.Choi C.S., Kim Y.W. A study of the correlation between organic matrices and nanocomposite materials in oyster shell formation. Biomaterials. 2000;21:213–222. doi: 10.1016/s0142-9612(99)00120-9. [DOI] [PubMed] [Google Scholar]
- 9.Chiuminatto U., Gosetti F., Dossetto P., Mazzucco E., Zampieri D., Robotti E., Gennaro M.C., Marengo E. Automated online solid phase extraction ultra high performance liquid chromatography method coupled with tandem mass spectrometry for determination of forty-two therapeutic drugs and drugs of abuse in human urine. Anal. Chem. 2010;82:5636–5645. doi: 10.1021/ac100607v. [DOI] [PubMed] [Google Scholar]
- 10.Gika H.G., Theodoridis G., Extance J., Edge A.M., Wilson I.D. High temperature-ultra performance liquid chromatography-mass spectrometry for the metabonomic analysis of Zucker rat urine. J. Chromatogr. B. 2008;871:279–287. doi: 10.1016/j.jchromb.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Kang J., Choi M.Y., Kang S., Kwon H.N., Wen H., Lee C.H., Park M., Wilklund S., Kim H.J., Kwon S.W., Park S. Application of a 1H nuclear magnetic resonance (NMR) metabolomics approach combined with orthogonal projections to latent structure-discriminant analysis as anefficient tool for discriminating between Korean and Chinese herbal medicines. J. Agric. Food. Chem. 2008;56:11589–11595. doi: 10.1021/jf802088a. [DOI] [PubMed] [Google Scholar]
- 12.Zhao N., Gao H.Y., Sun B.H., Wu L.J. Chemical constituents from the leaves of Camellia sinensis. J. Shenyang Pharm. Univ. 2007;24:211–214. [Google Scholar]
- 13.Sitkowski J., Stefaniak L., Nicol L., Martin M.L., Martin G.J., Webb G.A. Complete assignments of the 1H, 13C and 15N NMR spectra of caffeine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1995;51:839–841. [Google Scholar]
- 14.Daly J.W. Caffeine analogs: Biomedical impact. Cell. Mol. Life Sci. 2007;64:2153–2169. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathanson J.A. Caffeine and related methylxanthines: Possible naturally occurring pesticides. Science. 1984;226:184–187. doi: 10.1126/science.6207592. [DOI] [PubMed] [Google Scholar]
- 16.Fisone G., Borgkvist A., Usiello A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell. Mol. Life Sci. 2004;61:857–872. doi: 10.1007/s00018-003-3269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang S.S., Han K.S., Ku B.M., Lee Y.K., Hong J., Shin H.Y., Almonte A.G., Woo D.H., Brat D.J., Hwang E.M., et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res. 2010;70:1173–1183. doi: 10.1158/0008-5472.CAN-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskelinena M.H., Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimers Dis. 2010;20:S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- 19.Su J.Y., Long K.H., Jian Z.G. Chemical constituents of Chinese Gorgonia (V). A new marine C29-sterol from Echinogorgia pseudossapo (Koelliker) J. Sun Yat-sen Univ. (Nat. Sci.) 1984;1:97–101. [Google Scholar]
- 20.Imre S., Oztunc A., Celik T., Wagner H. Isolation of caffeine from the gorgonian Paramuricea chamaeleon. J. Nat. Prod. 1987;50:1187. doi: 10.1021/np50054a040. [DOI] [PubMed] [Google Scholar]
- 21.Espada A., Jimenez C., Debitus C., Riguera R. Villagorgin A and B. New type of indole alkaloids with acetylcholine antagonist activity from the gorgonian. Villagorgia rubra. Tetrahedron Lett. 1993;34:7773–7776. [Google Scholar]
- 22.Yang J., Qi S.H., Zhang S., Li Q.X. Chemical constituents from the south China sea gorgonian coral Subergorgia reticulate. J. Chin. Med. Mat. 2006;29:555–557. [PubMed] [Google Scholar]
- 23.Li T., Liao X.J., Xu S.H. Chemical constituents of gorgonian coral Paraplexaura sp. from south China sea. Chin. Pharm. J. 2010;45:420–422. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDF-Document (PDF, 1147 KB)