Abstract
One new pentacyclic sesterterpene, hippospongide A (1), and one new scalarane sesterterpenoid, hippospongide B (2), along with six previously reported known scalarane–type sesterterpenes (3–8), were isolated from a sponge Hippospongia sp. The structures of these compounds were elucidated on the basis of their spectroscopic data and comparison of the NMR data with those of known analogues. These metabolites are the first pentacyclic sesterterpene and scalarane-type sesterterpenes to be reported from this genus. Compounds 3–5 exhibited significant cytotoxicity against DLD-1, HCT-116, T-47D and K562 cancer cell lines.
Keywords: sesterterpenoid, scalarane, sponge, Hippospongia
1. Introduction
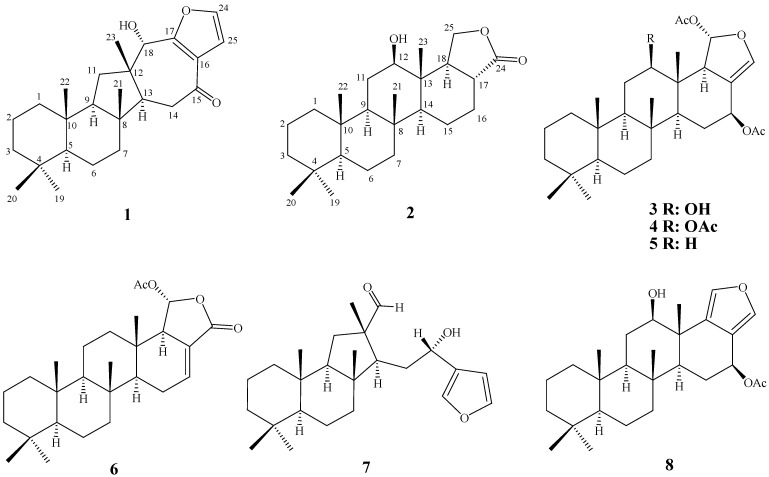
In previous reports, scalarane sesterterpenoids have been identified from sponges and nudibranchs [1]. Research into the pharmacological properties of this class of natural products is of particular interest. In fact, many scalarane metabolites show a variety of biological activities, such as antimicrobial, cytotoxic, antifeedant, ichthyotoxic, anti-inflammatory, antitubercular, platelet aggregation inhibition, RCE-protease inhibition and nerve growth factor synthesis-stimulating [1]. Our investigation of the chemical constituents of a sponge Hippospongia sp. (Figure 1) yielded one new pentacyclic sesterterpene, hippospongide A (1), and one new scalarane sesterterpenoid, hiposppongide B (2), along with six known sesterterpenoids, heteronemin (3) [2], heteronemin acetate (4) [3], hyrtiosin E (5) [4], 12-deacetoxyscalarin 19-acetate (6) [5], hyrtiosal (7) [6] and scalarafuran (8) [7]. The cytotoxicity of metabolites 1–8 against human colon adenocarcinoma (DLD-1 and HCT-116), hormone-dependent breast cancer (T-47D) and human chronic myelogenous leukemia (K562) cell lines was evaluated.
Figure 1.
Sponge Hippospongia sp.
2. Results and Discussion
The EtOAc extract of the freeze-dried specimen was fractionated by silica gel column chromatography and the eluted fractions were further separated utilizing normal phase HPLC to yield metabolites 1–8 (Chart 1).
Chart 1.
Structures of metabolites 1–8.
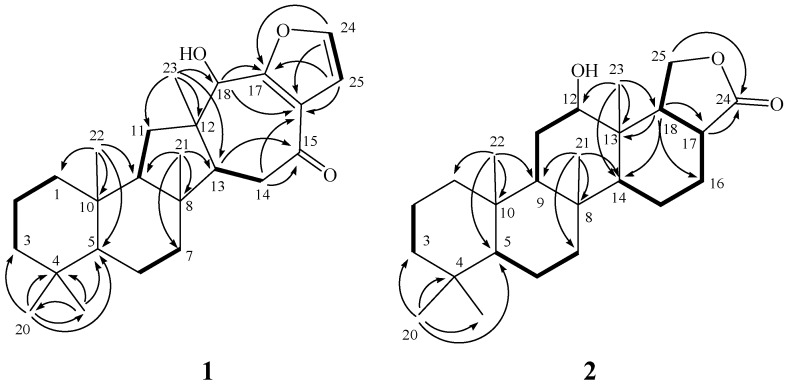
The new metabolite hippospongide A (1) had a molecular formula of C25H36O3 as determined by HRESIMS and NMR spectroscopic data. The IR spectrum of 1 showed absorption bands at 3386 cm−1, suggesting the presence of a hydroxy group. The 13C NMR data of 1 showed the presence of 25 carbons (Table 1): five methyls, seven sp3 methylenes, four sp3 methines (including one oxygenated carbon at δ 75.9), two sp2 methines, and four sp3 quaternary carbons. The remaining three signals appearing in the downfield region of the spectrum are due to the quaternary carbons of two olefinic carbons (δ 122.9 and 159.0) and one ketone carbonyl (δ 196.8). From the 1H NMR (Table 1) spectrum of 1, the 1H NMR data revealed the presence of two olefinic methine protons (δ 7.33 Hz; d, J = 1.5 Hz; 6.76 Hz; d, J = 1.5 Hz). Furthermore, one oxygenated methine (δ 4.58, s) was also designated from the 1H NMR signal. Careful analysis of the 1H–1H COSY correlations observed for 1 led to the establishment of five partial structures, as shown in Figure 2. The molecular framework of 1 was further established by a HMBC experiment (Figure 2). The five rings and their connectivities were elucidated on the basis of the following key HMBC correlations: both methyls H3-19 and H3-20 to C-3, C-4 and C-5, H3-21 to C-7, C-8, C-9 and C-13, H3-22 to C-1, C-5, C-9 and C-10, H3-23 to C-11, C-12, C-13 and C-18, H-13 to C-15, H-14 to C-15 and C-16, H-18 to C-17 and C-16, and both olefinic methines H-24 and H-25 to C-16 and C-17. Thus, 1 was found to possess two double bonds at C-16/C-17 and C-24/C-25, one hydroxy group at C-18, and one ketone group at C-15. Linking all the above functional groups to the sesterterpene skeleton thus yielded the gross structure of 1.
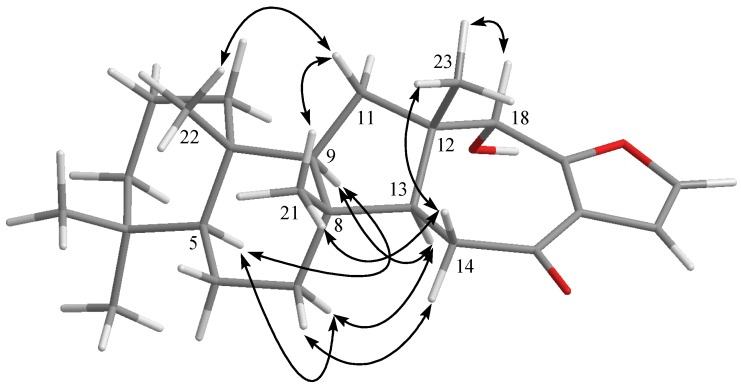
The relative configuration of 1, elucidated mainly from the NOESY spectrum, was corroborated by MM2 force field calculations, which suggested the most stable conformation to be that shown in Figure 2. In the NOESY spectrum, H-9 showed NOEs with H-5 and H-13 but not with three methyls H3-21, H3-22 and H3-23. Thus, assuming an α-orientation of H-5, both H-9 and H-13 must also be on the α face whilst the three methyls H3-21, H3-22 and H3-23 must be located on the β face. Moreover, the NOE correlations of H3-23 with H-18 indicated the β-orientation of H-18. On the basis of the above findings and other detailed NOE correlations (Figure 3), the relative structure of 1 was determined. After determining the structure of 1, we discovered that its molecular framework has been obtained as known sesterterpenoids salmahyrtisol A and similan A, which were isolated previously from sponges Hyrtios erecta [8] and Hyrtios gumminae [9], respectively.
Table 1.
1H and 13C NMR data for 1 and 2.
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) a | δC (mult.) b | δH (J in Hz) a | δC (mult.) b | |
| 1 | 1.46 m; 0.98 m | 40.2 (CH2) c | 1.65 m | 39.9 (CH2) |
| 2 | 1.65 m; 1.40 m | 18.4 (CH2) | 1.54 m; 1.38 m | 18.2 (CH2) |
| 3 | 1.38 m; 1.19 m | 42.5 (CH2) | 1.36 m; 1.12 m | 42.0 (CH2) |
| 4 | 33.1 (C) | 33.3 (C) | ||
| 5 | 0.92 m | 57.6 (CH) | 0.80 m | 56.5 (CH) |
| 6 | 1.57 m; 1.38 m | 18.8 (CH2) | 1.61 m; 1.42 m | 18.6 (CH2) |
| 7 | 1.68 m; 1.10 m | 40.1 (CH2) | 1.74 m; 0.90 m | 41.7 (CH2) |
| 8 | 44.8 (C) | 37.3 (C) | ||
| 9 | 1.45 m | 61.0 (CH) | 0.88 m | 58.9 (CH) |
| 10 | 36.8 (C) | 37.5 (C) | ||
| 11 | 1.99 d (6.0); 1.43 m | 35.0 (CH2) | 1.70 m; 1.45 m | 27.5 (CH2) |
| 12 | 43.0 (C) | 3.40 br d (10.5) | 80.5 (CH) | |
| 13 | 2.20 dd (13.0, 2.5) | 47.5 (CH) | 42.0 (C) | |
| 14 | 2.64 dd (13.5, 13.0) | 39.6 (CH2) | 0.80 m | 58.1(CH) |
| 2.54 dd (13.5, 2.5) | ||||
| 15 | 196.8 (C) | 1.78 m; 1.36 m | 20.0 (CH2) | |
| 16 | 122.9 (C) | 2.20 m; 1.22 m | 25.6 (CH2) | |
| 17 | 159.0 (C) | 2.22 m | 39.2 (CH) | |
| 18 | 4.58 s | 75.9 (CH) | 1.86 m | 55.3 (CH) |
| 19 | 0.85 s | 33.5 (CH3) | 0.84 s | 33.2 (CH3) |
| 20 | 0.84 s | 21.3 (CH3) | 0.80 s | 21.3 (CH3) |
| 21 | 0.85 s | 16.2 (CH3) | 0.84 s | 17.3 (CH3) |
| 22 | 0.87 s | 15.6 (CH3) | 0.84 s | 16.3 (CH3) |
| 23 | 1.14 s | 23.4 (CH3) | 0.91 s | 9.8 (CH3) |
| 24 | 7.33 d (1.5) | 142.3 (CH) | 177.8 (C) | |
| 25 | 6.76 d (1.5) | 110.9 (CH) | 4.38 dd (9.5, 7.0) | 70.0 (CH2) |
| 4.09 dd (11.0, 10.0) | ||||
a 500 MHz in CDCl3; b 125 MHz in CDCl3; c Numbers of attached protons were deduced by DEPT experiments.
Figure 2.
Selected 1H−1H COSY (▬) and HMBC (→) correlations of 1 and 2.
Figure 3.
Computer-generated model of 1 using MM2 force field calculations and key NOE correlations.
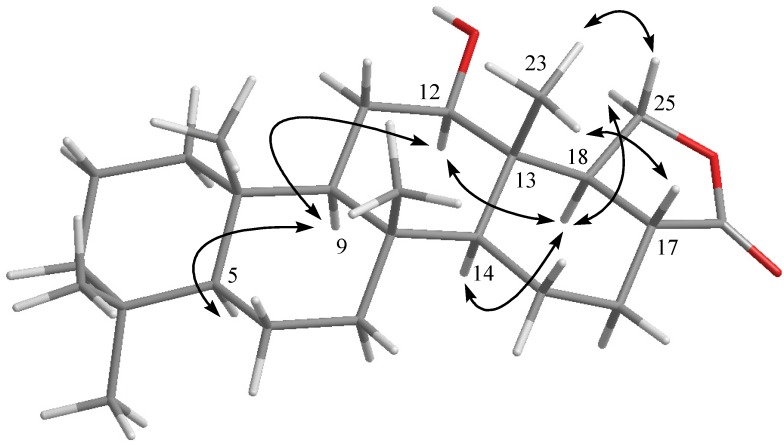
Hippospongide B (2) was isolated as a white powder with the molecular formula C25H40O3, which possesses six degrees of unsaturation, as indicated by HRESIMS (m/z 411.2878, [M + Na]+) and NMR spectroscopic data (Table 1). Moreover, it was found that the NMR data of the tricyclic skeleton (C-1 to C-14) of 2 were quite similar to those of 3 and 8, indicating the same substitution and stereochemistry at C-5, C-8, C-9, C-10, C-12, C-13 and C-14. Furthermore, analysis of the 1H–1H COSY and HMBC correlations established the remaining structure, including another two rings from C-13 to C-18 (Figure 2). Finally, the relative stereochemistries at C-17 and C-18 were resolved by careful interpretation of the NOE correlations (Figure 4). Key NOE correlations for 2 showed interactions between H-18 to H-12 and H-14. Thus, H-18 should be located on the α face. NOE correlations were also detected between H-17 and H3-23, revealing the β-orientation of H-17, as suggested by a molecular model of 2. After structural determination of 2, we found that this compound had been obtained previously by hydrogenation of the natural product hydroxylactone IV [10]. In the original report, the authors gave a planar structure. However, our study led to the isolation of 2 for the first time from natural sources. In addition, we successfully elucidated the full structure of 2. Moreover, our work also provides full assignment for the 1H and 13C NMR spectral data of 2.
Figure 4.
Key NOE correlations of 2.
The cytotoxicities of compounds 1–8 against DLD-1, HCT-116, T-47D and K562 cancer cells are shown in Table 2. The results showed that compounds 3–5 were found to exhibit cytotoxicity against all or part of the above carcinoma cell lines, while compound 3 (IC50 values 0.001, 0.001, 0.001 and 0.001 μM against the above carcinoma cell lines, respectively) was the most potent.
Table 2.
Cytotoxicity (IC50 μM) of compounds 1–5.
| Compound | Cell Lines | |||
|---|---|---|---|---|
| DLD-1 | HCT-116 | T-47D | K562 | |
| 1 | – a | – a | – a | – a |
| 2 | – a | – a | – a | – a |
| 3 | 0.001 | 0.001 | 0.001 | 0.001 |
| 4 | 2.4 | 2.7 | 0.3 | 0.05 |
| 5 | 1.1 | 8.0 | 0.7 | 0.7 |
| 6 | – a | – a | – a | – a |
| 7 | – a | – a | – a | – a |
| 8 | – a | – a | – a | – a |
| Actinomycin D | 1.9 | 0.2 | 0.6 | 0.03 |
a IC50 > 10 μM.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotation values were measured with a Jasco P-1010 digital polarimeter. IR spectra were recorded on a Varian Digilab FTS 1000 Fourier transform infrared spectrophotometer. The NMR spectra were recorded on a Varian Unity INOVA 500 FT-NMR instrument at 500 MHz for 1H NMR and 125 MHz for 13C NMR, respectively, in CDCl3. ESIMS data were obtained with a Finnigan LCQ ion-trap mass spectrometer. HRESIMS data were recorded on a LTQ Orbitrap XL mass spectrometer. Gravity column chromatography was performed on silica gel (230–400 mesh, Merck). TLC was carried out on pre-coated Kieselgel 60 F254 (0.2 mm, Merck) and spots were visualized by spraying with 10% H2SO4 solution followed by heating. High-performance liquid chromatography was performed using a system comprised of a Hitachi L-7100 pump and a Rheodyne 7725 injection port. A preparative normal phase column (250 × 21.2 mm, 5 μm) was used for HPLC.
3.2. Animal Material
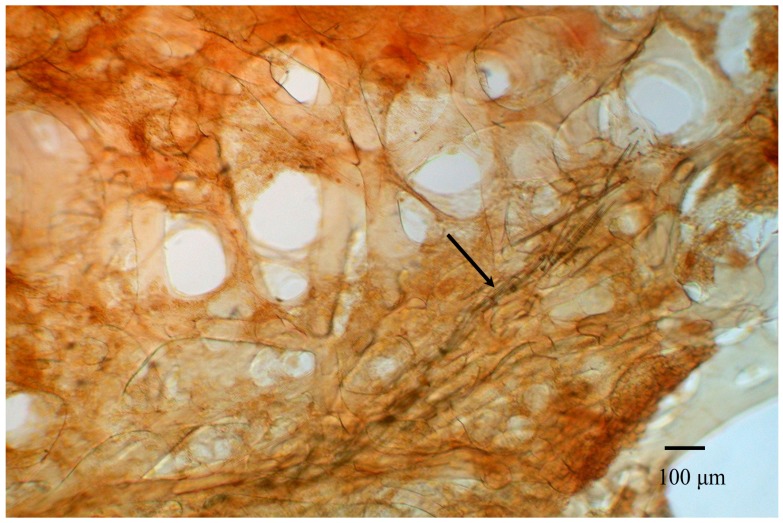
The specimen of Hippospongia sp. was collected by scuba diving at a depth of 20 m from coral reefs off the coast of Tai-tung, Taiwan. Voucher specimen was deposited in the National Museum of Marine Biology and Aquarium, Taiwan (specimen No. 2011SP-1). This genus is often confused with Hyattella (Lendenfeld, 1888), whereas Hippospongia is more elastic and compressible with fewer primary fibers (Figure 5). Taxonomic identification was performed by Li-Lian Liu of the National Sun Yat-sen University, Kaohsiung, Taiwan.
Figure 5.
Skeleton architecture of the Hippospongia sp. Arrow: foreign broken spicules in primary spongins.
3.3. Extraction and Separation
The frozen bodies of Hippospongia sp. (1.2 kg fresh wt) were collected and freeze-dried. The freeze-dried material (170 g) was minced and extracted exhaustively with EtOAc (5 × 1 L). The EtOAc extract (15.3 g) was chromatographed over silica gel by column chromatography and eluted with EtOAc in n-hexane (0–100%, stepwise), then with acetone in EtOAc (50–100%, stepwise) to yield 13 fractions. Fraction 3 (125.7 mg), eluted with n-hexane–EtOAc (10:1), was subjected to normal phase HPLC (n-hexane–EtOAc, 7:1) to afford four subfractions (A1–A4). Subfraction A4 (30.5 mg) was separated by normal phase HPLC using n-hexane–EtOAc (5:1) to afford 5 (5.9 mg, 0.039% dry wt. of extract) and 6 (2.1 mg, 0.014% dry wt. of extract). Fraction 4 (996 mg), eluting with n-hexane–EtOAc (8:1), was further purified by normal phase HPLC (n-hexane–EtOAc, 6:1) to afford five subfractions (B1–B5). Subfraction B1 (120 mg) was separated by normal phase HPLC using n-hexane–EtOAc (10:1) to afford 1 (1.7 mg, 0.011% dry wt. of extract), 7 (3.0 mg, 0.020% dry wt. of extract) and 8 (20.5 mg, 0.133% dry wt. of extract). Subfraction B2 (20 mg) was also purified by normal phase HPLC using n-hexane–EtOAc (7:1) to afford 4 (6.2 mg, 0.041% dry wt. of extract). Fraction 6 (10.5 g), eluting with n-hexane–EtOAc (3:1), was further separated by silica gel column chromatography with gradient elution (n-hexane–EtOAc, 3:1 to 1:1) to afford 3 (6 g, 39.2% dry wt. of extract). Fraction 8 (524 mg), eluted with n-hexane–EtOAc (2:1), was further separated by normal phase HPLC (n-hexane–EtOAc, 2:1) to yield six subfractions (C1–C6). Subfraction C3 was separated by normal phase HPLC using n-hexane–EtOAc (3:1) to afford 2 (0.8 mg, 0.005% dry wt. of extract).
Hippospongide A (1): white powder; mp 272–274 °C; 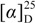 −66 (c 0.1, CHCl3); IR (neat) νmax 3386, 2922, 2854, 1715, 1642, 1455 and 1385 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 407 (100, [M + Na]+); HRESIMS m/z 407.2560 (calcd for C25H36O3Na, 407.2562).
−66 (c 0.1, CHCl3); IR (neat) νmax 3386, 2922, 2854, 1715, 1642, 1455 and 1385 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 407 (100, [M + Na]+); HRESIMS m/z 407.2560 (calcd for C25H36O3Na, 407.2562).
Hippospongide B (2): white powder; mp 289–291 °C; 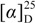 −3 (c 0.05, CHCl3); IR (neat) νmax 3436, 2927, 1753, 1461 and 1383 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 411 (80, [M + Na]+); HRESIMS m/z 411.2878 (calcd for C25H40O3Na, 411.2875).
−3 (c 0.05, CHCl3); IR (neat) νmax 3436, 2927, 1753, 1461 and 1383 cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 411 (80, [M + Na]+); HRESIMS m/z 411.2878 (calcd for C25H40O3Na, 411.2875).
Heteronmin (3): 13C NMR (CDCl3, 100 MHz) data: δ 171.3 (C, OAc), 170.1 (C, OAc), 135.3 (C, C-17), 114.4 (CH, C-24), 101.6 (CH, C-25), 80.5 (CH, C-12), 69.3 (CH, C-16), 64.1 (CH, C-18), 58.7 (CH, C-9), 56.5 (CH, C-5), 54.6 (CH, C-14), 42.7 (C, C-13), 42.0 (CH2, C-3), 41.8 (CH2, C-7), 39.9 (CH2, C-1), 38.0 (C, C-10), 37.4 (C, C-8), 33.2 (CH3, C-19), 33.2 (C, C-4), 28.0 (CH2, C-15), 27.2 (CH2, C-11), 21.3 (CH3, OAc), 21.2 (CH3, OAc), 21.0 (CH3, C-20), 18.6 (CH2, C-6), 18.1 (CH2, C-2), 17.3 (CH3, C-21), 16.3 (CH3, C-22), 8.7 (CH3, C-23). Selective 1H NMR (CDCl3, 400 MHz) data: δ 6.76 (1H, s, H-25), 6.16 (1H, s, H-24), 5.35 (1H, m, H-16), 3.42 (1H, d, J = 11.6 Hz, H-12), 2.43 (1H, s, H-18), 0.91 (3H, s, H3-21), 0.84 (6H, s, H3-19 and H3-22), 0.82 (3H, s, H-20).
Scalarafuran (8): 13C NMR (CDCl3, 125 MHz) data: δ 171.2 (C, OAc), 139.0 (CH, C-24), 137.3 (CH, C-25), 134.5 (C, C-18), 120.9 (C, C-17), 79.6 (CH, C-12), 68.1 (CH, C-16), 58.6 (CH, C-9), 56.6 (CH, C-5), 54.0 (CH, C-14), 42.0 (CH2, C-3), 41.6 (CH2, C-7), 40.1 (C, C-13), 39.8 (CH2, C-1), 37.4 (C, C-10), 37.4 (C, C-8), 33.3 (CH3, C-19), 33.2 (C, C-4), 27.8 (CH2, C-11), 24.6 (CH2, C-15), 21.3 (CH3, OAc), 21.3 (CH3, C-20), 18.8 (CH3, C-23),18.6 (CH2, C-6), 18.1 (CH2, C-2), 17.4 (CH3, C-21), 16.2 (CH3, C-22), Selective 1H NMR (CDCl3, 500 MHz) data: δ 7.53 (1H, d, J = 1.5 Hz, H-25), 7.26 (1H, s, H-24), 5.76 (1H, dd, J = 8.5, 8.0 Hz, H-16), 3.60 (1H, d, J = 10.5 Hz, H-12), 1.26 (3H, s, H3-23), 0.91 (3H, s, H3-21), 0.85 (3H, s, H3-22), 0.84 (3H, s, H3-19), 0.81 (3H, s, H3-20).
3.4. Cytotoxicity Testing
Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of compounds 1–8 were performed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method [11,12].
3.5. Molecular Mechanics Calculations
Implementation of the MM2 force filed in Chem3D Pro software [13] was used to calculate the molecular models.
4. Conclusions
Previous chemical investigations of sponges of the genus Hippospongia have led to the isolation and identification of various metabolites [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Some of these have been found to possess several kinds of biological activities, such as isocitrate lyase (ICL) inhibitory [14], RCE protease inhibitory [15] and cytotoxic [16,17,18,19,20,21] activities. In the present study, two new sesterterpenoids, hippospongides A and B (1 and 2), together with six known scalarane sesterterpenoids were isolated from the sponge Hippospongia sp. Compounds 3–5 showed significant cytoxicities against DLD-1, HCT-116, T-47D and K562 cell lines. However, the new compounds 1 and 2 and the other known compounds had no significant activity. Furthermore, it is worth mentioning that these compounds are the first pentacyclic sesterterpene and scalarane-type sesterterpenes to be reported from this genus. However, this genus is often confused with Hyattella and the sesterterpenoids are not likely to assist in chemical differentiation of the species.
Acknowledgements
This work was supported by grants from the Ministry of Education (00C030205) and National Museum of Marine Biology & Aquarium and the National Science Council (NSC 100-2320-B-291-001), Taiwan, awarded to J.-H. Su.
Supplementary Files
PDF-Document (PDF, 3039 KB)
Footnotes
Samples Availability: Not available.
References
- 1.González M.A. Scalarane sesterterpenoids. Curr. Bioact. Comp. 2010;6:178–206. [Google Scholar]
- 2.Kashman Y., Rudi A. The 13C NMR spectrum and stereochemistry of heteronemin. Tetrahedron. 1977;33:2997–2998. [Google Scholar]
- 3.Crews P., Bescansa P. Sesterterpenes from a common marine sponge, Hyrtios erecta. J. Nat. Prod. 1986;49:1041–1052. doi: 10.1021/np50048a012. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z.-G., Bi K.-S., Gue Y.-W. Hyrtiosins A–E, five new scalarane sesterterpenes from the South China Sea sponge Hyrtios erecta. HeIv. Chim. Acta. 2005;88:1004–1009. doi: 10.1002/hlca.200590070. [DOI] [Google Scholar]
- 5.Wonganuchitmeta S.-N., Yuenyongsawad S., Keawpradub N., Plubrukarn A. Antitubercular sesterterpenes from the Thai sponge Brachiaster sp. J. Nat. Prod. 2004;67:1767–1770. doi: 10.1021/np0498354. [DOI] [PubMed] [Google Scholar]
- 6.Iguchi K., Shimada Y., Yamada Y. Hyrtiosal, a new sesterterpenoid with a novel carbon skeleton from the Okinawan marine sponge Hyrtios erectus. J. Org. Chem. 1992;57:522–524. [Google Scholar]
- 7.Walker R.P., Thompson J.E., Faulkner D.J. Sesterterpenes from Spongia idia. J. Org. Chem. 1980;45:4976–4979. [Google Scholar]
- 8.Youssef D.T.A., Yamaki R.K., Kelly M., Scheuer P.J. Salmahyrtisol A, a novel cytotoxic sesterterpene from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2002;65:2–6. doi: 10.1021/np0101853. [DOI] [PubMed] [Google Scholar]
- 9.Mahidol C., Prawat H., Sangpetsiripan S., Ruchirawat S. Bioactive scalaranes from the Thai sponge Hyrtios gumminae. J. Nat. Prod. 2009;72:1870–1874. doi: 10.1021/np900267v. [DOI] [PubMed] [Google Scholar]
- 10.Fattorusso E., Magno S., Santacroce C., Sica D. Scalarin, a new pentacyclic C-25 terpenoid from the sponge Cacospongia scalaris. Tetrahedron. 1972;28:5993–5997. [Google Scholar]
- 11.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 12.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 13.Chem3D Ultra, version 9.0.1. CambridgeSoft Corporation; Cambridge, MA, USA: 2005. [Google Scholar]
- 14.Lee H.-S., Lee T.-H., Yang S.H., Shin H.J., Shin J., Oh K.-B. Sesterterpene sulfates as isocitrate lyase inhibitors from tropical sponge Hippospongia sp. Bioorg. Med. Chem. Lett. 2007;17:2483–2486. doi: 10.1016/j.bmcl.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 15.Craig K.S., Williams D.E., Hollander I., Frommer E., Mallon R., Collins K., Wojciechowicz D., Tahir A., van Soest R., Andersen R.J. Novel sesterterpenoid and norsesterterpenoid RCE-protease inhibitors isolated from the marine sponge Hippospongia sp. Tetrahedron Lett. 2002;43:4801–4808. [Google Scholar]
- 16.Liu H., Wang G., Namikoshi M., Kobayashi H., Yao X., Cai G. Sesquiterpene quinones from a marine sponge Hippospongia sp. that inhibit maturation of starfish oocytes and induce cell cycle arrest with HepG2 cells. Pharm. Biol. 2006;44:522–527. doi: 10.1080/13880200600883056. [DOI] [Google Scholar]
- 17.Shen Y.-C., Chen C.-Y., Kuo Y.-H. New sesquiterpene hydroquinones from a Taiwanese marine sponge, Hippospongia metachromia. J. Nat. Prod. 2001;64:801–803. doi: 10.1021/np000610c. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi M., Ohizumi Y., Cheng J.-f., Nakamura H., Hirata Y., Sasaki T., Kobayashi J. Metachromins A and B, novel antineoplastic sesquiterpenoids from the Okinawan sponge Hippospongia cf. metachromia. J. Org. Chem. 1988;53:2855–2858. [Google Scholar]
- 19.Musman M., Ohtani I.I., Nagaoka D., Tanaka J., Higa T. Hipposulfates A and B, new sesterterpene sulfates from an Okinawan sponge, Hippospongia cf. metachromia. J. Nat. Prod. 2001;64:350–352. doi: 10.1021/np000060i. [DOI] [PubMed] [Google Scholar]
- 20.Piao S.-J., Zhang H.-J., Lu H.-Y., Yang F., Jiao W.-H., Yi Y.-H., Chen W.-S., Lin H.-W. Hippolides A–H, acyclic manoalide derivatives from the marine sponge Hippospongia lachne. J. Nat. Prod. 2011;74:1248–1254. doi: 10.1021/np200227s. [DOI] [PubMed] [Google Scholar]
- 21.Oda T., Wang W., Fujita A., Mochizuki M., Ukai K., Namikoshi M. Promotion of IL-8 production in PMA-stimulated HL-60 cells by sesquiterpene quinones from a marine sponge, Hippospongia sp. J. Nat. Med. 2007;61:434–437. doi: 10.1007/s11418-007-0160-9. [DOI] [Google Scholar]
- 22.Madaio A., Piccialli V., Sica D., Corriero G. New polyhydroxysterols from the dictyoceratid sponges Hippospongia communis, Spongia officinalis, Ircinia variabilis, and Spongionella gracilis. J. Nat. Prod. 1989;52:952–961. doi: 10.1021/np50065a007. [DOI] [Google Scholar]
- 23.Madaio A., Notaro G., Piccialli V., Sica D. Minor 5,6-secosterols from the marine sponge Hisppospongia communis. Isolation and synthesis of (7Z,22E,24R)-24-methyl-5,6-secocholesta-7,22-diene-3β,5β,6-triol. J. Nat. Prod. 1990;53:565–572. [Google Scholar]
- 24.Cimino G., de Stefano S., Minale L. Furospongin-1, a new C-21 furanoterpene from the sponges Spongia officinalis and Hippospongia communzs. Tetrahedron. 1971;27:4673–4679. [Google Scholar]
- 25.Cimino G., de Stefano S., Minale L. Minor C-21 furanoterpenes from the sponges Spongia officinalis and Hippospongia communis. Tetrahedron. 1972;28:267–273. [Google Scholar]
- 26.Madaio A., Piccialli V., Sica D. Hipposterol, a unique trihydroxylated 5,6-secosterol from the marine sponge Hippospongia communis. Tetrahedron Lett. 1988;29:5999–6000. [Google Scholar]
- 27.Kobayashi J., Murayama T., Ohizumi Y. Metachromin C, a new cytotoxic sesquiterpenoid from the Okinawan marine sponge Hippospongia metachromia. J. Nat. Prod. 1989;52:1173–1176. doi: 10.1021/np50065a047. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi J., Naitoh K., Saaski T., Shigemori H. Metachromins D-H, new cytotoxic sesquiterpenoids from the Okinawan marine sponge Hippospongia metachromia. J. Org. Chen. 1992;57:5773–5776. [Google Scholar]
- 29.Kobayashi J., Shinonaga H., Shigemori H., Sasaki T. Untenospongin C, a new C21 furanoterpene from the Okinawan marine sponge Hippospongia sp. Chem. Pharm. Bull. 1993;41:381–382. [Google Scholar]
- 30.Rochfort S.J., Atkin D., Hobbs L., Capon R.J. Hippospongins A-F: New furanoterpenes from a Southern Australian marine sponge Hippospongia sp. J. Nat. Prod. 1996;59:1024–1028. [Google Scholar]
- 31.Kobayashi J., Ohizumi Y., Nakamura H., Hirata Y. Hippospongin, a novel furanosesterterpene possessing antispasmodic activity from the Okinawan marine sponge Hippospongia sp. Tetrahedron Lett. 1986;27:2113–2116. [Google Scholar]
- 32.Ohta S., Uno M., Tokumasu M., Hiraga Y., Ikegami S. Hippospongic acid A: An unusual triterpenoic acid from a marine sponge, Hippospongia sp., which inhibits gastrulation of starfish embryos. Tetrahedron Lett. 1996;37:7765–7766. [Google Scholar]
- 33.Guo Y.W., Trivellone E. Ent-untenospongin A, a new C21 furanoterpene from the Indian marine sponge Hippospongia sp. Chin. Chem. Lett. 2000;11:327–330. [Google Scholar]
- 34.Nakamura H., Deng S., Kobayashi J., Ohizumi Y. Dictyoceratin-A and -B, novel antimicrobial terpenoids from the Okinawan marine sponge Hippospongia sp. Tetrahedron. 1986;42:4197–4201. [Google Scholar]
- 35.Ishiyama H., Ishibashi M., Ogawa A., Yoshida S., Kobayashi J. Taurospongin A, a novel acetylenic fatty acid derivative inhibiting DNA polymerase and HIV reverse transcriptase from sponge Hippospongia sp. J. Org. Chem. 1997;62:3831–3836. [Google Scholar]
- 36.Guo Y.-W., Trivellone E. New hurgamides from a Red Sea sponge of the genus Hippospongia. J. Asian Nat. Prod. Res. 2006;2:251–256. doi: 10.1080/10286020008041363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDF-Document (PDF, 3039 KB)