Abstract
Background
Of 4,706 peripheral neuroblastic tumors (pNTs) registered on the Children’s Cancer Group and Children’s Oncology Group Neuroblastoma Study between 1989 and 2010, 51 cases (1.1%) had genotype-phenotype discordance characterized by MYCN amplification (indicating poor prognosis) and Favorable Histology (indicating better prognosis).
Procedure
To distinguish prognostic subgroups in the genotype-phenotype discordant pNTs, two subgroups, “conventional” and “bull’s eye”, were identified based on the nuclear morphology. The “conventional” tumors (35 cases) included: Neuroblastoma, Poorly differentiated subtype (NB-PD, 26 cases) with “salt-and-pepper” nuclei; Neuroblastoma, Differentiating subtype (4 cases); Ganglioneuroblastoma, Intermixed (3 cases); and Ganglioneuroma, Maturing subtype (2 cases). The “bull’s eye” tumors included NB-PD with prominent nucleoli (16 cases). Clinicopathologic characteristics of these two subgroups were analyzed. N-myc protein expression was tested immunohistochemically on available tumors.
Results
No significant difference was found between these two subgroups in the distribution of prognostic factors such as age at diagnosis, clinical stage, histopathology category/subtype, mitosis-karyorrhexis index, ploidy, 1p LOH, and unbalanced 11qLOH. However, prognosis of the patients with “conventional” tumors (5-year EFS 85.7±12.2%; OS 89.3±10.3%) was significantly better than those with “bull’s eye” tumors (EFS 31.3±13.0%; OS 42.9±16.2%) (P=0.0010 and 0.0008, respectively). Immunohistochemically all (11/11) tested “conventional” tumors were negative, and 10/11 tested “bull’s eye” tumors were positive for N-myc protein expression.
Conclusions
Based on the presence or absence of prominent nucleoli (the putative site of RNA synthesis/accumulation leading to N-myc protein expression), two prognostic subgroups, “conventional” with a better prognosis and “bull’s eye” with a poor prognosis, were distinguished among the genotype-phenotype discordant pNTs.
Keywords: neuroblastoma, International Neuroblastoma Pathology Classification, MYCN, genotype-phenotype correlation, prognosis, immunohistochemistry
INTRODUCTION
Peripheral neuroblastic tumors (pNTs; including neuroblastoma, ganglioneuroblastoma, and ganglioneuroma) offer one of the best models for investigating biologically and clinically significant relationships between molecular/genomic alterations and their morphologic/phenotypic manifestations. These tumors have well-established prognostic factors such as age at diagnosis, clinical stage, histopathology classification, MYCN status, chromosomal abnormalities, and ploidy pattern [1–10]. Among these prognostic factors, we have been investigating a reproducible correlation between MYCN status and histopathology classification [11–14].
MYCN amplification, one of the strongest indicators of aggressive tumor progression, is reported to be a powerful driving force preventing tumor maturation and increasing mitotic and karyorrhectic activities in pNTs [11,12]. Accordingly, MYCN-amplified tumors typically demonstrate the characteristic histology of neuroblastoma, undifferentiated/poorly differentiated subtype (NB-UD/PD) with a high mitosis-karyorrhexis index (MKI), and are classified into the Unfavorable Histology (UH) group. It is also reported that the presence of prominent nucleoli in neuroblastoma cells is a sign of MYCN amplification [13,14], with a high specificity and a low sensitivity, in pNTs [15].
During our central pathology review for the Children’s Cancer Group (CCG) and Children’s Oncology Group (COG) Neuroblastoma Biology Study, however, we encountered tumors in the extremely rare subset of genotype-phenotype discordance, i.e. MYCN-amplified tumors demonstrating histologic features classified into the Favorable Histology (FH) group. In this study, we summarized their clinicopathologic characteristics and reported the importance of recognizing unique nuclear morphologies and their correlation with N-myc protein expression for distinguishing two prognostic subgroups in this rare subset of pNTs. The results suggested that the presence or absence of prominent nucleoli (the putative site of RNA synthesis/accumulation), which is critical for subsequent N-myc protein expression in the neuroblastic cells, was the key for predicting the clinical behavior of the tumors in this subset.
METHODS
Patient Cohort
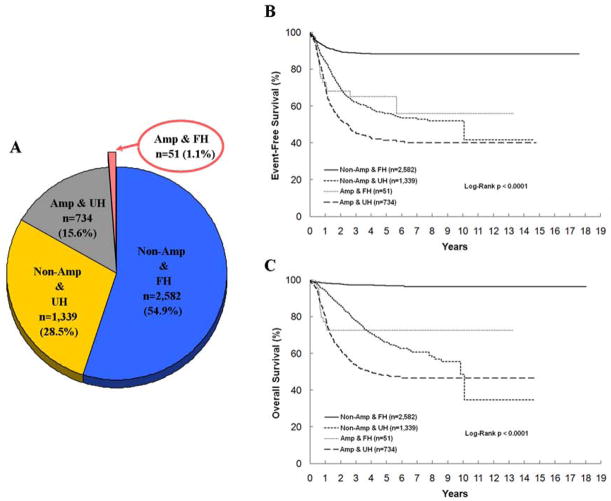
Among 5,962 cases registered in the CCG and COG Neuroblastoma Studies during a period from April 10, 1989 to May 31, 2010, a total of 4,706 cases had available data of both MYCN status (A=Amplified vs. NA=Non-Amplified) and histopathology classification (FH vs. UH) by the criteria of International Neuroblastoma Pathology Classification (INPC) [4,5]. Fifty-one cases were defined as genotype-phenotype discordant cases with the presence of A&FH tumors (1.1%; 5-year event-free survival [EFS] 65.2 ± 11.6 %, overall survival [OS] 72.6 ± 11.0%). As shown in Figure 1, there were 2,582 NA&FH (54.9%; 5-year EFS 88.3 ± 1.1%, OS 96.9 ± 0.6%), 1,339 NA&UH (28.5%; 5-year EFS 56.0 ± 2.3%, OS 66.6 ± 2.2%), and 734 A&UH (15.6%; 5-year EFS 41.4 ± 3.2%, OS 48.2 ± 3.2%) tumors.
Figure 1.
Four subsets of peripheral neuroblastic tumors by MYCN status and histopathology classification for all patients registered in the Children’s Cancer Group and the Children’s Oncology Group studies (n=4,706). A: Pie-graph illustrating the proportion of patients in those subsets with an arrow pointing to the genotype-phenotype discordant subset. B: Kaplan–Meier curve for event-free survival of patients in those subsets. C: Kaplan–Meier curve for overall survival of patients in those subsets; Non-Amp & FH, MYCN non-amplified and Favorable Histology subset; Non-Amp & UH, MYCN non-amplified and Unfavorable Histology subset; Amp & FH, MYCN amplified and Favorable Histology subset; Amp & UH, MYCN amplified and Unfavorable Histology subset.
All of those study patients were treated according to risk group classification: low-risk patients underwent primarily surgery alone; intermediate-risk patients had a moderate chemotherapy treatment in addition to biopsy/surgery; high-risk patients had intensive chemotherapy, surgery, radiation, and in many cases, myeloablative therapy as described in previous publications which included details of risk stratification and protocol assignment for the patients on CCG and COG studies [16–19]. Informed consent approved by the institutional review board was obtained for all patients at the time of enrollment on either a CCG or COG biology or therapeutic study.
MYCN Test
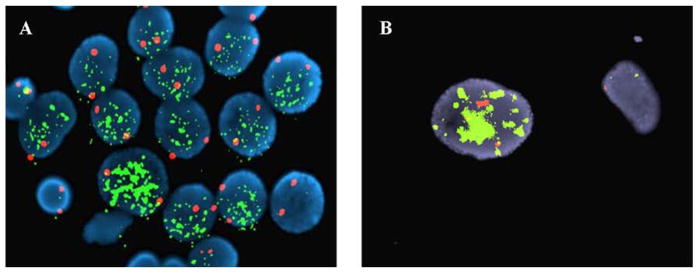
MYCN status of all the CCG and COG study cases was determined by the Reference Laboratory at St. Jude Research Hospital (Memphis, Tennessee), Dana-Farber Cancer Institute (Boston, Massachusetts), Children’s Hospital Los Angeles (Los Angeles, California), or Nationwide Children’s Hospital (Columbus, Ohio). Between 1989 and 1993, MYCN status was assessed via Southern blot analysis of gene copy numbers [6,7]. Since 1993, fluorescence in situ hybridization (FISH) has been used to analyze for MYCN gene copy numbers compared with a reference probe located on chromosome 2 [20]. In the majority of cases, the MYCN FISH test was performed on touch imprints of the snap frozen sample. A histologic section was prepared from the area where touch imprints were made and reviewed by the pathologist for confirmation. In cases where only paraffin-embedded slides were available, adjacent sections were used for FISH test and histologic review. Among the 51 genotype-phenotype discordant cases, MYCN amplification was confirmed by Southern blot analysis in 5 tumors and by FISH in 46 tumors. Figure 2 shows FISH images indicating amplified MYCN from two examples in this subset of genotype-phenotype discordance: (1) from a tumor of neuroblastoma (Schwannian stroma-poor), poorly differentiated subtype with a low MKI (Fig. 2A); and (2) from a tumor of ganglioneuroma (Schwannian stroma-dominant), maturing subtype (Fig. 2B).
Figure 2.
FISH images demonstrating MYCN amplification from the tumors in the genotype-phenotype discordant (MYCN amplified and Favorable Histology) subset. A: Tumor cells from a Neuroblastoma (Schwannian stroma-poor), poorly differentiated subtype with a low MKI tumor clearly demonstrating increased MYCN signals (green), compared to the signals of the reference probe on chromosome 2 (red), thus indicating amplification. B: Ganglioneuroma (Schwannian stroma-dominant), maturing subtype showing a ganglion cell (left) with increased MYCN signals (green) compared to the reference probe (red) and one possible Schwann cell (right) without MYCN amplification.
Pathology Review and Distinction of Two Subgroups
Pathology review of all the CCG and COG study cases was performed according to the INPC by the Neuroblastoma Pathology Reference Laboratory at the Department of Pathology and Laboratory Medicine, Children’s Hospital Los Angeles (Los Angeles, California). All pathology slides were obtained by either biopsy or surgery prior to starting chemotherapy/irradiation.
Re-review of the hematoxylin and eosin-stained (H&E) slides (1–38 slides per case; mean, 4.69; median, 2) from 51 genotype-phenotype discordant cases was conducted with a special reference to the nuclear morphology of individual tumors by the members of International Neuroblastoma Pathology Committee (GA, KB, CC, EDA, CG, JJ, VJ, SN, MP, and HS) during the meeting in Genoa, Italyin 2010, and by the reviewers at the COG Neuroblastoma Pathology Reference Laboratory (RS, LW, and HS).
The main focus was to identify the prominent nucleoli in neuroblastic cells of the tumors in this rare subset. The prominent nucleoli were identified according to our previous report as either single or few, prominent, well-defined, discrete, and eosinophilic structures found in medium-sized or large, often vesicular, and round nuclei [21]. These nucleoli should be found exclusively in the poorly differentiated neuroblasts associated with sparse or moderate amount of neuropil formation. In most of the cases, these prominent nucleoli were seen using low to medium-power magnification. In other cases, examination under an oil-immersion lens at 1,000x magnification was required for confirmation. Nucleoli in differentiating neuroblasts and ganglion cells which were related to neuronal differentiation were excluded.
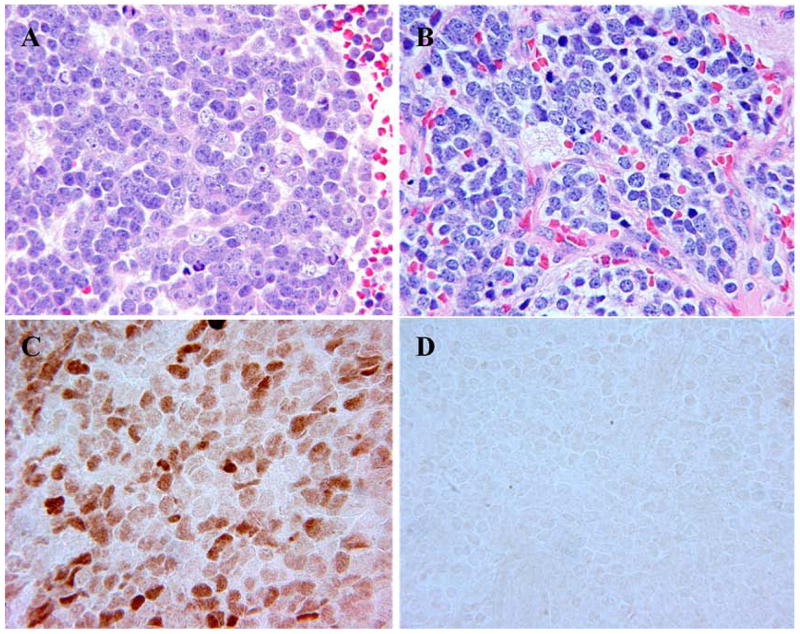
Based on the presence or absence of prominent nucleoli, two subgroups were distinguished in the genotype-phenotype discordant cases: These included (1) “bull’s eye” tumors, and (2) “conventional” tumors (Table I). The “bull’s eye” tumors were exclusively found in neuroblastoma (Schwannian stroma-poor), poorly differentiated subtype (NB-PD) with 10% or more of the neuroblastic cells containing prominent nucleoli (Fig. 3A). The “conventional” tumors included: NB-PD, typically characterized by the so-called “salt-and-pepper” nuclei (no or <10% of the neuroblastic cells with prominent nucleoli, Fig. 3B); Neuroblastoma (Schwannian stroma-poor), differentiating subtype (NB-D); Ganglioneuroblastoma, intermixed (Schwannian stroma-rich, GNB-Int); and Ganglioneuroma (Schwannian stroma-dominant), maturing subtype (GN-Mtrng). Tumors in neuroblastoma (Schwannian stroma-poor), undifferentiated subtype and those with a high MKI were not included in this study, since they were all classified into the UH group according to the INPC. No tumors in the category of ganglioneuroblastoma, nodular (Schwannian stroma-rich/stroma-dominant and stroma-poor) were found in this subset.
Table I.
Histology of pNTs with Genotype-Phenotype Discordance (n=51)
| Bull’s Eye Tumors
|
Conventional Tumors
|
||||||
|---|---|---|---|---|---|---|---|
| Category - Subtype - MKI | Age at Dx (months) | Prominent Nucleoli (%) | N-myc Protein | Category - Subtype - MKI | Age at Dx (months) | Prominent Nucleoli (%) | N-myc Protein |
| NB-PD-L | 12 | 90 | NB-PD-L | 1 | 5 | ||
| NB-PD-I | 13 | 80 | positive | NB-PD-L | 1 | 5 | negative |
| NB-PD-I | 17 | 70 | NB-PD-L | 2 | 5 | ||
| NB-PD-I | 10 | 50 | NB-PD-L | 3 | 5 | ||
| NB-PD-L | 12 | 50 | positive | NB-PD-L | 7 | 5 | |
| NB-PD-I | 10 | 40 | positive | NB-PD-I | 9 | 5 | |
| NB-PD-L | 13 | 40 | positive | NB-PD-I | 9 | 5 | |
| NB-PD-L | 3 | 30 | positive | NB-PD-I | 13 | 5 | |
| NB-PD-L | 11 | 25 | NB-PD-L | 15 | 5 | ||
| NB-PD-L | 13 | 25 | positive | NB-PD-L | 5 days | 0 | |
| NB-PD-I | 16 | 25 | NB-PD-I | 5 days | 0 | ||
| NB-PD-I | 5 | 20 | positive | NB-PD-L | 18 days | 0 | |
| NB-PD-I | 4 | 15 | negative | NB-PD-L | 1 | 0 | |
| NB-PD-I | 12 | 15 | positive | NB-PD-L | 2 | 0 | |
| NB-PD-L | 4 | 10 | positive | NB-PD-I | 3 | 0 | |
| NB-PD-L | 15 | 10 | positive | NB-PD-L | 5 | 0 | |
| NB-PD-L | 5 | 0 | |||||
| NB-PD-L | 5 | 0 | negative | ||||
| NB-PD-I | 6 | 0 | |||||
| NB-PD-L | 6 | 0 | |||||
| NB-PD-I | 6 | 0 | negative | ||||
| NB-PD-I | 7 | 0 | negative | ||||
| NB-PD-I | 8 | 0 | |||||
| NB-PD-L | 9 | 0 | negative | ||||
| NB-PD-I | 15 | 0 | |||||
| NB-PD-L | 17 | 0 | negative | ||||
| NB-D-L | 17 | 0 | negative | ||||
| NB-D-L | 17 | 0 | negative | ||||
| NB-D-L | 19 | 0 | |||||
| NB-D-L | 22 | 0 | negative | ||||
| GNB-I | 57 | 0 | |||||
| GNB-I | 70 | 0 | |||||
| GNB-I | 73 | 0 | negative | ||||
| GN-mtrng | 39 | 0 | |||||
| GN-mtrng | 74 | 0 | negative | ||||
pNTs, peripheral neuroblastic tumors; Age at Dx, age at diagnosis; NB, neuroblastoma; PD, poorly differentiated; D, differentiating; GNB-I, ganglioneuroblastoma intermixed; GN-mtrng, ganglioneuroma maturing; MKI, mitosis and karyorrhexis index; L, low; I, intermediate.
Figure 3.
A: “Bull’s eye” tumor of neuroblastoma (Schwannian stroma-poor), poorly differentiated subtype with prominent nucleoli (H&E, original 400x). B: “Conventional” tumor of neuroblastoma (Schwannian stroma-poor), poorly differentiated subtype with “salt and pepper” nuclei (H&E, original 400x). C: Nuclear staining for N-myc protein in a “bull’s eye” tumor (Immunostaining, original 400x). D: Negative staining for N-myc protein in a “conventional” tumor. (Immunostaining, original 400x).
Immunohistochemistry
N-myc protein expression was detected immunohistochemically using formalin-fixed, paraffin-embedded sections from 22 tumors based on the availability of unstained slides in the genotype-phenotype discordant cases filed at the Biopathology Center, Nationwide Children’s Hospital, Columbus, Ohio. As listed in Table 1, those cases included 11 “bull’s eye” tumors (all NB-PDs) and 11 “conventional” tumors (6 NB-PDs, 3 NB-Ds, one GNB-Int, and one GN-Mtrng). Immunostaining was performed with the polymer-based method [22]. The sections were treated with steamer antigen retrieval for 60 min in Tris-EDTA at pH8.5 and incubated with anti-MYCN polyclonal rabbit antibody (Proteintech Group, Inc., Chicago, Illinois) at a dilution of 1:50, and signals were detected with the ImmPRESS™ detection system (Vector Laboratories, Inc., Burlingame, California). Appropriate positive controls (4 A&UH tumors) and negative controls (4 NA&FH tumors, 4 NA&UH tumors, and all tumors stained without primary antibody) were also stained.
Statistical Analyses
The relationship between the two subgroups (“bull’s eye” tumors and “conventional” tumors) and prognostic factors, such as histopathology category/subtype (NB-PD, NB-D, GNB-Int, and GN-Mtrng), MKI (Low vs. Intermediate), age at diagnosis (< 18 months vs. ≥ 18 months), clinical stage by International Neuroblastoma Staging System (INSS) (1, 2, 3, 4, and 4s), ploidy (Hyperdiploid vs. Diploid), 1p [No Loss vs. Loss of heterozygosity (LOH)], and 11q (No Loss vs. Unbalanced LOH) was explored via a two-sided Fisher’s exact test. The association of the subgroups with outcome was also examined. All prognostic analyses for EFS and overall survival OS analyses from the time of diagnosis were performed using the methods of Kaplan and Meier [23] with standard errors per Peto et al [24]. Survival curves were compared using a log-rank test. Time to event was defined as the time from diagnosis until the time of first occurrence of relapse, progressive disease, secondary malignancy, or death, or until the time of last contact if no event occurred. For OS, death was the only event considered. P-values less than 0.05 were considered statistically significant. All patients with available measurements for a particular factor were included in a given analysis.
RESULTS
Among the 51 A&FH cases, 35 (69%) were “conventional” tumors and 16 (31%) were “bull’s eye” tumors. In the former subgroup, there were 26 NB-PD tumors with “salt-and-pepper” nuclei (16 tumors with a low MKI and 10 tumors with an intermediate MKI), 4 NB-D tumors (all tumors with a low MKI), 3 GNB-Int tumors, and 2 GN-Mtrng tumors. As defined above, the latter subgroup was composed entirely of NB-PD tumors (8 tumors with a low MKI and 8 tumors with an intermediate MKI). As shown in Table II, there was no statistically significant difference in distribution of prognostic factors, such as histopathology category/subtype, MKI, age at diagnosis, INSS stage, ploidy, 1p LOH and unbalanced 11q LOH, between these two subgroups in the genotype-phenotype discordant subset.
Table II.
Prognostic Factors of the Study Population and Association
| Prognostic Factor | Subgroup, No. of Patients
|
P- value | |
|---|---|---|---|
| Bull’s Eye | Conventional | ||
| Category-Subtype | |||
| NB-PD | 16 | 26 | |
| NB-D | 0 | 4 | 0.2855 |
| GNB-Int | 0 | 3 | |
| GN-Mtrng | 0 | 2 | |
| MKIa | |||
| Low | 8 | 20 | 0.347 |
| Intermediate | 8 | 10 | |
| Age at diagnosis | |||
| < 18 months | 16 | 28 | 0.0831 |
| ≥ 18 months | 0 | 7 | |
| Stageb | |||
| 1 | 1 | 9 | |
| 2 | 0 | 4 | 0.0953 |
| 3 | 2 | 5 | |
| 4 | 11 | 10 | |
| 4s | 2 | 6 | |
| Ploidyb | |||
| Hyperdiploid | 4 | 14 | 0.5059 |
| Diploid | 8 | 16 | |
| 1pc | |||
| No Loss | 2 | 7 | 0.5301 |
| LOH | 2 | 2 | |
| unbalanced 11qc | |||
| No Loss | 3 | 8 | 1 |
| LOH | 0 | 1 | |
NB-PD, neuroblastoma poorly differentiated; NB-D, neuroblastoma differentiating; GNB-Int, ganglioneuroblastoma intermixed; GN-Mtrng, ganglioneuroma maturing; MKI, mitosis and karyorrhexis index; LOH, loss of heterozygosity.
For neuroblastoma.
1 was missing stage and 9 were missing ploidy.
38 were missing 1p LOH and 39 were missing unbalanced 11q LOH, since these testings started in 2007.
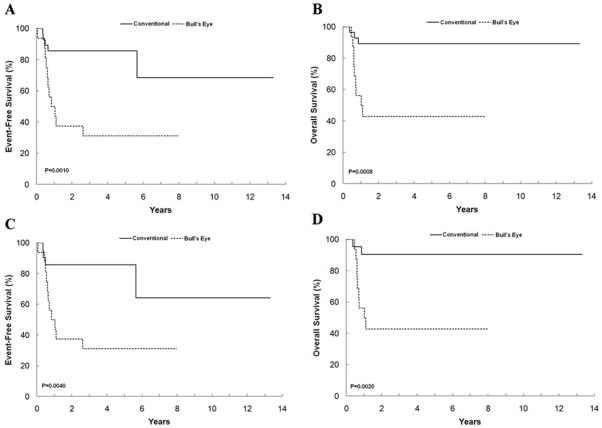
In this series, all 10 stage 1 cases were classified into the low-risk group. In contrast, all other non-stage 1 (stage 2, 3, 4, and 4S) cases were classified into the high-risk group. All 6 patients who were one year or younger at diagnosis had non-stage 1 (stage 4S – 3 cases; stage 3 – one case; stage 4 – 2 cases) disease and were also classified into the high-risk group. There was a significant difference in survival rates between these two subgroups: 5-year EFS and OS for patients with the “conventional” tumors were 85.7 ± 12.2% and 89.3 ± 10.3%, and for the patients with the “bull’s eye” tumors they were 31.3 ± 13.0% (P=0.001) and 42.9 ± 16.2% (P=0.0008), respectively (Fig. 4A, B). A significant prognostic difference was also observed between the patients with “conventional” tumors and “bull’s eye” tumors among the NB-PD subtype. As shown in Figures 4C–D, the 5-year EFS and OS for the patients with “conventional” NB-PD tumors were 85.7 ± 13.2% (P=0.004) and 90.5 ± 10.6% (P=0.002), respectively.
Figure 4.
Kaplan-Meier Curves for patients with genotype-phenotype discordance by two subgroups, “conventional” and “bull’s eye”. Event-free survival (A) and overall survival (B) for “conventional” tumors including all histologies (n=35) and “bull’s eye tumors (n=16). Event-free survival (C) and overall survival (D) for “conventional” neuroblastoma (Schwannian stroma-poor), poorly differentiated subtype tumors with “salt-and-pepper” nuclei (n=26) and for “bull’s eye” tumors (n=16).
Further analyses for the outcome comparisons (excluding the 10 Stage 1 patients, 9 of them had “conventional” tumors), also disclosed a significant difference in survival rates between the two subgroups: the 5-year EFS and OS were 85.7±14.5% and 85.7±14.5%, respectively, for patients with “conventional” tumors (n=26), and 26.7±13.2% (P=0.0012) and 38.9±17.6% (P=0.0039), respectively, for patients with “bull’s eye” tumors (n=15). A significant prognostic difference was also observed between patients with “conventional” tumors and “bull’s eye” tumors among the NB-PD subtype. Five-year EFS and OS for patients with “conventional” NB-PD tumors (n=21) were 88.2±13.5% and 88.2±13.5%, respectively, and for patients with “bull’s eye” NB-PD tumors (n=15) were 26.7±13.2% (P=0.0018) and 38.9±17.6% (P=0.0045), respectively.
As indicated in Table I, all 11 tested samples in the “conventional” tumors were negative for N-myc protein expression by immunohistochemistry, despite having MYCN amplification. In contrast, 10 of 11 tested samples in the “bull’s eye” tumors demonstrated positive N-myc protein expression in the nuclei of neuroblastoma cells. Only one “bull’s eye” tumor, in which 15% of neuroblasts had prominent nucleoli, showed negative staining for N-myc protein and the patient was reported alive with 8 years of follow-up after diagnosis. Examples of immunostaining for N-myc protein are shown in Figure 3.
DISCUSSION
This report successfully distinguishes two prognostic subgroups in genotype-phenotype discordant (A&FH) pNTs after analyzing the largest series of cases. It was noted through the CCG and COG Neuroblastoma Biology Study that the vast majority of pNTs in the FH group (2,582 of 2,633 tumors; 98.1%) had non-amplified MYCN, while MYCN amplified pNTs were almost always (734 of 785 tumors; 93.5%) classified into the UH group. pNTs with genotype-phenotype discordance, i.e., amplified MYCN (indicator of poor prognosis) and FH (indicator of better prognosis), were extremely rare and accounted for only 1.1% of all tumors. Atotal of 31 cases of A&FH pNTs, 14 of which were also included in this study, have been found in literature [25–29]. Prognosis of patients with A&FH pNTs was variable and 7 deaths were reported among those 31 cases.
MYCN amplified pNTs, typically having UH and associated with a poor clinical prognosis, are often characterized as NB-UD/PD having prominent nucleoli [13,14,21]. In the MYCN amplified pNTs, an excess amount of N-myc protein expression and subsequent MYC-MAX heterodimer formation is critical for initiating the molecular mechanism of aggressive behavior by changing the expression of multiple target genes [30–33]. RNA synthesis occurs within nucleoli, and myc transcripts have been shown to have nucleolar localization [34,35]. Thus, we hypothesize that the prominent nucleoli identified in MYCN amplified neuroblasts may result from the accumulation of increased quantities of MYCN transcripts.
The prominent nucleoli (“bull’s eye”) were seen in 31% of tumors with genotype-phenotype discordance, while the remaining 69% tumors had conventional histologic/cytologic features of NB-PD with “salt-and-pepper” nuclei, NB-D, GNB-Int, and GN-Mtrng despite MYCN amplification. Although there was no significant difference in the distribution of so-called prognostic factors between these two tumor subgroups, significantly better outcome was observed in the cohort of patients with “conventional” tumors compared to those with “bull’s eye” tumors.
The survival rates of the patients with “conventional” tumors were 85.7% EFS and 89.3% OS comparable to patients having NA&FH tumors with 88.3% EFS and 96.9% OS. Furthermore, the “conventional” tumors displayed neuroblasts with histologic evidence of age-appropriate maturation defined by INPC, from NB-PD to NB-D to GNB-Int to GN-Mtrng [4,36]. Surprisingly, none of the “conventional” tumors analyzed by immunohistochemistry expressed N-myc protein, presumably due to a lack of mRNA synthesis. This clearly indicated that the amplified MYCN gene in those tumors was not functional and also did not prevent them from proceeding through maturational sequences comparable to the biologically favorable pNTs [11,12]. In contrast, the survival rates of the patients with “bull’s eye” tumors were 31.3% EFS and 42.9% OS, similar to the outcome for patients having A&UH tumors with 41.4% EFS and 48.2%OS. All the “bull’s eye” tumors were NB-PD, and N-myc protein was detected in 10 of the 11 tumors tested, supporting our hypothesis that prominent nucleoli seen in these tumors contains MYCN transcripts.
As shown in Table I, the proportion of neuroblastic cells containing prominent nucleoli varied from tumor to tumor, especially among the “bull’s eye” tumor subgroup. This variability may be attributable to disparities in mRNA synthesis or functional status. In this study, we determined that having 10% or more “bull’s eye” cells was the optimal cut-off for distinguishing the level of N-myc protein expression and survival between the two histologic phenotypes.
The prominent nucleoli associated with neuroblastic differentiation should be carefully and strictly distinguished from the prominent nucleoli in the “bull’s eye” tumors. The nuclei seen in differentiating neuroblasts and ganglion cells are typically single and situated in the eccentrically located vesicular nuclei [4]. In those cells, both nucleolar and cytoplasmic enlargement are seen, and cytoplasmic diameter is usually more than twice the nuclear diameter.
In conclusion, among the A&FH tumors, two prognostic subgroups were identified based on the nuclear morphology and immunohistochemistry. The “conventional” tumors, where N-myc protein expression was not detected, were associated with an excellent prognosis. In contrast, the “bull’s eye” tumors with prominent nucleoli, where N-myc protein expression was observed, were associated with an aggressive behavior and a poor prognosis. Although prospective studies are needed, this histologic/immunohistochemical distinction could be useful for refining risk and treatment stratification in this rare cohort of patients.
Acknowledgments
The authors thank Xingchao Wang, BS, HT (ASCP) at Children’s Hospital Los Angeles for his technical support in immunohistochemistry. We also thank Akira Toki, MD, PhD, (Division of Pediatric Surgery, Department of Surgery, Showa University School of Medicine, Tokyo, Japan) for his encouragement and discussion during the project. Many thanks also go to Dr. Nilsa Ramirez and Ms. Wanda Smith at Nationwide Children’s Hospital for their continuous support in this project. This study was supported by the NIH grants U10 CA98413 (COG SDC grant) and U10 CA98543 (COG Chair’s grant), the Merit Award to Hiroyuki Shimada from the CHLA Research Institute and the Showa University Research Grant for Young Researchers.
Grant sponsor: National Institutes of Health; grant number: U10 CA98413 (COG SDC grant) and U10 CA98543 (COG Chair’s grant), Merit Award to Hiroyuki Shimada from the CHLA Research Institute and Showa University Research Grant for Young Researchers
Footnotes
Conflict of Interest Statement: Nothing to declare.
Presented in part at the Advances of Neuroblastoma Research Meeting 2006 in Los Angeles, California, May 17–20, 2006 and at the Society for Pediatric Pathology Fall Meeting 2011 in Milwaukee, Wisconsin, September 19 – October 2, 2011
References
- 1.Evans AE, D’Angio GJ, Propert K, et al. Prognostic factors in neuroblastoma. Cancer. 1987;59:1853–1859. doi: 10.1002/1097-0142(19870601)59:11<1853::aid-cncr2820591102>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 3.Evans AE, D’Angio GJ, Randolph J. A proposed staging for children with neuroblastoma. Cancer. 1971;27:374–378. doi: 10.1002/1097-0142(197102)27:2<374::aid-cncr2820270221>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Shimada H, Ambros IM, Dehner LP, et al. Terminology and morphologic criteria of neuroblastic tumors: Recommendations by the International Neuroblastoma Pathology Committee. Cancer. 1999;86:349–363. [PubMed] [Google Scholar]
- 5.Shimada H, Ambros IM, Dehner LP, et al. The International Neuroblastoma Pathology Classification (the Shimada System) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 6.Brodeur GM, Seeger RC, Schwab M, et al. Amplification of N-myc in untreated human neuroblastoma correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 7.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastoma. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 8.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11p deletions and outcome in neuroblastoma. N Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 9.Schwab M, Westermann F, Hero B, et al. Neuroblastoma: biology and molecular and chromosomal pathology. Lancet Oncol. 2003;4:472–480. doi: 10.1016/s1470-2045(03)01166-5. [DOI] [PubMed] [Google Scholar]
- 10.Look AT, Hayes FA, Shuster JJ, et al. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: APediatric Oncology Group study. J Clin Oncol. 1991;9:581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- 11.Shimada H, Stram DO, Chatten J, et al. Identification of subsets of neuroblastomas by combined histopathologic and N-myc analysis. J Natl Cancer Inst. 1995;87:1470–1476. doi: 10.1093/jnci/87.19.1470. [DOI] [PubMed] [Google Scholar]
- 12.Goto S, Umehara S, Gerbing RB, et al. Histopathology (International Neuroblastoma Pathology Classification) and MYCN status in patients with peripheral neuroblastic tumors. Cancer. 2001;92:2699–2708. doi: 10.1002/1097-0142(20011115)92:10<2699::aid-cncr1624>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Tornoczky T, Kalman E, Kajtar PG, et al. Large cell neuroblastoma: A distinct phenotype of neuroblastoma with aggressive clinical behavior. Cancer. 2004;100:390–397. doi: 10.1002/cncr.20005. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi C, Monforte-Munoz HL, Gerbing RB, et al. Enlarged and prominent nucleoli may be indicative of MYCN amplification: A study of neuroblastoma (Schwannian stroma–poor), undifferentiated/poorly differentiated subtype with high mitosis-karyorrhexis index. Cancer. 2005;103:174–180. doi: 10.1002/cncr.20717. [DOI] [PubMed] [Google Scholar]
- 15.Thorner PS, Ho M, Chilton-MacNeill S, et al. Use of chromogenic in situ hybridization to identify MYCN gene copy number in neuroblastoma using routine tissue sections. Am J Surg Pathol. 2006;30:635–642. doi: 10.1097/01.pas.0000202163.82525.5c. [DOI] [PubMed] [Google Scholar]
- 16.Matthay KK, Perez C, Seeger RC, et al. Successful treatment of stage III neuroblastoma based on prospective biologic staging: A Children’s Cancer Group study. J Clin Oncol. 1998;16:1256–1264. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 17.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 18.Perez CA, Matthay KK, Atkinson JB, et al. Biologic variables in the outcome of stage I and II neuroblastoma treated with surgery as primary therapy: A Children’s Cancer Group study. J Clin Oncol. 2000;18:18–26. doi: 10.1200/JCO.2000.18.1.18. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein JL, Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. Oncologist. 2003;8:278–292. doi: 10.1634/theoncologist.8-3-278. [DOI] [PubMed] [Google Scholar]
- 20.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. British Journal of Cancer. 2009;100:1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambros IM, Hata J, Joshi VV, et al. Morphologic features of neuroblastoma (Schwannian stroma-poor tumors) in clinically favorable and unfavorable groups. Cancer. 2002;94:1574–1583. doi: 10.1002/cncr.10359. [DOI] [PubMed] [Google Scholar]
- 22.Heras A, Roach CM, Key ME. Enhanced polymer detection system for immunohistochemistry. Lab Invest. 1995;72:165. (abstract) [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part II: Analysis and Examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohn SL, Look AT, Joshi VV, et al. Lack of correlation of N-myc gene amplification with prognosis in localized neuroblastoma: A Pediatric Oncology Group Study. Cancer Research. 1993;55:721–726. [PubMed] [Google Scholar]
- 26.Fabbretti G, Valenti C, Loda M, et al. N-myc gene amplification/expression in localized stroma-rich neuroblastoma (ganglioneuroblastoma) Hum Pathol. 1993;24:294–297. doi: 10.1016/0046-8177(93)90040-n. [DOI] [PubMed] [Google Scholar]
- 27.Alvarado CS, London WB, Look AT, et al. Natural history and biology of stage A neuroblastoma: A Pediatric Oncology Group study. J Pediatr Hematol Oncol. 2000;22:197–205. doi: 10.1097/00043426-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 28.George RE, Variend S, Cullinane C, et al. Relationship between histopathological features, MYCN amplification, and prognosis: A UKCCSG study. Med Pediatr Oncol. 2001;36:169–176. doi: 10.1002/1096-911X(20010101)36:1<169::AID-MPO1041>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa A, Matsuoka K, Okita H, et al. Neuroblastomas with discordant genotype-phenotype relationship: Report of four cases with MYCN amplification and favorable histology. Pediatr Dev Pathol. 2011;14:87–92. doi: 10.2350/08-12-0579.1. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel A, Cziepluch C, Hamann U, et al. The N-Myc oncoprotein is associated in vivo with the phosphoprotein Max(p20/22) in human neuroblastoma cells. ENMO J. 1991;10:3703–3712. doi: 10.1002/j.1460-2075.1991.tb04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kretzner L, Blackwood EM, Eisenman RN. Myc and Max proteins possess distinct transcriptional activities. Nature. 1992;359:426–429. doi: 10.1038/359426a0. [DOI] [PubMed] [Google Scholar]
- 32.Luscher B, Larsson LG. The basic region/helix-loop-helix/leucine zipper domain of Myc proto-oncoproteins: Function and regulation. Oncogene. 1999;18:2955–2966. doi: 10.1038/sj.onc.1202750. [DOI] [PubMed] [Google Scholar]
- 33.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 34.Bond VC, Wold B. Nucleolar localization of myc transcripts. Mol Cell Biol. 1993;13:3221–3230. doi: 10.1128/mcb.13.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arabi A, Rustum C, Hallberg E, et al. Accumulation of c-Myc and proteasomes at the nucleoli of cells containing elevated c-Myc protein levels. J Cell Sci. 2003;116:1707–1717. doi: 10.1242/jcs.00370. [DOI] [PubMed] [Google Scholar]
- 36.Okamatsu C, London WB, Naranjo A, et al. Clinicopathological characteristics of ganglioneuroma and ganglioneuroblastoma: A report from the CCG and COG. Pediatr Blood Cancer. 2009;53:563–569. doi: 10.1002/pbc.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]