Abstract
We have previously reported on calcium transport mechanisms in American lobster, Homarus americanus, using 45Ca2+ coupled with vesicle preparations of hepatopancreatic endoplasmic reticulum. The active transport of calcium across membranes bordering calcium-sequestering stores such as sarcoplasmic or endoplasmic reticulum is catalyzed by membrane-spanning proteins, the sarco-endoplasmic Ca2+-ATPases (SERCAs). In the study described here we used advanced bioinformatics and molecular techniques to clone SERCA from the economically important Caribbean spiny lobster, Panulirus argus. We report the complete cloning of a full-length SERCA from P. argus antenna cDNA (GenBank accession number AY702617). This cDNA has a 1020-amino acid residue open reading frame which is 90% identical to published sequences of other crustacean SERCA proteins. Our data support the hypothesis that one crustacean and three vertebrate genes controlling calcium transport were derived from a common ancestral gene.
Keywords: Spiny lobster, Cloning, Polymerase chain reaction, Rapid amplification of cDNA ends, Sequencing, Real time PCR
Introduction
Ca2+ is an essential regulatory ion in living cells. Cellular Ca2+ signaling plays a pivotal role in signal-transduction processes. Extracellular Ca2+ concentrations are in the 1–3 mM range where as cytoplasmic Ca2+ concentration in cells at rest is commonly 100–300 nM (Kretsinger 1976). In presence of stimulus, Ca2+ enters into the cytoplasm and concentrations reaches from 1 to 10 μM (Maruyama et al. 1989). Changes in the cytosolic concentration of Ca2+ ion play a key role in controlling a host of different physiological processes in every animal cell. The uncontrolled increase in cytosolic Ca2+ concentration would lead to cell death.
Eukaryotes utilize Ca2+ ions as an intracellular signal messenger to coordinate several cellular events. Cell activation promotes Ca2+ signals generated by the concerted operation of different Ca2+-transporting and Ca2+-binding proteins. In neuronal cells, Ca2+ plays critical roles in neuronal excitability, axonal transport, synaptic transmission, plasticity, and neuro-degenerative disorders (Mattson et al. 2000). In most cases, the endoplasmic reticulum (ER) plays important role in Ca2+ storage and regulation site and role of plasma membrane primarily remains in the reduction of overall Ca2+ concentrations. These activities are primarily achieved by two ion transport systems: the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA), which transfers Ca2+ ions into the endoplasmic reticulum and the plasma membrane Ca2+-ATPase (PMCA) which pumps intracellular Ca2+ to the extracellular medium (Sepúlveda et al. 2004). The loss or malfunction of specific Ca2+ pump isoforms is associated with defects such as ataxia, deafness or heart failure.
Sarco-endoplasmic reticulum Ca2+-ATPases (SERCA) are membrane-spanning proteins catalyzing the active transport of Ca2+ across membrane bordering Ca2+-sequestering stores such as sarcoplasmic or endoplasmic reticulum. Mammalian SERCAs are encoded by a family of three homologous genes which through alternative splicing, encode several isoforms and was initially cloned and sequenced from muscle, where it is highly abundant and functions to re-sequester Ca2+ during relaxation (MacLennan et al. 1985; Brandl et al. 1986, 1987). Each, of these three SERCA isoforms, have about a 75% sequence homology between one another. Subsequently, SERCA has been cloned from other vertebrate including birds, frogs and fishes. Invertebrate SERCA have been cloned and characterized from two arthropods, the fruit fly Drosophila melanogaster (Magyar and Varadi 1990) and the brine shrimp Artemia franciscana (Palmero and Sastre 1989) and two crustaceans, the crayfish Procambrus clarkii (Zhang et al. 2000) and terrestrial isopod Porcellio scaber (Hagedorn et al. 2003) and recently from pearl oyster Pinctada fucata (Fan et al. 2007).
Here we report the complete cloning of a full-length SERCA from antennae cDNA of P. argus using nested PCR and rapid amplification of cDNA ends (RACE) techniques. P. argus SERCA cDNA confirmed 1,020 amino acid residues lengthy open reading frame sequence which is 90% identical to published sequences of other crustacean SERCAs proteins. Also in our study we have tried to find out the relative expression of SERCA in different organ systems of both lobsters (H. americanus, P. argus) using the Real Time Polymerase Chain Reaction (RT-PCR) and mRNA expression in H. americanus using in situ hybridization.
Materials and methods
Animal material
The Caribbean spiny lobster, P. argus were obtained from Florida Key Laboratory (FLM, Layton, FL, USA) and maintained in sea water at room temperature at the Whitney laboratory for Marine Biosciences (St. Augustine, FL, USA). The Maine lobster H. americanus were obtained from local market and maintained in University of North Florida biology department in an aquarium at 10°C. Tissue samples were removed and frozen immediately in liquid nitrogen and stored at −80°C from both the lobsters.
Total RNA isolation
Total RNA from different tissues were isolated by acid guanidium thiocyanate-phenol-chloroform extraction (Chomczynski and Sacchi 1987) and purification was done by adding three volumes of 100% ethanol. RNA were quantified by using spectrophotometer at wavelengths of 260 and 280 nm. The integrity of RNA was confirmed on a 0.72 mol/l formaldehyde/1% agarose denaturing gel run in MOPS buffer (5 mmol/l sodium acetate, 1 mmol/l EDTA, 20 mmol/l MOPS, pH 6.6).
Isolation of partial SERCA cDNA sequence from antennae total RNA
The first strand of cDNA was synthesized by SuperScript II reverse transcriptase with an oligo(dT) primer, TRsa. The second strand cDNA was synthesized using the Marathon kit (CLONTECH). cDNA amplification were done by using CLONTECH kit following manufacturer’s method modified at Whitney laboratory (Matz 2003). The double-stranded cDNA were ligated to pseudo-double-stranded adaptor LUST-Rsa and were purified by using Qiaquick kit (Qiagen) and were amplified using Advantage polymerase mix (CLONTECH) under the following conditions: 94°C, 30 s; 66°C, 1 min; 72°C, 2 min 30 s; 15 cycles. PCR (Polymerase chain reaction) screening of P. argus cDNA were done by using a set of exact primers. Conserved region-specific degenerate primers were generated using CODEHOP software (COnsensus-DEgenerate Hybrid Oli-gonucleotide Primers) and used to screen a high-performance lobster cDNA from selected tissues. These primers were manually designed for selected conserved motives of different invertebrate SERCA. These primers targeted a fragment of approximately 700 bp.
Degenerate primers set for nested PCR
The degenerate region (core) is printed in lower case; the non-degenerate region (clamp) is printed in upper case.
| #1F_KEYEPEMGK oligo:5′-TGAAGGAGTACGAGCCCgaratgggnaa-3′ #2F_AVNQDKKN oligo:5′-GGGCCGTGAACCAGgayaaraaraa-3′ #3F_LQQKLDEFG oligo:5′-GCTGCAGCAGAAGCTGgaygarttygg-3′ |
| Species selective priming sight VIKQKWKKEFT oligo:5′-GTGATGAAGCAGAAGTGGAAGaargarttyac-3′ |
| #4R_YFKIAVAL atraarttytaGCGGCACCGGGAC oligo: 5′-CAGGGCCACGGCGatyttraarta-3′
|
| #XR_EFDDLSPEE_reserved ctyaarctrctAGACTGGGGCCTCCTC oligo:5′-CTCCTCCGGGGTCAGAtcrtcraaytc-3′ |
| #5R_KEFTLEFSR ttyctyaartgGGACCTCAAGAGGGCG oligo:5′-GCGGGAGAACTCCAGGgtraaytcytt-3′ #6R_MKQFIRYLIS tacttygtyaaGTAGGCGATGGACTAGAGG oligo:5′-GGAGATCAGGTAGCGGATGaaytgyttcat-3′ |
Touchdown hot-start PCR (Hecker and Roux 1996) were performed using Taq DNA Polymerase in 1.5 mM MgCl2 solution. Total volume of 50 μl PCR reaction included 2 μl of cDNA, 1 μl of LA-Taq polymerase in an MJ Research thermal cycler. PCR started with denaturation of cDNA at 94°C for 1 min, followed by 30 cycles of 94°C for 1 min, 63°C for 1 min, 72°C for 1 min and a final cycle of 72°C for 7 min. Negative controls in which reactions contained only one primer or no template cDNA were included. PCR products were analyzed by 1% agarose gel electrophoresis with 0.5 μg of ethidium bromide in 1× TAE buffer (40 mmol/l Tris, 40 mmol/l sodium acetate and 1 mmol/l EDTA) and the fragment was visualized under ultraviolet light. Molecular weight was determined by using 1 kb marker. A ~1,000 bp PCR product was ligated to a vector pCR 2.1-TOPO and sequenced from University of Florida, Gainesville, sequencing facility by using M13 primers. Homology screening was done by BLASTp of protein databases.
Amplification of full-length SERCA cDNA by using Marathon rapid amplification of cDNA ends (RACE) techniques
RACE was employed to complete the 3′ and 5′ regions of the antennae SERCA. First, an amplified, adapter ligated cDNA library was constructed from antennae total RNA, as described by Matz (2002) with two rounds of step-out and nested PCR (Matz et al. 2003). Exact primers, based on the partial sequences from SERCA cDNA were used in combination with cDNA-flanked TRsa and LU4 adapters (Matz 2002) for RACE. As a template, 2 μl of cDNA library (diluted 1:50) was used for a 20-μl PCR mixture. Agarose gels of the PCR products are shown in Fig. 3. The PCR product were ligated into a pGEM—T vector (Promega) for transformation into DH—5α cells (Invitrogen). Each clone was digested with the appropriate restriction enzymes and sub-cloned for sequencing.
Fig. 3.
a Unrooted Neighbor-Joining phylogenetic tree of selected SERCA proteins. Protein sequence alignments were completed by using ClustalX. Phylogenetic tree re-construction were prepared using quartet maximum likelihood TreePuzzle-5 software. Phylogenetic tree of selected SERCA proteins from published sequences including our results from P. argus. As shown in this figure, the spiny lobster SERCA sequence is very similar (>90% identical) to those of both the crayfish (P. clarkii) and the isopod (P. scaber). Species abbreviations: hs, Homo sapiens; ce, Caenorhabditis elegans; dm, Drosophila melanogaster; ps, Porcellio scaber; pc, Procambarus clarkii; pa, Panulirus argus, Florida spiny lobster SERCA. b Neighbor-Joining dendrogram (Thompson et al. 1997) of P-type ATPases found in genomic models including: Homo sapience (hs) and two arthropods, Drosophila melanogaster (dm) and Anopheles gambiae (ag). Cloned crustacean SERCAs assigned (ps) and (pc) for Porcellio scaber (Hagedorn et al. 2003) and Procambarus clarkii (Zhang et al. 2000), respectively
DNA sequencing
The cDNA clones were sequenced by the dideoxynucleotide method by using automated sequencer at University of Florida core sequencing facility, Gainesville, FL, USA.
DNA and amino acid sequence analysis
The complete sequence was analyzed with SeqMan II (DnaStar software, Madison, WI, USA). Sequence homology was revealed through a GenBank data base search using BLAST (Altschul et al. 1990). Hydropathy analysis was performed with TopPRED2 software at remote server (http://bioweb.pasteur.fr/seqanal/interfaces/toppred.html).
Phylogenetic analysis
ClustalW (Thompson et al. 1997), as implemented in the Biological Workbench (https://workbench.sdsc.edu), was used to align full-length SERCA sequences. Phylogenetic and evolutionary analyses were conducted using neighbour joining algorithm (Saitou and Nei 1987). Species and their GenBank accession numbers are given in Fig. 3a.
Quantitative real-time PCR (qRT-PCR)
Relative expression levels of SERCA in both the lobster tissues were determined by RT-PCR using SYBR Green dye technology on an ABI prism 7000 sequence Detection system (Applied Biosystems). P. argus and H. americanus 18s ribosomal RNA was selected as an endogenous control to normalize quantitation of the mRNA target for differences in the amount of cDNA added to each reaction. Primers were designed based on P. argus SERCA sequences (Mandal et al. 2005b) using primer express v.2.0 software (Applied Biosystems) with the following sequences.
| paSERCA-316F | GTGTGGCAGGAGCGCAAT |
| paSERCA-391R | GGACCACCTTGCCCATCTC |
| pa18S rRNA-1656F | CGTCCCTGCCCTTTGTACAC |
| pa18S rRNA-1720R | CCGAAGCCTCACTAAATCATTCA |
| haSERCA-11F | GGCAAGGTCGTGGTAGTTGTC |
| haSERCA-81R | AGCCATCTGGGTACGGATCTT |
| ha18S rRNA-238F | GGCGCCGCTTCTTTCAA |
| ha18S rRNA-302R | GCCATTGTAGGCGCATAACC |
PCR reactions were performed in triplicate in a total volume of 25 μl containing 12.5 μl SYBR green PCR Master Mix (Applied Biosystems), 200 nM (SERCA) or 300 nM (18s rRNA) of each primer, and approximately 10 ng cDNA template. Control runs included a subset of PCR components lacking the cDNA template. The PCR program was run as follows: 50°C for 2 min, 95°C for 15 s, 60°C for 20 s, and 95°C for 15 s. Dissociation curve analysis were performed to confirm the specificity of the reaction products. Relative expression values were calculated according to the formula: 2−ΔΔCt. Ct refers to the cycle number at which the fluorescence rises above a set of threshold. Expression of SERCA in each tissue was normalized to that found in antennal gland which was set to a value of one.
In situ hybridization
Probe preparation
Briefly, a pGEM-T H. americanus SERCA was linearized with NotI restriction enzymes to obtain full-length transcripts using SP6 and T7 promoters for anti-sense and sense (control) probes. DIG-labeled probes were transcribed in vitro using a DIG RNA labeling kit (Roche company).
Tissue preparation
Thin sections of the antennal gland tissues from H. americanus were prepared on glass slides by using the cryomicrotome instrument at Whitney lab and in situ hybridizations were done by following the protocol used previously (Meleshkevitch et al. 2006). The slides with the tissue sections were fixed in 4% paraformaldehyde in PBS on ice for 3 h followed by washing three times with 1× PBS, 0.1% Tween 20 (PTw) for 5 min each. Then the tissues on the slides were dehydrated by being incubated in 3:1, 1:1, 1:3 PTw/methanol for 10 min each and in 100% methanol for 5 min followed by rehydrated in 3:1, 1:1, 3:1 PTw/methanol solution for 10 min each and were incubated for PTw alone for 10 min. Then the slides were treated with Proteinase K (10 μg/ml) in PTw at room temperature for 10 min and were fixed in 4% Para formaldehyde in PBS at 4°C for 20 min followed by washing twice with glycine (2 mg/ml) in PTw at room temp for 10 min and fixed in 4% Paraformaldehyde in PBS at 4°C for 20 min followed by washing with 0.1 M triethanolamine hydrochloride, pH 8 and with acetic anhydride 2.5 μl/ml and three times with PTw.
Hybridization
Prehybridization of those tissues were done at 50°C for 6–8 h in hybridization solution (50% formamide, 5 mmol/l EDTA, 5× SSC, 1× Denhardt’s solution, 0.1% Tween-20, 0.5 mg/ml yeast tRNA), and hybridization were performed by incubation of 1 μg DIG-labeled RNA probe in hybridization solution at 50°C for 12–14 h and washing were performed following several steps: slides were incubated in 50% formamide/5× SSC/1% SDS at 60°C for 30 min; 50% formamide/2× SSC/1% SDS at 60°C for 30 min; 0.2× SSC at 55°C for 30 min twice; washed three times in PBS + Tween 20 (PBT) and incubated in alkaline phosphatase-conjugated DIG-antibodies in 1% normal goat serum at 4°C for overnight. Slides were washed three times with PBT, incubated twice in the detection buffer followed by dark incubation at 4°C with NBT/BCIP for 24 h. The slides were washed after staining with 4% paraformaldehyde in methanol at 4°C for 1 h followed by washing twice with 100% ethanol for 10 min each and added a drop of Permount® and covered with cover glass.
Results
Degenerated primers 1 and 2 were successful in amplification of prominent product from P. argus antennae cDNA. The size of PCR product was ~700 bp (data not shown), and the nested PCR product was ~1,000 bp (data not shown) matching the expected size on the basis of P. scaber SERCA sequences. A search of GenBank confirmed that the nucleotide sequences display a high homology to an earlier cloned P. scaber SERCA (~90%). This partial sequence provided crucial DNA sequence information required for 5′–3′ RACE cloning of the complete SERCA cDNA. On the basis of 1,000 bp partial sequences, two gene specific primers, RACE 1 and RACE 2, were used together with adaptor primers LU 4—TRsa to perform RACE of P. argus antennae cDNA. A major band of 1.5 kb (data not shown) was obtained from 5′ RACE and a major band of 3 kb (data not shown) was obtained from 3′ RACE. Both the products were cloned into pGEM (Promega) vector.
The complete nucleotide sequence and deduced amino acid sequence of P. argus SERCA is shown in Fig. 1. This 3,060 nucleotide sequence contains an open reading frame of 3056 bp coding for 1,020 amino acid residues with a molecular mass of 110 kDa. The predicted amino acid sequence displays structural features common to other SERCA pumps. The hydropathy plot of P. argus SERCA (Fig. 2a) reveals ten distinct regions of hydrophobicity representing sections of amino acid sequence. It appears that five of these putative transmembrane domains are located near the N-terminal region and the remaining 5 five are located near the C terminus, with an extracellular loop in between. The pattern of the trans-membrane arrangement is very similar to that of other crustaceans and mammalian SERCAs. The hydropathy profile (Fig. 2a) of P. argus SERCA shows similarity with the transmembrane organization of all other cloned SERCAs, suggesting a common protein secondary structure. There are two transmembrane segments in the NH2-terminal region followed by an extracellular loop, and three transmembrane segments followed by small cytosolic loop. A large extracellular segment lies between transmembrane regions five and six. The other five transmembrane regions that precedes in the COOH-terminal region are part of the polypeptide. Predicted domain, motifs and trans-membrane domains of P. argus SERCA are shown in Tables 1 and 2.
Fig. 1.
The complete nucleotide sequence (top) and deduced amino acid sequence (bottom) of spiny lobster P. argus antennae SERCA cDNA. Nucleotide and amino acids are numbered to the right of the sequences
Fig. 2.
a Hydrophobicity plot of spiny lobster P. argus SERCA. Hydrophobicity values were determined by the method of Kyte and Doolittle 1982. Putative transmembrane segments are indicated by longitudinal bar lines. b Putative secondary structure (a) of paSERCA by TopPredII (http://bioweb.pasteur.fr/seqanal/tmp/toppred)
Table 1.
Candidate membrane-spanning segments
| Name | Begin | End | E value |
|---|---|---|---|
| Pfam:Cation_ATPase_N | 1 | 77 | 9.00e-25 |
| Pfam:E1-E2_ATPase | 93 | 341 | 3.00e-121 |
| Pfam:Hydrolase | 345 | 723 | 2.40e-17 |
| Pfam:Cation_ATPase_C | 818 | 990 | 1.10e-78 |
Table 2.
Trans membrane domain
| Helix | Begin–end | Score certainty |
|---|---|---|
| 1 | 60–80 | 1.727 |
| 2 | 88–108 | 1.639 |
| 3 | 215–235 | 1.197 |
| 4 | 259–279 | 1.277 |
| 5 | 298–318 | 1.164 |
| 6 | 764–784 | 1.212 |
| 7 | 830–850 | 1.704 |
| 8 | 882–902 | 1.428 |
| 9 | 922–942 | 1.976 |
| 10 | 988–1008 | 1.469 |
A search of the GenBank database using BLAST algorithm (Altschul et al. 1990) revealed 30 high score matches with the putative P. argus SERCA amino acid sequence. These matches were exclusively SERCA sequences from variety of invertebrates and vertebrates (Zhang et al. 2000; Brandl et al. 1986; Karin et al. 1989; Lytton et al. 1989; Palmero and Sastre 1989; Magyar and Varadi 1990; Wu and Lytton 1993). The deduced amino acid sequences of spiny lobster SERCA (Fig. 6) shares highest homology with both crayfish SERCA sequences. The cloning and functional characterization of the heart muscle isoform of SERCA from crayfish was described using RT-PCR and RACE (Chen et al. 2002). This isoform, consists of 4,495 bp with a 3060 bp open reading frame, coding for 1020 amino acids, differs from the previously identified axial abdominal muscle SERCA. Phylogenetic tree (Fig. 3a) of selected SERCA proteins from published sequences including our results from P. argus shows this lobster SERCA sequence is very similar (>90% identical) to those of both P. clarkii and P. scaber.
Fig. 6.
Protein sequence alignment of selected SERCAs. Species abbreviation: hs, Homo sapiens; dm, Drosophila melanogaster; ps, Porcellio scaber; pc, Procambarus clarkii; pa, Panulirus argus, Florida spiny lobster; ce, Caenorhabditis elegans
Real-time PCR data (Fig. 4) confirmed significant SERCA expression in muscle and heart and comparatively less expression in intestine, gills and hepatopancreas. Result analysis indicated that the SERCA expression in H. americanus was 8,000-fold higher in the muscle and 1,000-fold higher in the heart tissues, where as in P. argus it is 27-fold higher in the muscle and 12-fold higher in the heart tissues compared to the antennal gland expression. Expression of SERCA in H. americanus hepatopancreas was significantly lower than in the antennal gland and the gills. SERCA localization in H. americanus antennal gland tissue sections by in situ hybridization confirmed the expression of P. argus SERCA mRNA antisense probe (Fig. 5).
Fig. 4.
Relative expression of SERCA in different tissues of H. americanus and P. argus. Relative expression values were calculated according to the formula: 2−ΔΔCt. Expression of SERCA in each tissue was normalized to the expression values found in antennal gland which was set to a value of one
Fig. 5.
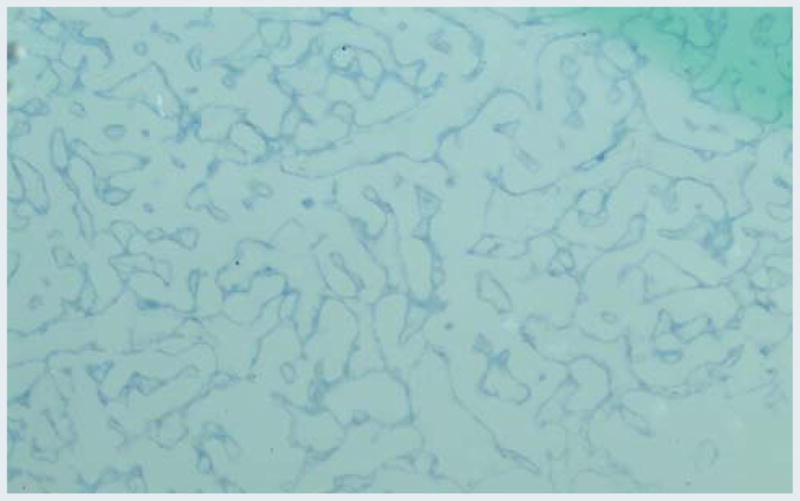
Computer images of in situ hybridization P. argus antisense probe in H. americanus antennal gland sections. mRNA abundance is illustrated by increasing intensity of blue
Discussion
Physiology of H. americanus hepatopancreatic calcium transport mechanism were reported earlier from our lab (Ahearn and Franco 1993; Zhuang and Ahearn 1998; Mandal et al. 2005a). SERCA is a key player in regulating intracellular Ca2+. Present study reports for the first time regarding the molecular cloning of SERCA specific to neural antennae tissue of P. argus including the expression in H. americanus by real time PCR and tissue distribution by in situ hybridization. Among arthropods earlier studies have reported characterization of SERCA type Ca2+ ATPase from Drosophila melanogaster (Magyar and Varadi 1990). Unlike vertebrates, Drosophila has a single highly conserved SERCA gene, making analysis of the physiological contribution of SERCA particularly feasible. SERCA cloning and characterizations were also done in crustacean Artemia franciscana (Palmero and Sastre 1989) and in axial muscle of freshwater crustacean Procambarus clarkii (Zhang et al. 2000). Also smooth endoplasmic reticulum Ca2+ ATPase cloning and characterizations were done in another crustacean, i.e. sternum tissue of isopod Porcellio scaber (Hagedorn et al. 2003). Recent studies have reported SERCA cloning from mollusk pearl oyster, Pinctada fucata (Fan et al. 2007).
Large number of P-type ATPases has been cloned and/or identified by cyber analysis, which creates perfect situation for cloning of the orthologous proteins from any particular species, including crustaceans. Our cyber analysis indicated high probability of presence of seven functionally distinct subfamilies of crustacean P-type ATPases. Only SERCA has been cloned and characterized from three crustacean species. Four other subfamilies apparently are defined in vertebrates. In addition, two ORPHAN clusters with unclear functional significance are present (Fig. 3b). The spiny lobster P. argus antennae SERCA gene encodes a protein of 1,020 amino acid residues with molecular mass of 112 kDa. The deduced amino acid sequence reveals that it contains all the conserved functional domains similar to other P-type ATPases.
When the amino acid sequences are aligned with known SERCAs from P. clarkii (pcAAB82291 and pcAAB82290), P. scaber (psAAN77377), D. melanogaster (dmNP_47683), Caenorhabditis elegans (ceCAA09985), and Homo sapiens (hsNP_00516), the differences are concentrated at the NH2 terminus, the central loop region and the COOH terminus (Fig. 6). Two regions that are highly conserved among those species are residues 270–368 and 700–840; both showed greater than 96% amino acid similarity. These may be the polypeptide regions that anchor the cytoplasmic domain to the membrane and are thought to be important for energy transduction.
Phylogenetic tree in Fig. 3a shows very close (>90%) relationship between our full-length P. argus SERCA with three other crustacean SERCAs. Further work will focus on the expression of SERCA in heart since these sites are continuously active and/or other claw muscle which atrophies in moulting. RT-PCR technique has the ability to monitor the progress of the PCR as it occurs (i.e. in real time). Data is therefore collected throughout the PCR process. In RT-PCR, reactions are characterized by the point in time during cycling when amplification of a target is supposed to be detected rather than the amount of target accumulated after a fixed number of cycles. The higher the copy number of the nucleic acid target the sooner a significant increase in fluorescence is observed. Result showed increased expression of SERCA in muscle tissues of both species H. americanus and P. argus in comparison to other tissues. SERCA expression in H. americanus antennal gland tissues suggests that this protein may play a role by reabsorbing Ca2+ in antennal gland during moulting stage. However, SERCA protein level is a critical determinant of intracellular Ca2+ homeostasis and change in SERCA expression are not without consequences.
In situ hybridization showed expression of P. argus SERCA in H. americanus antenna gland tissue sections which supports the prior report about SERCA mRNA abundance in crustacean tissues (Hagedorn et al. 2003). The crustacean SERCA are encoded by one gene leads to the hypothesis that one crustacean and three vertebrate genes derived from a common ancestral gene (Escalante and Sastre 1996). The phylogenetic tree in Fig. 3a, showing the relationship between full-length crustacean and some other invertebrate and vertebrate SERCAs, supports the hypothesis. Our results reinforce, develop and further refine our understanding of the role of this key protein.
The SERCA sequence from spiny lobster P. argus antennae tissue has been accepted by GenBank (Accession number AY702617).
Acknowledgments
This work was supported by NSF grant IBN04-21986 and NIH grant 2RO1 A1030464-13A1. The first two authors made equal contributions to the laboratory experiments. We thank Dr. Melissa Miller of Whitney laboratory, UF for her help in doing RT-PCR; and Mr. David Wilson of CIRT, UNF for computer help.
Contributor Information
A. Mandal, Email: anita.mandal@ewc.edu, Biology Program, Department of Mathematics and Sciences, Edward Waters College, 1658 Kings Road, Jacksonville, FL 32209, USA
S. C. Arunachalam, Department of Biology, University of North Florida, 1, UNF Drive, Jacksonville, FL 32224, USA
E. A. Meleshkevitch, The Whitney Laboratory for Marine Bioscience, University of Florida, 9505 Ocean Shore Blvd., St Augustine, FL 32080, USA. Department of Physiology and Biophysics, The Chicago Medical School, Rosalind Franklin University of Medicine and Science, 3333 Green Bay Road, North Chicago, IL 60064, USA
P. K. Mandal, Biology Program, Department of Mathematics and Sciences, Edward Waters College, 1658 Kings Road, Jacksonville, FL 32209, USA
D. Y. Boudko, The Whitney Laboratory for Marine Bioscience, University of Florida, 9505 Ocean Shore Blvd., St Augustine, FL 32080, USA. Department of Physiology and Biophysics, The Chicago Medical School, Rosalind Franklin University of Medicine and Science, 3333 Green Bay Road, North Chicago, IL 60064, USA
G. A. Ahearn, Department of Biology, University of North Florida, 1, UNF Drive, Jacksonville, FL 32224, USA
References
- Ahearn GA, Franco P. Ca2+ transport pathways in brush-border membrane vesicles of crustacean antennal glands. Am J Physiol. 1993;264:R1206–R1213. doi: 10.1152/ajpregu.1993.264.6.R1206. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Brandl CJ, DeLeon S, Martin DR, MacLennan DH. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986;44:597–607. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- Brandl CJ, DeLeon S, Martin DR, MacLennan DH. Adult forms of the Ca2+ ATPase of sarcoplasmic reticulum: expression in developing skeletal muscle. J Biol Chem. 1987;262:3768–3774. [PubMed] [Google Scholar]
- Chen D, Zhang Z, Wheatly MG, Gao Y. Cloning and characterization of the heart muscle isoform of sarco/endoplasmic reticulum Ca2 + ATPase (SERCA) from crayfish. J Exp Biol. 2002;205:2677–2686. doi: 10.1242/jeb.205.17.2677. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Escalante R, Sastre L. Tissue specific expression of two Artemia franciscana sarco/endoplasmic reticulum Ca-ATPase isoforms. J Histochem Cytochem. 1996;44:321–325. doi: 10.1177/44.4.8601691. [DOI] [PubMed] [Google Scholar]
- Fan W, Li C, Li S, Feng Q, Xie L, Zhang R. Cloning, characterization, and expression patterns of three sarco/endoplasmic reticulum Ca2+-ATPase isoforms from pearl oyster (Pinctada fucata) Acta Biochim Biophys Sin (Shanghai) 2007;39(9):722–730. doi: 10.1111/j.1745-7270.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Weihrauch D, Towle DW, Ziegler A. Molecular characterization of the smooth endoplasmic reticulum Ca2+-ATPase of Porcellio scaber and its expression in sternal epithelia during the moult cycle. J Exp Biol. 2003;206:2167–2175. doi: 10.1242/jeb.00380. [DOI] [PubMed] [Google Scholar]
- Hecker KH, Roux KH. High and low annealing temperatures increase both specificity and yield in touchdown and stepdown PCR. Biotechniques. 1996;20(3):478–485. doi: 10.2144/19962003478. [DOI] [PubMed] [Google Scholar]
- Karin NJ, Kaprielian Z, Fambrough DM. Expression of avian Ca2+-ATPase in cultured mouse myogenic cells. Mol Cell Biol. 1989;9(5):1978–1986. doi: 10.1128/mcb.9.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger RH. Calcium-binding proteins. Ann Rev Biochem. 1976;45:239–266. doi: 10.1146/annurev.bi.45.070176.001323. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying hydrophobic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lytton J, Zarain-Herzberg A, Periasamy M, MacLennan DH. Molecular cloning of the mammalian smooth muscle sarco(endo)plasmic reticulum Ca2+-ATPase. J Biol Chem. 1989;264(12):7059–7065. [PubMed] [Google Scholar]
- MacLennan DH, Brandl CJ, Korczak B, Green NM. Aminoacid sequence of a Ca2+ Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985;316:696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Magyar A, Varadi E. Molecular cloning and chromosomal localization of a sarco/endoplasmic reticulum-type Ca2+-ATP-ase of Drosophila melanogaster. Biochem Biophys Res Comm. 1990;173:872–877. doi: 10.1016/s0006-291x(05)80867-8. [DOI] [PubMed] [Google Scholar]
- Mandal PK, Mandal A, Ahearn GA. Physiological characterization of 45Ca2+ and 65Zn2+ transport by lobster hepatopancreatic endoplasmic reticulum. J Exp Zool A Comp Exp Biol. 2005a;303(7):515–526. doi: 10.1002/jez.a.186. [DOI] [PubMed] [Google Scholar]
- Mandal A, Meleshkevitch E, Mandal PK, Boudko D, Ahearn GA. Cloning of sarco-endoplasmic reticulum Ca2+ ATP-ase (SERCA) from caribbean Spiny lobster Panulirus argus. FASEB J. 2005b;19(4):A215. doi: 10.1007/s00360-008-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Clarke DM, Fujii J, Loo TW, MacLennan DH. Expresion of mutation of Ca2+ ATPase of the sarcoplasmic reticulum. Cell Motil Cytoskelet. 1989;14:26–34. doi: 10.1002/cm.970140107. [DOI] [PubMed] [Google Scholar]
- Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD. Calcium signaling in the RE: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- Matz MV. Amplification of representative cDNA samples from microscopic amounts of invertebrate tissue to search for new genes. Methods Mol Biol. 2002;183:3–18. doi: 10.1385/1-59259-280-5:003. [DOI] [PubMed] [Google Scholar]
- Matz MV. Amplification of representative cDNA pools from microscopic amounts of animal tissue. Methods Mol Biol. 2003;221:103–116. doi: 10.1385/1-59259-359-3:103. [DOI] [PubMed] [Google Scholar]
- Matz MV, Alieva NA, Chenichik A, Lukyanov SA. Amplification of cDNA ends using PCR suppression effect and step-out PCR. Methods Mol Biol. 2003;221:41–49. doi: 10.1385/1-59259-359-3:41. [DOI] [PubMed] [Google Scholar]
- Meleshkevitch EA, Assis-Nascimento P, Popova LB, Miller MM, Kohn AB, Phung EN, Mandal A, Harvey WR, Boudko DY. Molecular characterization of the first aromatic nutrient transporter from the sodium neurotransporter symporter family. J Exp Biol. 2006;209:3183–3198. doi: 10.1242/jeb.02374. [DOI] [PubMed] [Google Scholar]
- Palmero I, Sastre L. Complementary DNA cloning of a protein highly homologous to mammalian sarcoplasmic reticulum Ca2+-ATPase from the crustacean Artemia. Mol Biol. 1989;210:737–748. doi: 10.1016/0022-2836(89)90106-x. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sepúlveda MR, Hidalgo-Sánchez M, Mata AM. Localization of endoplasmic reticulum and plasma membrane Ca2+-ATPases in subcellular fractions and sections of pig cerebellum. Eur J Neurosci. 2004;19:542–551. doi: 10.1111/j.0953-816x.2003.03156.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DJ. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25(24):4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KD, Lytton J. Molecular cloning and quantification of sarcoplasmic reticulum Ca2+-ATPase isoforms in rat muscles. Am J Physiol. 1993;264:C333–C341. doi: 10.1152/ajpcell.1993.264.2.C333. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen D, Wheatly MG. Cloning and characterization of Sarco/Endoplasmic reticulum Ca2+ ATPase (SERCA) from crayfish axial muscle. J Exp Biol. 2000;203:3411–3423. doi: 10.1242/jeb.203.22.3411. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Ahearn GA. Energized Ca2+ transport by hepatopancreatic basolateral plasma membranes of Homarus americanus. J Exp Biol. 1998;201:211–220. doi: 10.1242/jeb.201.2.211. [DOI] [PubMed] [Google Scholar]







