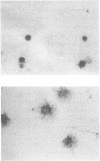
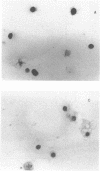
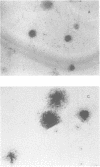
Abstract
The position of replication origins and replication forks relative to the nuclear matrix was analysed by autoradiography. Analysis of 2M NaCl-extracted extracted BHK-nuclei, prepared on coverslips, showed that after brief pulses grains were exclusively found over the central core of the residual nuclei, which corresponds to positions in the nuclear matrix. In asynchronous cells these grains were found to migrate into the DNA-halo surrounding the matrix during a subsequent chase. When the pulse had been administered to synchronous cells at the onset of S-phase, it was observed, however, that in the majority of the structures no such migration had occurred. From this, and from the fact that label incorporated later in S-phase could be chased into the halo, we conclude that, contrary to DNA in replication forks, DNA containing replication origins is permanently attached to the nuclear matrix.
Full text
PDF


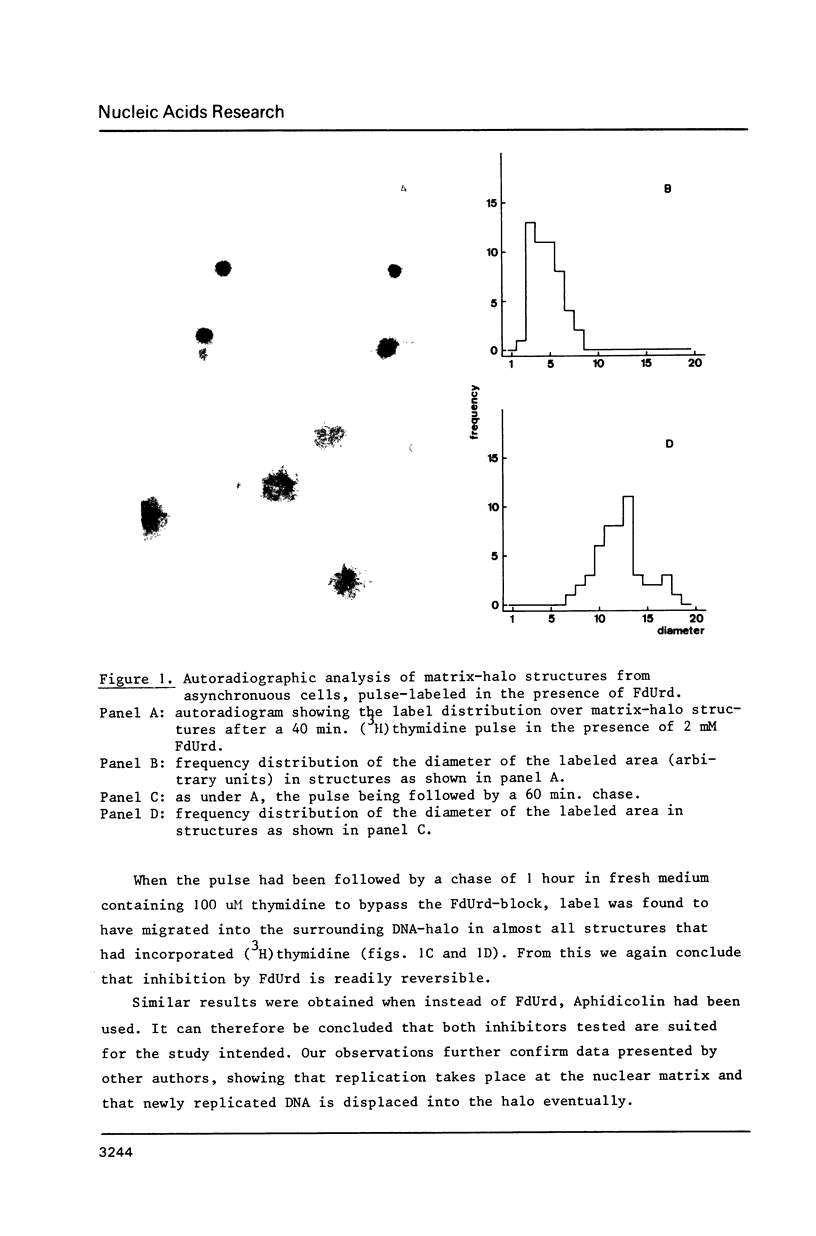
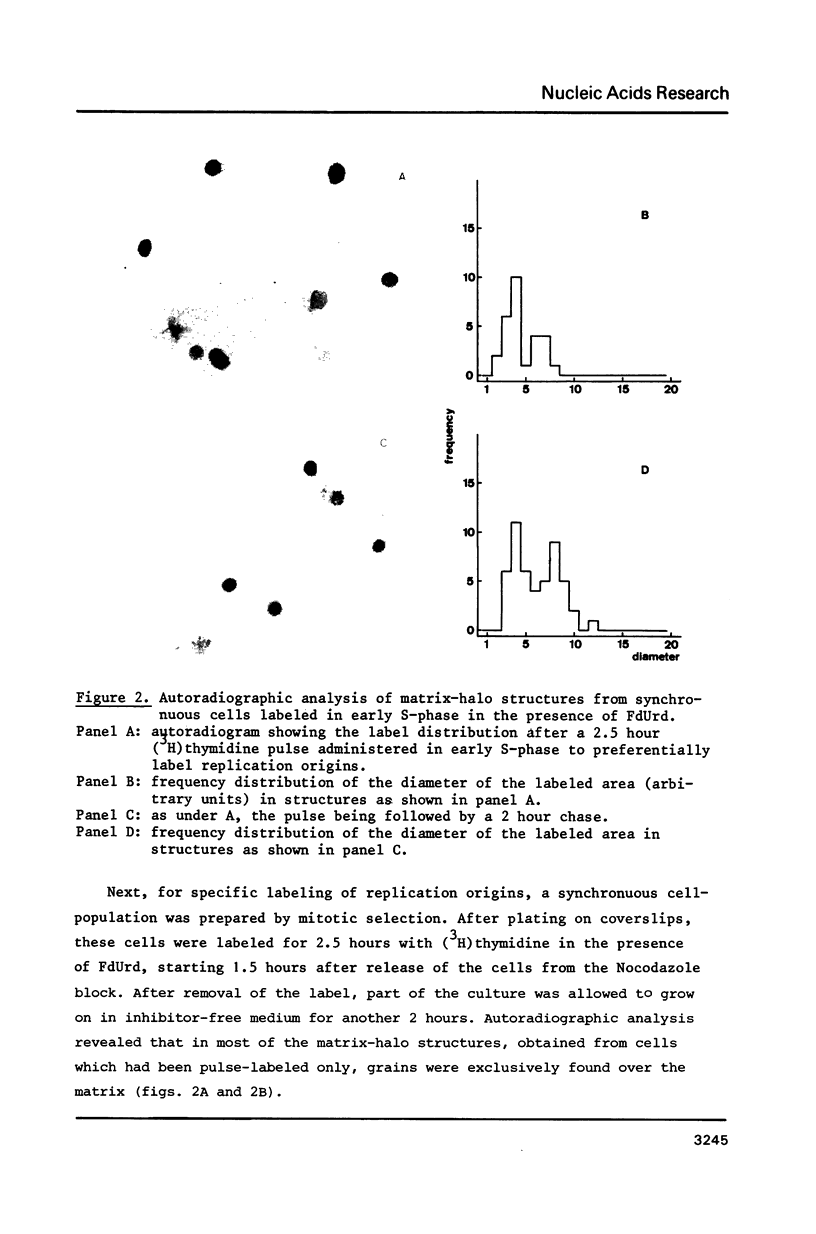
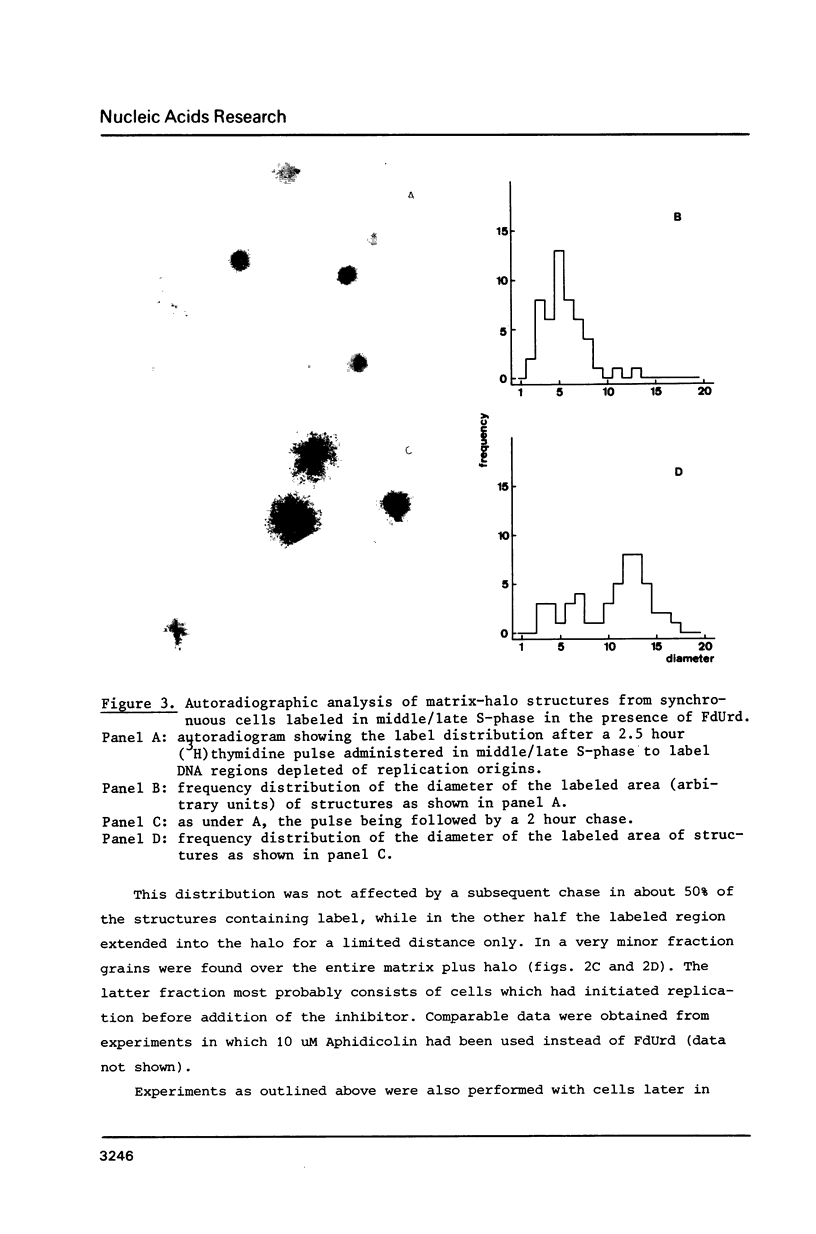



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aelen J. M., Opstelten R. J., Wanka F. Organization of DNA replication in Physarum polycephalum. Attachment of origins of replicons and replication forks to the nuclear matrix. Nucleic Acids Res. 1983 Feb 25;11(4):1181–1195. doi: 10.1093/nar/11.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear protein matrix: association with newly synthesized DNA. Science. 1975 Jul 25;189(4199):291–293. doi: 10.1126/science.1145202. [DOI] [PubMed] [Google Scholar]
- Buongiorno-Nardelli M., Micheli G., Carri M. T., Marilley M. A relationship between replicon size and supercoiled loop domains in the eukaryotic genome. Nature. 1982 Jul 1;298(5869):100–102. doi: 10.1038/298100a0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Conformational constraints in nuclear DNA. J Cell Sci. 1976 Nov;22(2):287–302. doi: 10.1242/jcs.22.2.287. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Lang J. The spatial organization of sequences involved in initiation and termination of eukaryotic DNA replication. Nucleic Acids Res. 1984 Jan 25;12(2):1069–1075. doi: 10.1093/nar/12.2.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupido M., Simons J. W. A re-appraisal of the BHK 21 clone-13 cell transformation system with respect to the assay conditions and the mutational origin of the transformed phenotype. Carcinogenesis. 1984 Jul;5(7):857–862. doi: 10.1093/carcin/5.7.857. [DOI] [PubMed] [Google Scholar]
- Dijkwel P. A., Mullenders L. H., Wanka F. Analysis of the attachment of replicating DNA to a nuclear matrix in mammalian interphase nuclei. Nucleic Acids Res. 1979 Jan;6(1):219–230. doi: 10.1093/nar/6.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C. W. Bidirectional chromosome replication: some topological considerations. J Theor Biol. 1974 Jan;43(1):187–195. doi: 10.1016/s0022-5193(74)80052-4. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Harland R. M. Replication origins in the eucaryotic chromosome. Cell. 1981 May;24(2):283–284. doi: 10.1016/0092-8674(81)90316-0. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Godwin J., Mason D. W., Brazell I. A., Cook P. R. DNA is replicated at the nuclear cage. J Cell Sci. 1980 Dec;46:365–386. doi: 10.1242/jcs.46.1.365. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Ockey C. H., Saffhill R. The comparative effects of short-term DNA Inhibition on replicon synthesis in mammalian cells. Exp Cell Res. 1976 Dec;103(2):361–373. doi: 10.1016/0014-4827(76)90272-x. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B., Coffey D. S. A fixed site of DNA replication in eucaryotic cells. Cell. 1980 Feb;19(2):527–536. doi: 10.1016/0092-8674(80)90527-9. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. C., Berezney R. DNA polymerase alpha is tightly bound to the nuclear matrix of actively replicating liver. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1541–1547. doi: 10.1016/s0006-291x(80)80041-6. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Wanka F., Mullenders L. H., Bekers A. G., Pennings L. J., Aelen J. M., Eygensteyn J. Association of nuclear DNA with a rapidly sedimenting structure. Biochem Biophys Res Commun. 1977 Jan 24;74(2):739–747. doi: 10.1016/0006-291x(77)90364-3. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Turnbull D., Mullins J. M., McIntosh J. R. Production of large numbers of mitotic mammalian cells by use of the reversible microtubule inhibitor nocodazole. Nocodazole accumulated mitotic cells. Exp Cell Res. 1980 Apr;126(2):397–405. doi: 10.1016/0014-4827(80)90279-7. [DOI] [PubMed] [Google Scholar]
- van der Velden H. M., van Willigen G., Wetzels R. H., Wanka F. Attachment of origins of replication to the nuclear matrix and the chromosomal scaffold. FEBS Lett. 1984 Jun 4;171(1):13–16. doi: 10.1016/0014-5793(84)80451-2. [DOI] [PubMed] [Google Scholar]
- veer Reddy G. P., Pardee A. B. Inhibitor evidence for allosteric interaction in the replitase multienzyme complex. Nature. 1983 Jul 7;304(5921):86–88. doi: 10.1038/304086a0. [DOI] [PubMed] [Google Scholar]