Abstract
Anal cancer used to be a rare cancer traditionally associated with elderly women. There are approximately 5260 cases per year in the U.S. (1). The onslaught of the Human Immunodeficiency Virus (HIV) virus has led to a change in anal cancer demographics. Anal cancer is on the rise in the U.S and the number of anal cases documented has quadrupled in the past 20 yrs correlating with the rise of the HIV epidemic. The incidence of anal cancer is 40 to 80 fold higher in the HIV positive (HIV+) population when compared to the general population (2). With the advent of highly active antiretroviral therapy (HAART), HIV+ patients are living longer as less are progressing to AIDS. As a consequence non AIDS defining cancers such as anal cancer are on the rise. Factors implicated in the etiology of anal cancer in HIV+ patients include (Human papillomavirus) HPV virus status, sexual habits, and a history of smoking. HPV 16 and receptive anal intercourse (RAI) increase the risk of anal cancer by 33% over the general population. In the general population, the rate of anal cancer is approximately 0.9 cases per 100,000. In patients with a history of RAI, the rate approaches 35 cases per 100,000 which is equivalent to the prevalence of cervical cancer (3). Smokers are eight times more likely to develop anal cancer. There has been much discussion about tailoring treatment decisions in HIV+ patients with anal cancer. This review focuses on squamous cell carcinomas of the anal canal which comprise 80 to 90% of all anal cancers diagnosed and highlight key issues in the management of HIV+ anal cancer patients including recent clinical trials.
Keywords: Anal cancer, HIV, Anal canal
Incidence
Anal cancer is on the rise in the US especially in the HIV positive population. In the early 1990’s the incidence of anal cancer was approximately one case per 100,000 person-years (4). By 2004, the number of cases increased 30% (4). In the HIV positive population the rate of anal cancer increased from 19 to 78.2 per 100,000 person-years (4). 80% of anal cancers involve the anal canal with the majority being squamous cell carcinomas (5).
One major factor implicated in the increase in anal cancer from the 1990’s to now is the HIV virus. It is known that the incidence of anal cancer is 40 to 80 times higher in the HIV+ population. HIV+ patients tend to get anal cancer at a younger age, are more frequently men, and more frequently homosexual men who practice RAI (6). Cancer has been associated with HIV since the first reports of AIDS in the 1980s. Three different cancers are AIDS defining: Kaposi’s sarcoma, Non-Hodgkin’s Lymphoma (B cell), and invasive cervical cancer. Anal cancer is part of a group of non-AIDS defining cancers which include Hodgkin’s lymphoma, lung adenocarcinoma, hepatocellular carcinoma, oropharyngeal carcinoma, kidney carcinoma, melanoma, and conjunctival carcinoma (primarily sub-Saharan Africa) (4). These non AIDS defining cancers have a two to three fold higher incidence in the HIV+ population (7).
There are other factors implicated in the etiology of anal cancer in addition to HIV. Anal cancer, similar to cervical cancer, is known to be associated with the HPV virus, sexual behavior, and tobacco use. HPV-16 is the most prevalent subtype associated with anal cancer and precancerous lesions followed by HPV-33 and HPV-39 (8). HPV is known to play a definitive role in the development of anal and cervical squamous cell cancer. HIV+ patients are more likely to be co-infected with HPV, approximately 2 to 6 fold higher probably secondary to similar risk factors such as sexual behavior. In the HIV+ population, men have an increased relative risk of developing anal cancer compared to women (37.9 versus 6.8) (9). That risk increases depending on sexual practices. Men who has sex with men (MSM) is associated with a higher incidence of anal cancer. HIV+ MSM have doubled the rate of anal cancer as compared to HIV- MSM (70-100 per 100,000 versus 35 per 100,000) (10).
HPV infection persists in HIV+ patients compared to immunocompetent patients. HIV+ patients are seven times more likely to have persistent HPV infection. There is a suggestion that immunosuppression in HIV+ patients prevents clearance of HPV and subsequent higher risk of developing anal cancer.
The discovery and subsequent use of antiretroviral drugs (HAART) in the late 1990’s has led to a significant decrease in AIDS defining cancers. In the U.S, recommendations set forth by the United States Department of Health and Human Services for initiating HAART treatment include: all HIV positive patients who present with AIDS defining illness, and HIV positive patients with CD4<200 (cluster of differentiation 4) (11). It is also recommended that clinicians consider treatment for asymptomatic HIV positive patients with CD4 counts between 201-350 (11). However, HAART has not reduced the incidence of non-AIDS defining cancers such as anal cancer. One theory is that immunosuppression plays a role in the development of anal cancer. It has been suggested that immunosuppression not only leads to increased risk of non AIDS defining cancers but also increases the aggressive nature of such cancers (7). A French study examined the incidence of cancer in a cohort of HIV+ patients and found that a CD4 count less than 200 cells per uL and HIV viral load >100,000 copies per mL were associated with an increased risk of anal cancer. The majority of patients (93%) diagnosed with anal cancer had been treated with antiretroviral therapy for over 6 months (12).
Screening
Anal cancer and cervical cancer share many similar characteristics. Both anal cancer and cervical cancer develop from precursor lesions: anal intraepithelial neoplasia (AIN) and cervical intraepithelial neoplasia (CIN) respectively. The incidence and mortality from cervical cancer in the U.S. has significantly diminished with the routine use of cytology screening with the Papanicolau (Pap) smear test. Pap smears identify precancerous lesions and early treatment of these lesions has been shown to prevent the development of cervical cancer. As a result the rate of cervical cancer dramatically decreased in the U.S. In countries where screening for cervical cancer is not routinely done the incidence and mortality of cervical cancer is much greater.
Squamous cell carcinoma of the anus is thought to arise from a precancerous lesion. The etiology of this precancerous lesion is thought to involve integration of HPV into the patient’s genome. Similar to cervical cancer, a Bethesda staging criteria has been devised for precursor anal lesions (13). AIN1 is thought to be low grade squamous intraepithelial lesion (LSIL) whereas AIN 2, 3 are high grade squamous intraepithelial lesion (HSIL). Similar to cervical cancer, treatment is recommended for high grade precancerous (HSIL) anal lesions.
Studies have identified additional risk factors in the development of AIN. Wilkin et al (2004) studied the risk of developing AIN in HIV+ men (14). Almost three-quarters of men with abnormal anal cytology had co-infection with a high risk HPV serotype (HPV 16>>52>18>45) (14). Multivariate analysis indicated that abnormal cytology was more likely in patients with a history of RAI and no HAART treatment. AIN histology on biopsy was more likely in patients with history of RAI, history of no HAART use, young age (<40) and low CD4 count (<350). CD4 count was the most significant prognostic factor. Patient who were on HAART and had persistent low CD4 counts were also more likely to have AIN. The relationship between AIN and HAART use, CD4 count, and viral load is probably confounded as patients with lower CD4 counts are more likely to have high viral loads and to be started on HAART.
Recently physicians have begun to advocate anal cancer screening in the high risk HIV+ population. Studies support the high incidence of precancerous lesions in the HIV+ population. Wilkin et al. (2004) analyzed the prevalence of anal precursor lesions in HIV+ men and reported that almost ½ of patients had abnormal cytology on the anal Pap smear and subsequently 40% of these patients were found to have AIN histology by biopsy (14). Kreuter et al (2010) prospectively examined a population of 400 HIV+ MSM over a period of 5 yrs and determined that over 70% had some degree of AIN (10). 35% had high grade AIN and 2.5% had anal cell cancer detected on screening. More importantly Kreuter et al (2010) demonstrated that untreated AIN can progress to anal cancer in as little as 8 months (10). Previous studies in the mid 1990’s had showed AIN progressing to anal cancer over 3-5 years (14), (15). Also studies indicate that that the incidence of AIN has increased with the widespread use of HAART in the HIV+ population (15).
The feasibility of screening for anal cancer has been research extensively over the past decade. New York State has established screening guidelines for anal cancer in HIV+ patients (16). They recommend that all HIV+ patients undergo screening for anal cancer and propose a similar screening scheme to cervical cancer. Initially patients should have an annual visual and digital rectal exam plus an anal PAP. If PAP reveals abnormal anal cytology then a high-resolution anoscopy (HRA) should be performed similar to the colposcopy in cervical cancer. One caveat to anal cancer screening is that while the test is sensitive it is not specific. Both Palefsky et al (1997) and Goldstone et al (2001) showed that over 70%-90% of HIV+ MSM had some abnormal cytology on anal pap (17), (18). The correlation of abnormal pap with HSIL biopsy was poor. Therefore, all lesions noted on HRA should be biopsied. If HSIL is detected treatment should be offered, either medical ablation or surgical excision. If LSIL is detected the recommendation is to have repeat anal pap smears in 3-6 months. If persistent abnormal pap, these patients should have yearly HRA.
Mount Sinai implemented this practice in 2007 for all HIV+ patients (19). Researchers believe such practices are both cost effective and efficacious. Goldie et al (1999) performed a cost analysis on screening for AIN and found that screening increased quality-adjusted life expectancy for all HIV+ patients (20). Goldie et al (1999) calculated that screening with anal pap tests every year around time of diagnosis of HIV resulted in an incremental cost-effectiveness ratio of $16,600 per quality-adjusted life year saved (20). Screening more frequently than yearly did not provide any additional benefit.
Once HSIL histology is confirmed, there are a couple of treatment options. However there is still debate on the most efficacious treatment for precursor anal lesions. A study by Chang et al (2002) showed that surgery for treatment of AIN was effective in preventing recurrence of AIN in 8 HIV negative men studied but only effective in 23/29 HIV positive patients (21). Singh et al (2009) evaluated the efficacy of nonsurgical treatments (trichloroacetic acid, i.e. TCA) (22). Overall TCA worked well in younger patients (<48yo). For HIV+ patients specifically, those with two or fewer HSIL lesions responded the best. 32% had no residual lesion on follow up. HIV negative patients had a much better chance of clearance of AIN lesions than HIV+ patients. 75% of HIV+ patients had recurrence after clearance of the initial AIN lesion treated with TCA within 6 months suggesting that close follow up is needed in this high risk population (22). The treatment of AIN with surgery or with non surgical methods such as TCA is not without morbidity. Studies do show a low incidence of morbidity with possible side effects such as fibrosis and anal sphincter stenosis (3).
The risk of progression from AIN to anal cancer is high, ranging from 10-50% in HIV+ patients (23). Most experts at this time advocate screening of all HIV+ patients and treatment for all HGIL. The ease and cost effectiveness of screening seem to justify its use even though there are not prospective randomized trials proving a reduction in mortality. Treatment for AIN should be tailored based upon size, number, and location of the lesion. Both surgical and non surgical treatment options exist. There are recent and ongoing clinical trials for the detection and treatment of AIN conducted by the AIDS Associated Malignancies Clinical Trials Consortium which are documented on the NCI webpage (24). One such study is: Companion Study of Anogenital Human Papillomavirus Infection and Anogenital Squamous Intraepithelial Lesions in HIV-Positive Patients Participating in AIDS-Related Malignancy Clinical Trials (24).
Treatment of anal cancer
In 1974, Nigro was the first to report that squamous cell carcinoma of the anus responded favorably to combined chemotherapy and radiation. Since that time the standard of care has sifted from surgery which left all patients with a colostomy to a sphincter sparing approach of definitive concurrent chemotherapy and radiation therapy (RT) with surgery as salvage (25), (26).
The standard treatment for squamous cell carcinoma of the anus is concurrent mitomycin C (MMC), 5-fluorouracil (5-FU), and RT. There have been multiple prospective randomized trials that have shown improvement in local control, disease free survival, and sphincter preservation with the addition of chemotherapy to RT (27)-(30), (33). There have been 4 randomized trials that have established concurrent MMC and 5-FU with RT as the standard of care. The initial UKCCR (United Kingdom Coordinating Committee of Cancer Research) trial (ACT I) compared concurrent MMC and 5-FU with RT to RT (27). RT was prescribed to 45Gy given over 4 to 5 weeks with the inguinal lymph nodes and anus included in the target. A boost was given 6 weeks later if the patient had a > 50% response or a complete response. The boost was given either with external beam (15Gy) or Ir192 implant (25Gy). Clinical response was measured at 2 months after completion of the boost. The combined modality arm consisted of the same RT schedule with 2 cycles of chemotherapy; bolus MMC (12-10mg/m2) and continuous venous infusion 5FU (1000-750mg/m2/day for 4 days) commencing at the start of RT. An update was recently reported with a median follow up of 13 years. The hazard ratio for local regional relapse (HR=0.46, P<0.001), disease free survival (HR=0.70, P<0.001), anal cancer mortality (HR=0.61, P<0.001), and colostomy free survival (HR=0.76, P=0.004) all favored the combined modality arm. The hazard ratio for overall survival was 0.86 and not statistically significant (P=0.12). There was an increase in non-anal cancer deaths in the first 5 years in the combined modality arm which almost disappeared at 10 years. Also there were more deaths due to second malignancy in the combined modality arm (P=0.03). Again acute toxicity was higher in the concurrent arm but there were no differences in late toxicity (28).
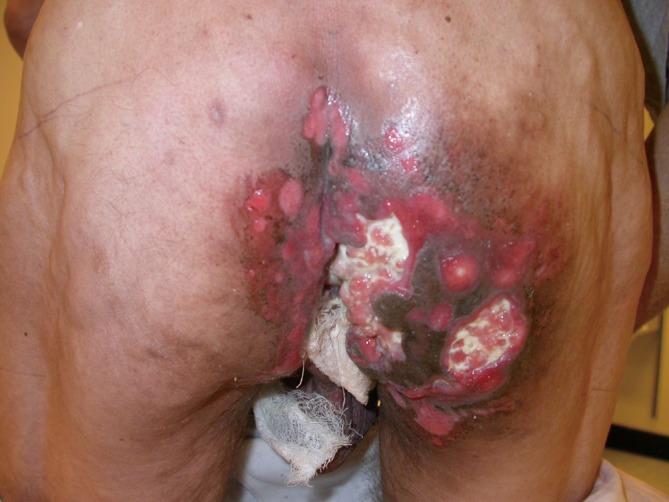
Figure 1. Acute cutaneous toxicity from chemoradiotherapy for a clinical T4 bulky, locally advanced anal canal carcinoma.
The EORTC (European Organization for Research and Treatment of Cancer) confirmed the results in a similar study comparing concomitant RT with 5FU and MMC versus RT alone (29). The locoregional control rate was improved by almost one-fifth and the colostomy free rate improved by one-third in the combined modality arm at 5 yrs. Both results were statistically significant. There was no difference between the two arms in overall survival. Again acute toxicity was worse in the combined modality arm (29).
The importance of MMC in the treatment of anal cancer was confirmed in the RTOG 87-04 (Radiation Therapy Oncology Group) trial which randomized patients to RT with MMC and 5FU versus RT and 5FU alone (30). RT total doses ranged from 45 to 54Gy. This trial showed that the MMC arm had superior colostomy free survival (71%vs 59%), disease free survival (73% vs. 51%), and fewer post treatment positive biopsies (7.7% vs. 15%) at 4years. All were statistically significant. Grade 4 and 5 acute toxicity were greater in the MMC arm (23% versus 7%) (30).
MMC is known to be toxic and the question was tested if a potentially less toxic agent, cisplatin, could be substituted. Specifically, the hemolytic uremic syndrome and thrombocytopenia are not uncommon life-threatening toxicities that result from use of MMC (31), (32). RTOG 98-11 tested concurrent MMC and 5FU versus induction cisplatin and 5FU followed by concurrent RT and cisplatin and 5FU. RT total doses ranged from 45-59 Gy. At five years the cisplatin arm had statistically significant inferior colostomy rate of 19% versus 10%. Other end points measured such as disease free survival, overall survival, local regional recurrence, and distant metastasis were not statistically different. Cisplatin was associated with less hematologic toxicity (33). The ACT II trial from the UK which also analyzed if cisplatin could replace MMC showed that there was no difference in clinical complete response or OS with either MMC or cisplatin (34). The ACT II trial also studied if maintenance therapy after definitive chemoradiation would be of benefit and reported that maintenance therapy did not improve overall survival or recurrence free survival. Follow-up for the ACT II trial is only at 3 yrs so further study is needed to determine if cisplatin could substitute for MMC (34). Currently concurrent 5FU + MMC with radiation is still the standard of care.
Treatment breaks due to toxicity lead to prolonged RT treatment duration and at times patients are unable to complete the planned RT. A report from Boston University Medical Center demonstrated that RT dose (≥ 54Gy) was significantly associated with local control and overall survival and treatment time less than 40 days also showed a trend toward improved outcome (35). Other studies confirmed that greater overall treatment time was associated with worse outcomes and local failure (36). This is a clinical example of accelerated repopulation of residual tumor clonagens, in all likelihood (37). Roohipour et al 2008 in a multivariate analysis of 131 patients with anal squamous cell carcinoma showed the inability to complete definitive RT and disease stage at diagnosis were both predictive of relapse free survival (38). A recent analysis of 2 RTOG trials showed that for every 2 weeks of treatment interruption there was a 9.4% increase in the hazard ratio for colostomy failure (39). The success of sphincter sparing treatment is in part dependent upon getting the patient through treatment without interruptions for treatment side effects.
It should be noted that during the development of the aforementioned prospective trials, there was a consensus among those designing the studies before the HAART era that an unfavorable therapeutic ratio would be seen in HIV+ patients. Hence this study population was heretofore excluded from participation onto such studies.
HIV positive patients and treatment for anal cancer
There have been concerns that HIV+ patients have increased toxicity and tolerate treatment less than the HIV negative patient. As a result physician bias has trended toward reducing the dose/amount of concurrent chemoradiation treatment for HIV+ patients for fear of causing unacceptable toxicity and a suboptimal therapeutic ratio. Such changes in practice can lead to treatment failures in the HIV+ population. Retrospective studies have been conducted to address such concerns.
Early reports likely contributed to the perception that HIV+ patients do not tolerate aggressive combined modality treatment. Peddada et al (1997) in a limited study demonstrated that 8 HIV+ patients with anal cancer could be treated with concurrent 5FU/MMC but used a lower dose of RT, 30Gy instead of the standard of care > 50Gy (40). All 8 patients achieved a clinical complete response. One out of eight 8 had a grade 4 hematologic toxicity. 50% of the patients were alive and disease free. However the remaining four patients died from AIDS related illnesses in the 3 year follow up (40).
Around the same time Kim et al (George Washington University, 2001) showed that HIV+ patients had worse outcomes and tolerance than HIV negative patients in the treatment of anal cancer using the standard RT dose of 50-54 Gy and concurrent full dose chemotherapy (6). In this study, analyzed patients were from 1985 to 1998 a period of time before the advent of HAART. The HIV+ patients included patients with AIDS defining illnesses, and low CD4 counts. These patients tended to have a lower performance status. Overall 5/13 HIV+ patients analyzed failed initial definitive chemoradiation compared to 9/60 HIV negative patients. If the patients with known AIDS were removed from the analysis, the differences in treatment outcomes between the two groups are reduced. The only patients that required treatment breaks were the patients with AIDS. Each required an unscheduled 3-4 week treatment break due to grade 4 toxicity (1-skin ulcer, 1-thrombocytopenia). Furthermore, in the Kim analysis, late toxicity (poor skin healing) was higher in the HIV+ group 4/10 versus 5/33. Another early study also suggested that HIV+ patients with AIDS may not tolerate anal cancer treatment. Clinicians at the Beth Israel Medical Center in NY (1987-1991) commented on the results of treating 9 HIV+ patients, 3 of which had AIDS (41). The authors reported over 50% needed more than 2 weeks of unscheduled treatment break due to toxicity. Over 50% had grade 3-4 skin toxicity. However, 7/9 did have a clinical complete response despite the toxicity. At least one of the two patients that did not have a complete response also had AIDS. The author do mention that an early antiretroviral, zidovudine was given concurrently with chemoradiation in patients and was well tolerated.
Delineation of subgroups in the HIV+ population can help identify HIV+ patients that may not tolerate treatment from those that can tolerate standard combined modality therapy. In 1999, Hoffman et al (UCSF) published a report on a small cohort of HIV+ patients treated for anal cancer (42). Hoffman et al (1999) stratified patients based on CD4 count and showed that values greater than 200 portend to superior treatment outcomes and tolerance (42). Patients with higher CD4 counts were more likely to receive the standard of care in terms of chemotherapy and RT dosing. These authors suggested that fear of toxicity caused physicians to empirically alter chemotherapy regimens in the HIV+ population. The mean RT dose was similar between the 2 groups ∼51 Gy. However even with an altered chemotherapy regimen the group with CD4 counts less than 200 still experienced more toxicity such as moist desquamation and hematologic suppression. In this group these side effects required longer treatment breaks and early treatment termination. An intact immune system appears to be critical to tolerating anal cancer treatment. A study from Emory University also found that HIV+ patients with CD4<200 did worse with anal cancer treatment (43). Of 17 HIV+ patients with anal cancer documented at Emory from 1994-2004, only those with CD4<200 were unable to complete treatment (43).
Antiretroviral drugs play a key role in controlling the HIV virus and helping bolster CD4 counts. Therapy for HIV changed dramatically in the mid 1990s with the implementation of HAART (highly active anti retroviral therapy). HAART therapy includes a combination of protease inhibitors (discovered/designed in 1995) and non nucleosidase reverse transcriptase inhibitors (1996). Widespread use of HAART came around 1999-2000. Papers discussing the use of HAART to aid in anal cancer treatment are thus limited. Hoffman et al (1999) at UCSF suggested that one patient in their cohort of 17 who initially had a CD4 count less than 200 tolerated the standard of care treatment for anal cancer due to the addition of a protease inhibitor which bolstered the CD4 count to greater than 200 (42). A later study done by Stadler et al (UT Southwestern 2004) demonstrated a trend toward improved efficacy of anal cancer treatment in HIV+ AIDS patient treated with HAART (44). Stadler et al (2004) compared outcomes in patients treated for anal cancer preHAART and post HAART (44). The UT Southwestern study differs from the other studies in that the chemotherapy used was 5FU/cisplatin instead of 5FU/MMC. The RT dose was similar at 54 Gy. In this study all patients had AIDS at time of diagnosis. Overall, 14 patients were analyzed, including 6 pre HAART and 8 on HAART. Stadler et al (2004) suggested a trend towards better treatment tolerability and outcome in patients treated with HAART. 2 year OS in patients on HAART was 67% vs. 17% in the pre-HAART era. 1yr and 3 yr mortality pre HAART was only 12% and 40% respectively compared to 67% and 80% for patients on HAART. The success of definitive treatment for HIV+ patients on HAART seems to fare the same as HIV negative patients in the randomized control trials. Moreover there was more toxicity in the preHAART patients (60%) compared to the HAART treated patients (50%) (44). It suggests that the HAART and increased CD4 count help patients tolerate treatment.
Recent single institutional studies have shown that as long as HIV+ patients can tolerate the standard of care treatment for anal cancer and do not have AIDS (i.e. CD4<200), the efficacy and durability of treatment is similar to immunocompetent patients. A group from Paris (Blazy et al 2005) reported on a cohort of 9 HIV+ men all on HAART treated with chemoradiation (45). They found no correlation between CD4 count and toxicity. Clinical outcome was similar to immunocompetent historical controls (45). Yet another single institutional study from St. Vincent’s (1997-2005) also reported that 32 HIV+ patients on HAART with mean CD4 count of 350 also did as well as immunocompetent patients (46). Similar to immunocompetent patients’ tumor size and treatment duration correlated with local regional control (46). A study at Case Western (1999-2007) compared treatment efficacy of immunocompetent and immunodeficient individuals (47). 14/36 patients were HIV+. The authors demonstrated similar efficacy of treatment and toxicity profile for both HIV+ and HIV negative patients. 3yr OS was 84-92%. The authors showed no correlation between CD4 count and response to treatment however the caveat being that 10/14 patients were on HAART and mean CD4 count was 190 (HIV1 RNA 16,670copies/ml) (47). Also HIV+ patients on RTOG 92-08 without treatment breaks did just as well as immunocompetent patients (48).
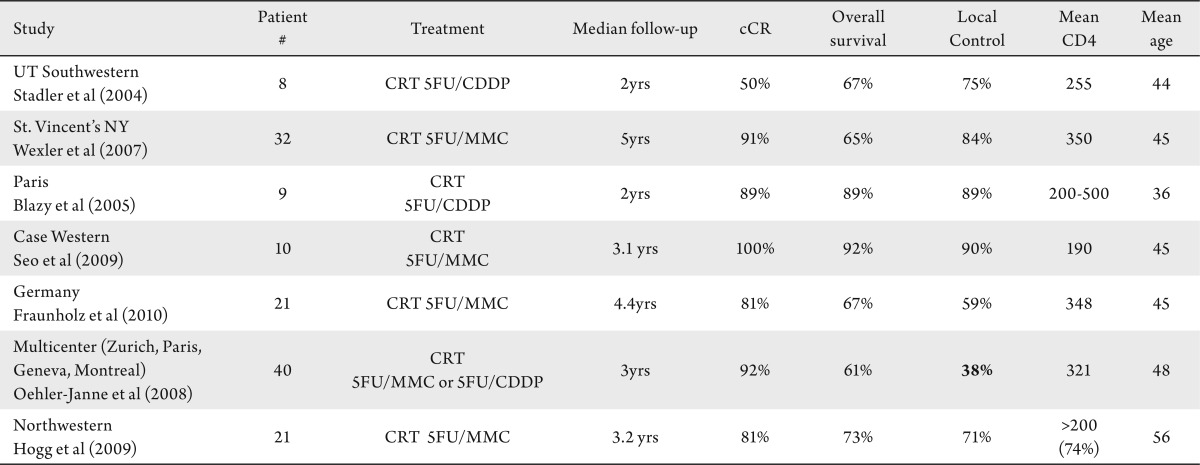
Table 1. Summary of anal cancer treatment outcomes of HIV positive patients on HAART. Table summarizes the results of only HIV positive patients on HAART. Generally all studies correlated to give a high overall survival and local control except for the local control reported from the multicenter trial from Europe. It is unclear if this is due to changes in chemotherapy practices due to fear of causing additional toxicity to the HIV positive patient or if these patients are inherently different than others reported. CRT: chemoradiation. MMC: mitomycin C. CDDP: cisplatin.
Another group from Germany (Fraunholz et al 2010) reported on a cohort of 21 HIV+ patients with anal cancer all on HAART (1997-2008) (49). While on HAART, all patients were able to complete the standard chemoradiation therapy for anal cancer. Only 5 cases required interruptions (median of 4 days). 81% had a complete clinical response, 5yr OS was 67%. Interestingly, this paper noted that CD4 counts dropped during treatment and one-third of patients had increases in HIV viral load (49). Both returned to baselines values at follow-up. It is unclear at this time what a transient increase in HIV viral load does to the overall disease progression in HIV+ patients. HAART appears to help HIV+ patients tolerate anal cancer treatment. However it has been observed that anal cancer treatment can cause immunosuppresion and patients need close monitoring during treatment. This immunosuppression may lead to the development of a specific pathologic subtype of anal cancer. The German study by Fraunholz et al (2010) noted that HIV+ patients on HAART had a large cell histology subtype of squamous cell carcinoma over 90% of the time compared to only 67% of HIV negative patients (49).
Not all reports state that HIV+ patients on HAART do fine with treatment. There are a couple of reports that show that HIV+ patients on HAARRT do worse than HIV negative patients. A multicenter cohort from Europe (Zurich, Paris, Geneva, Montreal, 1997-2006) reported on 40 HIV+ patients on HAART and 80 HIV negative patients (50). Overall there was >90% complete response. HIV+ patients on HAART had larger duration of treatment and more toxicities than immunocompetent patients. The 5 yr local control was only 38% for HIV+ patients on HAART compared to 87% for HIV negative patients (50). The Zurich author’s single institutional report stated that it was difficult for HIV+ patients to adhere to chemotherapy regimens and that chemotherapy had been decreased or changed in their cohort (51). This may be one explanation for the differences in outcomes. Northwestern (Hogg et al 2009) also suggested that HIV+ patients on HAART have more local recurrences and less response to therapy (52). Still there was >80% complete response and ∼70% overall survival in their single institutional experience (52).
The standard of care even for HIV+ patient is concurrent MMC and 5FU with high dose RT. Treatment modification may be necessary in patients with AIDS and a CD4 count of less than 200. It has been shown that treatment breaks lead to increased risk of failing definitive chemoradiation, likely a result, in part, from accelerated repopulation. The only curative option for RT failures is salvage surgery which results in a permanent colostomy. Only 50% of patients can be salvaged with surgery. Patients should be given the opportunity to participate in the AIDS Malignancy Consortium protocol: Phase II Study of Cisplatin, Fluorouracil, Cetuximab, and Radiotherapy in Patients With HIV-Associated Stage I-IIIB Anal Carcinoma (53). In aggregate, combined-modality approaches still holds the most promise for cure with sphincter preservation in the HIV+ patient.
Reducing treatment toxicity
Major acute toxicities in the treatment of anal cancer include diarrhea, skin desquamation, and immunosuppression. Severe acute toxicities require the radiation oncologist to break treatment while patients recover. RTOG 98-11 and UKCCR ACT I both used conventional RT in their study (27), (33). In the concurrent 5FU and MMC arm of RTOG 98-11 48% of the patients had grade 3 or 4 skin toxicity (33). ACT I reported 57% grade 3 or 4 skin toxicity in their concurrent arm (27). Reducing the volume of normal tissue exposed to high dose RT may minimize these toxicities. IMRT (intensity modulated radiation therapy) is a new RT delivery technique that allows for sculpting of the radiation dose (54), (55). This technique allows the radiation oncologist to reduce the volume of normal tissue exposed to high dose RT (26).
Trials using IMRT have been conducted to determine if this new technique still provides the same effective treatment outcome as conventional external beam RT while minimizing toxicities. Single institution studies seem to suggest encouraging results with IMRT. A study by Duke (Pepek et al 2010) demonstrated that out of 47 patients treated, the hematologic toxicity was 27%; there were no grade 3 skin toxicities and only 9% grade 3 GI toxicity (56). Only 18% of patients required treatment breaks. Again efficacy was in the 80% range (56). However long term follow up is lacking with a median follow up of only one year. Milano et al (2005) reported on 17 patients treated at the University of Chicago with similar results to the Duke trial (57). There were no treatment breaks from skin or GI toxicity and the authors were able to minimize toxicity to genitalia and small bowel. There was still 38% hematologic toxicity (57). These single institution studies show encouraging results with IMRT reducing acute toxicities and making treatment more tolerable.
Multi institutional trials using IMRT have also been conducted. A trial from the Mayo Clinic, University of California at San Diego, Emory University, Loyola University, and the University of Chicago was conducted to determine the efficacy of IMRT in treating anal cancer. 53 patients were analyzed, 8/53 were HIV+ (58). Treatment efficacy was similar to historical controls in that overall survival and colostomy free survival was in the 80% range (58). Median follow up time is only slightly over one year. In this study all HIV+ patients had a complete response. IMRT did help minimize GI toxicity (15% grade 3) however they still reported high rates of dermatologic (38%) and hematologic toxicity (34%). 41.5% of patients had to take treatment break (58). This trial recently reported the first volumetric study following IMRT implementation. There results suggest if the volume of small bowel receiving 30 Gy is less than 450cc then there is a 3 fold reduction in toxicity. IMRT had no effect on limiting bone marrow toxicity as 58% of patients had grade 3-4 leukopenia (59). This past year, data from a RTOG multiinstitutional phase II trial analyzing IMRT in the treatment of anal cancer were published in abstract form (60). Hong et al (2010) observed that IMRT was feasible and that IMRT decreased skin toxicity as well as high grade GI/GU toxicities more than 15% as compared to historic controls from the RTOG 9811 paper describing the standard of care for anal cancer (33),(60).
IMRT appears to be promising in reducing acute toxicities. Reducing acute toxicity and treatment breaks should improve outcomes. These benefits may be most important in the patient population most susceptible to acute toxicities. Long term follow up is needed to ensure that treatment efficacy is not compromised.
Anal cancer follow-up
The randomized control trials have demonstrated that concurrent chemoradiation (5FU/MMC) is efficacious in curing disease and preventing need for colostomy approximately 60-80% of the time (27)-(30). Preventing a colostomy is important in preserving a patient’s quality of life. The studies from the RTOG and EORTC have shown that time to colostomy and rate of colostomy is significantly improved with the use of concurrent chemoradiation, specifically 5FU/MMC +RT compared to RT alone or 5FU/cisplatin + RT. Close follow-up is needed to determine if patients are responding effectively to definitive chemoradiation.
Currently, the National Cancer Comprehensive Network (NCCN) guidelines state that patients should have a digital rectal exam 8-12 weeks after chemoradiation to determine response to treatment (61). If there is question of disease progression or no response, then a clinical biopsy is warranted. If biopsy is positive, the NCCN still recommends waiting an additional 4 weeks to assess response to chemoradiation. If persistent disease is biopsy positive then restaging is indicated. If disease is local (i.e. no distant metastases) then an APR with colostomy is recommended with/without groin dissection based on nodal positivity for salvage cure. Salvage surgery is effective approximately 50-60% of the time (62).
Otherwise best case scenario is complete remission of disease. If there is complete response to chemoradiation then clinical exams are recommended every 3-6 months for the next 5 years (61).
Research in anal cancer
Another avenue of research in anal cancer treatment involves elucidating specific molecular targets. Three genes well-known in carcinogenesis, EGFR, c-Met, and VEGFR1, are overexpressed in anal cancers especially in HIV+ patients potentially providing specific molecular targets for therapy (63). Specific protease inhibitors a component of HAART have been shown to be radiosensitizers in tumors with an active PI3kinase/akt pathway in vitro. Brunner et al (2008) demonstrated nelfinavir’s, a protease inhibitor, efficacy in the treatment of HIV+ pancreatic cancer patients (64). It may be beneficial to identify if the PI3kinase/AKT pathway is overexpressed in HIV+ anal cancer tumor cells. Another protease inhibitor, saquinavir, has been shown to increase apoptosis in a variety of cancer cell lines via inhibition of the proteasome pathway suggesting another pathway which may be targeted (65). Research into how HAART affects chemotherapy needs to be undertaken. Through future research, the oncologist may individually tailor both the cancer treatment and the HAART regimen to maximize treatment outcomes and minimize toxicities.
Conclusion
Anal cancer, once a rare entity, is increasing in incidence especially in the HIV+ population. Aggressive chemoradiation treatment is still the key to controlling the disease while preserving quality of life (i.e. preventing a colostomy). Patients with CD4>200 have the best treatment outcome as they can tolerate the most aggressive treatment. Accordingly, worse treatment outcomes in HIV+ patients include patients who are unable to complete the prescribed radiotherapy dose in a timely manner, refuse HAART, do not respond to HAART and/or have larger tumors (>3cm) at diagnosis. Thus screening for anal precursor lesions in the HIV+ population is important and should be performed yearly to prevent the development of anal cancer. The National Comprehensive Cancer Network recommendations for anal cancer treatment of HIV positive patients state that patient should be treated with concurrent chemoradiation preferably the standard 5FU + MMC with radiation. Dose escalation of radiation is advised if tumor is large (i.e. >5cm). If there is an indication that the HIV+ patient may not tolerate full treatment due to CD4 counts or AIDS related sequelae dose reduction or omission of MMC may be considered (61). Patients should also be given the opportunity to participant in the AIDS Malignancy Consortium protocol: Phase II Study of Cisplatin, Fluorouracil, Cetuximab, and Radiotherapy in Patients With HIV-Associated Stage I-IIIB Anal Carcinoma (53). Otherwise the standard of care for the treatment of anal cancer in the HIV+ population remains concurrent MMC or cisplatin plus 5FU with concomitant RT. This treatment still holds the most promise for cure with sphincter preservation in the HIV+ patient.
Footnotes
No potential conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 Jul 7; doi: 10.3322/caac.20073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Melbye M, Rabkin C, Frisch M, Biggar RJ. Changing patterns of anal cancer incidence in the United States, 1940-1989. Am J Epidemiol. 1994;139:772–80. doi: 10.1093/oxfordjournals.aje.a117073. [DOI] [PubMed] [Google Scholar]
- 3.Klencke B. American Society of Clinical Oncology Educational Book. ASCO, Alexandria, Va; 2002. pp. 260–5. [Google Scholar]
- 4.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 5.Kauh J, Koshy M, Gunthel C, Joyner MM, Landry J, Thomas CR., Jr Management of anal cancer in the HIV-positive population. Oncology (Williston Park) 2005;19:1634–8. discussion 1638-40, 1645 passim. [PubMed] [Google Scholar]
- 6.Kim JH, Sarani B, Orkin BA, Young HA, White J, Tannebaum I, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum. 2001;44:1496–502. doi: 10.1007/BF02234605. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet F, Chêne G. Evolving epidemiology of malignancies in HIV. Curr Opin Oncol. 2008;20:534–40. doi: 10.1097/CCO.0b013e32830a5080. [DOI] [PubMed] [Google Scholar]
- 8.Cañadas MP, Darwich L, Sirera G, Bofill M, Piñol M, Garcia-Cuyas F, et al. Human papillomavirus 16 integration and risk factors associated in anal samples of HIV-1 infected men. Sex Transm Dis. 2010;37:311–5. doi: 10.1097/OLQ.0b013e3181c9c23f. [DOI] [PubMed] [Google Scholar]
- 9.Klencke B, Palefsky J. Anal cancer: an HIV-associated cancer. Hematol Oncol Clin North Am. 2003;17:859–72. doi: 10.1016/s0889-8588(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 10.Kreuter A, Potthoff A, Brockmeyer NH, Gambichler T, Swoboda J, Stücker M, et al. Anal carcinoma in human immunodeficiency virus-positive men: results of a prospective study from Germany. Br J Dermatol. 2010 Feb 22; doi: 10.1111/j.1365-2133.2010.09712.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.United States Department of Health and Human Services (DHHS) Developed by the DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council (OARAC) Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2009 [Google Scholar]
- 12.Guiguet M, Boué F, Cadranel J, Lang JM, Rosenthal E, Costagliola D, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–9. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 13.Bean SM, Chhieng DC. Anal-rectal cytology: A Review. Diagnostic Cytopathology. 2009;38:538–46. doi: 10.1002/dc.21242. [DOI] [PubMed] [Google Scholar]
- 14.Wilkin TJ, Palmer S, Brudney KF, Chiasson MA, Wright TC. Anal intraepithelial neoplasia in heterosexual and homosexual HIV-positive men with access to antiretroviral therapy. J Infect Dis. 2004;190:1685–91. doi: 10.1086/424599. [DOI] [PubMed] [Google Scholar]
- 15.Palefsky JM, Holly EA, Efirdc JT, Da Costa M, Jay N, Berry JM, et al. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–14. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 16.Neoplastic complications of HIV infection. Available from: http://www.hivguidelines.org/clinical-guidelines/adults/neoplastic-complications-ofhiv-infection/#V.%20ANAL%20DYSPLASIA%20AND%20CANCER. [DOI] [PubMed]
- 17.Palefsky JM, Holly EA, Hogeboom CJ, Berry JM, Jay N, Darragh TM. Anal cytology as a screening tool for anal squamous intraepithelial lesions. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:415–22. doi: 10.1097/00042560-199704150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Goldstone SE, Winkler B, Ufford LJ, Alt E, Palefsky JM. High prevalence of anal squamous intraepithelial lesions and squamous-cell carcinoma in men who have sex with men as seen in a surgical practice. Dis Colon Rectum. 2001;44:690–8. doi: 10.1007/BF02234568. [DOI] [PubMed] [Google Scholar]
- 19.Tider DS, Caprio GR, Gaisa M, Klein RS, Goldstone SE. Successful initiation of an anal cancer screening and treatment program at a New York City HIV clinic. AIDS. 2010;24:1085–1086. doi: 10.1097/QAD.0b013e328336e978. author reply 1086-7. [DOI] [PubMed] [Google Scholar]
- 20.Goldie SJ, Kuntz KM, Weinstein MC, Freedberg KA, Welton ML, Palefsky JM. The clinical effectiveness and cost-effectiveness of screening for anal squamous intraepithelial lesions in homosexual and bisexual HIV-positive men. JAMA. 1999;281:1822–9. doi: 10.1001/jama.281.19.1822. [DOI] [PubMed] [Google Scholar]
- 21.Chang GJ, Berry JM, Jay N, Palefsky JM, Welton ML. Surgical treatment of high-grade anal squamous intraepithelial lesions: a prospective study. Dis Colon Rectum. 2002;45:453–8. doi: 10.1007/s10350-004-6219-8. [DOI] [PubMed] [Google Scholar]
- 22.Singh J, Kuohung V, Palefsky J. Efficacy of trichloroacetic acid in the treatment of anal intraepithelial neoplasia in HIV-positive and HIV-negative men who have sex with men. J Acquir Immune Defic Syndr. 2009;52:474–9. doi: 10.1097/QAI.0b013e3181bc0f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleshner PR, Chalasani S, Chang GJ, Levien DH, Hyman NH, Buie WD, et al. Practice parameters for anal squamous neoplasms. Dis Colon Rectum. 2008;51:2–9. doi: 10.1007/s10350-007-9093-3. [DOI] [PubMed] [Google Scholar]
- 24.Berry JM. AIDS Associated Malignancies Clinical Trials Consortium. Companion Study of Anogenital Human Papillomavirus Infection and Anogenital Squamous Intraepithelial Lesions in HIV-Positive Patients Participating in AIDS-Related Malignancy Clinical Trials. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=590397&protocolsearchid=5021159&version=patient, http://clinicaltrials.gov/ct2/show/NCT00622440, http://clinicaltrials.gov/ct2/show/NCT00428285, http://www.ncbi.nlm.nih.gov/pubmed/19050387]
- 25.Nigro N, Vaitkevicius V, Considine BJ. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17:354–6. doi: 10.1007/BF02586980. [DOI] [PubMed] [Google Scholar]
- 26.Chan E, Kachnic LA, Thomas CR., Jr Anal cancer: Progress on combined-modality and organ preservation. Curr Prob Cancer. 2009;33:295–326. doi: 10.1016/j.currproblcancer.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–54. [PubMed] [Google Scholar]
- 28.Northover J, Glynne-Jones R, Sebag-Montefiore D, James R, Meadows H, Wan S, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer. 2010;102:1123–8. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–9. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 30.Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–39. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 31.Thomas CR, Jr, Carter IK, Leslie WT, Sutton F. Common emergencies in cancer medicine: Hematologic and gastrointestinal syndromes. J Natl Med Assoc. 1992;84:165–76. [PMC free article] [PubMed] [Google Scholar]
- 32.Givens ML, Crandall J. Renal complications in oncologic patients. Hem Onc Clin N Am. 2010;24:567–75. doi: 10.1016/j.hoc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–21. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 34.James R, Wan S, Glynne-Jones R, Sebag-Montefiore D, Kadalayil L, Northover J, et al. A randomized trial of chemoradiation using mitomycin or cisplatin, with or without maintenance cisplatin/5FU in squamous cell carcinoma of the anus (ACT II) J Clin Oncol. 2009;27:18s. suppl; abstract. [Google Scholar]
- 35.Constantinou EC, Daly W, Fung CY, Willett CG, Kaufman DS, DeLaney TF. Time-dose considerations in the treatment of anal cancer. Int J Radiat Oncol Biol Phys. 1997;39:651–7. doi: 10.1016/s0360-3016(97)00329-5. [DOI] [PubMed] [Google Scholar]
- 36.Graf R, Wust P, Hildebrandt B, Gögler H, Ullrich R, Herrmann R, et al. Impact of overall treatment time on local control of anal cancer treated with radiochemotherapy. Oncology. 2003;65:14–22. doi: 10.1159/000071200. 12837978 [DOI] [PubMed] [Google Scholar]
- 37.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams and Wilkins; 2006. Chapter 22; pp. 384–95. [Google Scholar]
- 38.Roohipour R, Patil S, Goodman KA, Minsky BD, Wong WD, Guillem JG, et al. Squamous-cell carcinoma of the anal canal: predictors of treatment outcome. Dis Colon Rectum. 2008;51:147–53. doi: 10.1007/s10350-007-9125-z. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Josef E, Moughan J, Ajani J, Flam M, Gunderson L, Pollock J, et al. The impact of overall treatment time on survival and local control in anal cancer patients: a pooled data analysis of RTOG trials 8704 and 9811. IJROBP. 2009;75(supplement) doi: 10.1200/JCO.2010.29.1351. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peddada AV, Smith DE, Rao AR, Frost DB, Kagan AR. Chemotherapy and low-dose radiotherapy in the treatment of HIV-infected patients with carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 1997;37:1101–5. doi: 10.1016/s0360-3016(96)00596-2. [DOI] [PubMed] [Google Scholar]
- 41.Chadha M, Rosenblatt EA, Malamud S, Pisch J, Berson A, et al. Squamouscell carcinoma of the anus in HIV-positive patients. Dis Colon Rectum. 1994;37:861–5. doi: 10.1007/BF02052589. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman R, Welton ML, Klencke B, Weinberg V, Krieg R. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol Biol Phys. 1999;44:127–31. doi: 10.1016/s0360-3016(98)00528-8. [DOI] [PubMed] [Google Scholar]
- 43.Edelman S, Johnstone P. Combined modality therapy for HIV-infected patients with squamous cell carcinoma of the anus: outcomes and toxicities. Int J Radiat Oncol Biol Phys. 2006;66:206–11. doi: 10.1016/j.ijrobp.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 44.Stadler RF, Gregorcyk SG, Euhus DM, Place RJ, Huber PJ, Simmang CL, et al. Outcome of HIV-infected patients with invasive squamous-cell carcinoma of the anal canal in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2004;47:1305–9. doi: 10.1007/s10350-004-0584-1. [DOI] [PubMed] [Google Scholar]
- 45.Blazy A, Hennequin C, Gornet JM, Furco A, Gérard L, Lémann M, et al. Anal carcinomas in HIV-positive patients: high-dose chemoradiotherapy is feasible in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2005;48:1176–81. doi: 10.1007/s10350-004-0910-7. [DOI] [PubMed] [Google Scholar]
- 46.Wexler A, Berson AM, Goldstone SE, Waltzman R, Penzer J, Maisonet OG, et al. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2008;51:73–81. doi: 10.1007/s10350-007-9154-7. [DOI] [PubMed] [Google Scholar]
- 47.Seo Y, Kinsella MT, Reynolds HL, Chipman G, Remick SC, Kinsella TJ. Outcomes of chemoradiotherapy with 5-Fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys. 2009;75:143–9. doi: 10.1016/j.ijrobp.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 48.John M, Pajak T, Flam M, Hoffman J, Markoe A, Wolkov H, et al. Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92-08. Cancer J Sci Am. 1996;2:205–11. [PubMed] [Google Scholar]
- 49.Fraunholz I, Weiss C, Eberlein K, Haberl A, Rödel C. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for invasive anal carcinoma in human immunodeficiency virus-positive patients receiving highly active antiretroviral therapy. Int J Radiat Oncol Biol Phys. 2010;76:1425–32. doi: 10.1016/j.ijrobp.2009.03.060. [DOI] [PubMed] [Google Scholar]
- 50.Oehler-Jänne C, Huguet F, Provencher S, Seifert B, Negretti L, Riener MO, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26:2550–7. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 51.Oehler-Jänne C, Seifert B, Lütolf UM, Ciernik IF. Local tumor control and toxicity in HIV-associated anal carcinoma treated with radiotherapy in the era of antiretroviral therapy. Radiat Oncol. 2006;1:29.. doi: 10.1186/1748-717X-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hogg ME, Popowich DA, Wang EC, Kiel KD, Stryker SJ, Halverson AL. HIV and anal cancer outcomes: a single institution’s experience. Dis Colon Rectum. 2009;52:891–7. doi: 10.1007/DCR.0b013e31819eefa6. [DOI] [PubMed] [Google Scholar]
- 53.Sparano J, Kachnic L, Aboulafia D. AIDS associated malignancies clinical trials consortium. Phase II study of Cisplatin, Fluorouracil, Cetuximab, and radiotherapy in patients with HIV-associated stage I-IIIB anal carcinoma. Available from: http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=440065&version=HealthProfessional&protocolsearchid=2709686.
- 54.Fuller CD, Thomas CR., Jr Chemoradiation for anal cancer: the more things change, the more they stay the same. Oncology (Williston Partk) 2010;25:427–30. [PubMed] [Google Scholar]
- 55.Herman J, Thomas CR., Jr Intensity modulated radiation therapy (IMRT) for anal cancer: an obvious yet complicated transition. Oncology (Williston Park) 2010 in-press. [PubMed] [Google Scholar]
- 56.Pepek JM, Willett CG, Wu QJ, Yoo S, Clough RW, Czito BG. Intensity-Modulated Radiation Therapy for Anal Malignancies: A Preliminary Toxicity and Disease Outcomes Analysis. Int J Radiat Oncol Biol Phys. 2010 Mar 16; doi: 10.1016/j.ijrobp.2009.09.046. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Milano MT, Jani AB, Farrey KJ, Rash C, Heimann R, Chmura SJ. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2005;63:354–61. doi: 10.1016/j.ijrobp.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 58.Salama JK, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007;25:4581–6. doi: 10.1200/JCO.2007.12.0170. [DOI] [PubMed] [Google Scholar]
- 59.Devisetty K, Mell LK, Salama JK, Schomas DA, Miller RC, Jani AB, et al. A multi-institutional acute gastrointestinal toxicity analysis of anal cancer patients treated with concurrent intensity-modulated radiation therapy (IMRT) and chemotherapy. Radiother Oncol. 2009;93:298–301. doi: 10.1016/j.radonc.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 60.Hong TS, Tsai HK, Coen J, Blaszkowsky LS, Hartshorn KL, Kwak EL, et al. Dose-painted intensity-modulated radiation therapy for anal cancer: A multi-institutional report of acute toxicity and response to therapy. J Clin Oncol. 2010;28(s15_suppl):4144. doi: 10.1016/j.ijrobp.2010.09.030. Abstract. [DOI] [PubMed] [Google Scholar]
- 61.National Comprehensive Cancer Network Guidelines for Treatment of Anal Cancer. 2010. Available from: http://www.nccn.org/professionals/physician_gls/PDF/anal.pdf.
- 62.Pocard M, Tiret E, Nugent K, Dehni N, Parc R. Results of salvage abdominoperineal resection for anal cancer after radiotherapy. Dis Colon Rectum. 1998;41:1488–93. doi: 10.1007/BF02237294. [DOI] [PubMed] [Google Scholar]
- 63.Walker F, Abramowitz L, Benabderrahmane D, Duval X, Descatoire V, Hénin D, et al. Growth factor receptor expression in anal squamous lesions: modifications associated with oncogenic human papillomavirus and human immunodeficiency virus. Hum Pathol. 2009;40:1517–27. doi: 10.1016/j.humpath.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Brunner TB, Geiger M, Grabenbauer GG, Lang-Welzenbach M, Mantoni TS, Cavallaro A, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008;26:2699–706. doi: 10.1200/JCO.2007.15.2355. [DOI] [PubMed] [Google Scholar]
- 65.Pajonk F, Himmelsbach J, Riess K, Sommer A, McBride WH. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002;62:5230–5. [PubMed] [Google Scholar]