Abstract
Pelvic and distant recurrences in rectal cancer can be associated with substantial morbidity, and patients with stage II and III disease are at increased risk for both local and distant failure when compared to patients with earlier stage disease. Refinement of surgical techniques have helped to reduce the risk of recurrence, and adjuvant therapies such as radiation to the tumor and regional lymph nodes and 5-fluorouracil-based systemic therapies have helped to further provide local control and may have an impact on overall survival. Numerous studies have been completed internationally in an effort to determine the optimal treatment regimen for this patient population. The importance of pre-therapy staging is of key importance as sequencing of therapy appears to significantly impact outcome. In the United States, patients with stage II/III rectal cancer are recommended to undergo preoperative concurrent pelvic radiation and chemotherapy followed by surgery several weeks later in order to maximize treatment response, which is then followed by approximately 4 months of adjuvant 5-fluorouracil-based systemic therapy. In Europe, there is substantial evidence supporting the use of neoadjuvant radiation therapy, however the role of concurrent chemotherapy remains a question of debate. Regardless of definitive management strategy, close follow-up in the post-treatment setting is important for early tumor detection and for managing treatment-related side-effects.
Keywords: SStage II/III rectal cancer, Combined modality therapy, neoadjuvant therapy, Radiation Therapy, 5-Fluorouracil
Introduction
It is estimated that over 40,000 patients were diagnosed with rectal cancers in 2009 in the United States alone (1). While curative surgery remains the mainstay of definitive management, local and distant recurrence rates for rectal cancer after curative resection can be significant. The main prognostic determinant is stage, with those patients exhibiting invasion through the muscle wall or lymph node involvement (American Joint Committee on Cancer [AJCC] stage II or III) being at increased risk for both locoregional and systemic failure (2). More so than in colon cancer, locoregional failure is a significant concern due to the lack of serosa in the rectum and the technical difficulties of rectal cancer surgery. Compounding this, recurrence in the pelvis carries substantial morbidity (3). Secondary to these concerns, there has been refinement in surgical technique along with a variety of adjuvant therapies and therapy schedules being investigated over the last several years. The results of several randomized trials have been published attempting to determine the ideal sequence and technique of surgery, radiation therapy, and systemic therapy. This manuscript reviews the current clinical practice guidelines for patients with stage II/III rectal cancer and the published data supporting these recommendations.
Patient work-up/staging
In the treatment of rectal cancer, there are many different treatment paradigms depending on the extent of disease, making initial staging and work-up extremely important. And with recent investigations showing the importance of treatment sequence, inaccurate initial staging can potentially have a considerable impact on treatment outcome.
For patients with a newly diagnosed rectal cancer, a full colonoscopy should be performed to ensure that there are no other lesions in the large intestine that would impact management. In addition, a rigid proctoscopy should be performed by the surgeon in order to determine the size and location of the tumor, particularly the distance of the lesion from the anal verge. Additional work-up includes a full physical examination, computed tomography (CT) of the chest, abdomen, and pelvis, and a carcinoembryonic antigen (CEA) level (4). Ideally, each patient should also undergo either an endoscopic ultrasound or magnetic resonance imaging (MRI) in order to more precisely assess both tumor depth and the presence of adjacent lymph nodes. Both ultrasound and MRI have been found on meta-analysis to be more sensitive than CT for determining depth of tumor invasion on pre-treatment examination, while all three modalities had similar sensitivity and specificity in determining lymph node involvement (5). Following assessment of clinical stage, it is recommended that the patient’s case be presented in a multidisciplinary fashion with input from surgical, medical, and radiation oncologists, pathologists, and radiologists to reach a consensus on the recommended course of treatment.
Surgery
The anatomy of the bony pelvis makes complete tumor resection and dissection of mesenteric nodes at risk for metastasis within the mesorectum technically difficult (6). In addition, the close proximity of soft-tissue organs such as the bladder, vagina, and ureters, along with the absence of a serosal barrier allowing for early tumor extension into the perirectal tissue, further impacting the complexity level. Early randomized trials of patients with stage II/III disease found local recurrence (LR) rates of approximately 25-30% for patients treated with surgery alone (7)-(10). Surgery in these studies often relied on blunt dissection of the rectal fascia, a technique that many times failed in removing all tumor-bearing tissue, particularly at the circumferential margin (11),(12).
These relatively high recurrence rates led to a focus on a more anatomically precise surgery; the total mesorectal excision (TME). With TME, sharp dissection of the entire mesentery of the hindgut is performed. In several reports, results of TME were shown to be quite impressive both in terms of LR and overall recurrence (11)-(14). While TME is a more extensive surgery, data suggest no significant differences in operative mortality or complications between TME and conventional surgery (15). Maximal radial margin should be attained during surgery, as positive and close margins have been shown to increase rates of both local and distant recurrence (16)-(18).
In the United States, it is recommended that patients with stage II/III rectal cancers undergo transabdominal resection, and sphincter preservation is preferable if technically feasible. For those patients with tumors in the upper rectum, a low anterior resection (LAR) can be performed, extended several centimeters past the tumor distally with subsequent creation of a colorectal anastomosis. For those tumors in the low rectum, it is recommended that patients undergo TME with colorectal or coloanal anastomosis or alternatively, an abdominoperineal resection (APR) with the creation of a colostomy (4),(19).
Rationale for radiation therapy
In patients with stage II/III disease treated with conventional surgery, radiation therapy has often been employed, with and without systemic therapy, in order to reduce the risk of local recurrence. The timing (preoperative versus [vs.] postoperative), treatment dose and duration, and its sequence with adjuvant systemic therapy have all been investigated in this patient population. In Europe, 25 Gray (Gy) in 5 daily treatment fractions delivered preoperatively followed immediately by surgery has been extensively studied. While many studies showed a benefit in terms of local control (LC), there was no large randomized trial showing significant difference in overall survival (OS) between patients treated neoadjuvantly with radiation therapy and patients treated with surgery alone until the completion of the Swedish Rectal Cancer Trial (20). In this trial, the neoadjuvant arm received short course RT followed by surgery within 1 week of finishing RT. At 5 years, local recurrence was reduced from 27% to 11% (p<0.001) and OS was improved from 48% to 58% (p=0.004) with the addition of neoadjuvant irradiation (10).
Earlier trials including the Swedish Rectal Cancer Trial have been criticized for not using standardized surgery techniques. Proponents of TME argued that with improvements in surgical technique, radiation therapy was of marginal benefit (11),(12),(14). This led to the Dutch CKVO 95-04 trial randomizing patients with clinically resectable rectal cancer to surgery alone by TME, or short course radiation followed by TME (21). In this study, there was no significant difference in OS, but LR was decreased with short course neoadjuvant radiation (12% vs. 6% at 5 years). Patients with stage III disease randomized to surgery alone, had a 15% LR at 2 years compared to 4.3% for patients receiving neoadjuvant therapy (21),(22). Meta-analyses comparing surgery alone to neoadjuvant radiation and surgery have confirmed the LC benefit, but there remains debate over whether this translates into an improvement in OS (23),(24).
There are questions regarding the radiobiological limitations of short course neoadjuvant radiation (25)-(27). High dose-per-fraction short course radiation has been found to induce a relatively high rate of acute toxic reactions and increases perioperative morbidity (28),(29). Acute toxicity in the Dutch trial included 10% of patients with neurotoxicity, 12% with postoperative anastomotic leaks, and 29% with perineal wound complications (30). Also, with larger fraction sizes (5 Gy) there is a possibility for increased late side effects as seen in the Swedish Trial. In that study, a number of patients experienced neurogenic symptoms in the gluteal and hamstring region, leading to chronic pain and difficulty with ambulation (31). Despite the potential for increased toxicity, short course neoadjuvant radiation therapy is convenient for patients, leads to timely surgery, and contains cost, leading to many European institutions to adopt this regimen in patients with stage II/III disease (21).
Rationale for chemotherapy and chemoradiotherapy
Systemic therapies, particularly those featuring 5-Fluorouracil (5-FU), have been widely studied as adjuvant treatment in stage II/III rectal cancer. 5-FU serves as a radiosensitizer to improve the therapeutic ratio of radiation therapy, and also works to reduce microscopic systemic disease (32). The United States National Institutes of Health (NIH) recommended in 1990 that all patients with stage II or III rectal cancer should receive adjuvant chemoradiotherapy i.e. combined modality therapy (CMT) following the publication of results from a series of prospective randomized trials showing the efficacy of postoperative radiation therapy in combination with 5-FU-based chemotherapy (7),(8),(33)-(36). The Gastrointestinal Tumor Study Group (GITSG) 7175 study showed improved LC and OS in patients receiving postoperative irradiation (40-44 Gy) with concurrent 5-FU followed by maintenance chemotherapy (7). The National Surgical Adjuvant Breast and Bowel Project (NSABP) R-01 showed a reduction in LC with adjuvant radiation therapy alone and improved OS in males receiving adjuvant 5-FU-based chemotherapy alone (9). The North Central Cancer Treatment Group (NCCTG) 79-47-51 trial compared postoperative radiation therapy to 5-FU-based postoperative CMT, with the CMT group having statistically significant advantages in LC, control of distant metastases, and OS (34). NSABP R-02 compared postoperative chemotherapy alone to CMT and found the rate of LC was significantly improved in the CMT group (37).
In Europe, the role of systemic therapy in the neoadjuvant setting has been investigated. In the French FFCD 9203 study, patients with resectable T3/T4 tumors neoajuvantly received either radiation therapy alone (45 Gy in 25 fractions) or the same radiation concurrent with bolus 5-FU/leucovorin, with all patients undergoing surgery 3-10 weeks after therapy, followed by all patients receiving postoperative 5-FU/leucovorin (38). Grade 3/4 acute toxicity was more frequent with CMT (14.6% vs. 2.7%; p<0.05) and there was no difference in sphincter preservation. However, pathologic complete response (CR) was more frequent with CMT (11.4% vs. 3.6%; p<0.05). And while there was no significant impact on OS, at 5 years, the rate of LR was lower with CMT (8.1% vs. 16.5%; p<0.05).
In the European Organization for Research and Treatment of Cancer (EORTC) 22921 study, patients with clinical T3 or T4 resectable rectal lesions were randomized to preoperative radiation therapy, preoperative CMT, preoperative radiation therapy and postoperative chemotherapy, or preoperative CMT with postoperative chemotherapy. Radiation therapy consisted of 45 Gy in 25 fractions, chemotherapy consisted of bolus 5-FU and leucvorin (for 2 cycles when given preoperatively and for 4 cycles when given postoperatively) (39). The addition of preoperative chemotherapy allowed for a significant increase in tumor downstaging (p<0.0001) at the time of surgery, but did not have a significant effect on sphincter preservation (p=0.47) (40). Among the 4 groups, there was no significant difference in OS. However, the addition of chemotherapy did significantly affect the rate of LR, with 5-year LR rates of 8.7%, 9.6%, and 7.6% in the groups that received chemotherapy preoperatively, postoperatively, or both, respectively, and 17.1% in radiation therapy-only group (p=0.002).
Not all studies have confirmed a therapeutic benefit for neoadjuvant CMT. In a phase III study by the Polish Rectal Cancer Group, patients with resectable clinical T3 or T4 disease were treated with either preoperative short-course radiation (25 Gy in 5 fractions) and surgery within a week or preoperative CMT (50.4 Gy in 28 fractions and bolus 5-FU and leucovorin) and surgery 4-6 weeks later (41). While the early analysis of the trial showed a higher pathological CR rate, reduction in positive circumferential margins and increased downstaging at surgery in the CMT arm, further analysis revealed that among the two groups, there were no significant benefits in terms of sphincter preservation, OS, DFS, LC, or rate of late toxicity (41). In addition, the preoperative CMT arm had a significantly higher rate of acute toxicity (18.2% versus 3.2%; p<0.001).
Sequencing of adjuvant therapy
Preoperative radiation therapy (with or without systemic therapy) offers certain theoretical advantages that postoperative radiation therapy or CMT does not. In lesions of the distal rectum, preoperative therapy may allow for sphincter preservation. And for locally advanced (T4) lesions that may be otherwise unresectable, preoperative therapy may allow for the possibility of tumor downstaging and resection. Preoperative radiation therapy also allows for better definition of gross tumor volumes during radiation planning and may allow for smaller treatment portals. With preoperative radiation therapy, the perineum is often avoided from treatment and potentially less small bowel is irradiated since it is more mobile, and the anastomosis is not in the treatment field. In addition radiation before surgery can potentially sterilize the operative field, thus decreasing the risk of tumor cells spilling during surgery. Irradiating preoperatively has increased radiosensitivity compared to postoperative therapy due to preserved vasculature thus allowing for better tumor oxygenation (25). Therefore, preoperative radiation should theoretically improve the therapeutic ratio over postoperative therapy (25)-(27).
Three large randomized trials were designed to compare preoperative and postoperative CMT in stage II/III rectal cancer. All three used conventional doses of daily radiation and concurrent 5-FU-based chemotherapy with pretreatment assessment of the planned surgical procedure. Two of the trials (NSABP R-03 and Intergroup 0147) were closed early due to low accrual and thus the data from these studies is limited. Preliminary results of the NSABP R-03 trial demonstrated that 23% of patients treated neoadjuvantly had a clinical CR and a larger proportion of neoadjuvant patients underwent sphincter sparing operations compared to patients treated postoperatively (42).
The third study, the German Rectal Cancer Trial CAO/ARO/AIO-94, reached targeted accrual (43). In this study, stage II/III patients in the neoadjuvant arm received 50.4 Gy in 28 fractions while receiving 5-FU as 120-hour continuous venous infusion (CVI) of 1000 mg/m2/day during the 1st and 5th week of treatment. TME was then scheduled 4-6 weeks after completion of preoperative therapy. Three to 4 weeks after surgery, the patients went on to receive 4 additional cycles of 5-FU as bolus injection of 500 mg/m2/day for 5 consecutive days repeated every 4 weeks. In the postoperative arm, patients began CMT within 4 weeks after surgery, with the same concurrent chemotherapy and radiation therapy schedule as the neoadjuvant CMT arm. After the completion of the initial 50.4 Gy, a 5.4 Gy boost in 3 fractions was delivered to the tumor bed, followed by 4 cycles of bolus 5-FU as in the preoperative CMT arm. Five-year LR was significantly lower in the preoperative arm (6% vs. 13%, p=0.006), while there was no significant difference in DFS and OS. Eight percent of patients had a pathological CR, and there was a greater percentage of sphincter-preserving operations performed (39% vs. 19%, p=0.004) in the preoperative group. Acute grade 3 or 4 toxicity was significantly less in the neoadjuvant group (27% vs. 40%, p=0.001), as was the rate of late grade 3 or 4 toxicity (14% vs. 24%, p=0.01). It should be noted that 18% of patients in the immediate surgery arm were found to have stage I disease upon pathologic assessment of the surgical specimen. Since all patients were staged before treatment and were felt to have stage II/III disease, the authors concluded that this number (18%) represents the approximate number of patients at risk of overtreatment with neoadjuvant CMT, again stressing the importance of accurate pre-treatment staging (43).
The results of the Medical Research Council (MRC) CR07 study were recently published, evaluating the merits of short-course preoperative radiation (44). In this randomized study, patients were treated with 25 Gy in 5 fractions followed by surgery or were treated with immediate resection with selective postoperative CMT (45 Gy in 25 fractions with concurrent 5-FU) in patients with positive circumferential surgical margin. It should also be noted that all patients found to have stage III disease were to receive postoperative 5-FU. In patients receiving preoperative radiation, there was a 61% reduction in the relative risk of LR (hazard ratio [HR]: 0.39, 95% confidence interval [CI] 0.27-0.58, p<0.0001), with 3-year LR of 4.4% in the preoperative radiation therapy arm vs. 10.6% in the selective postoperative CMT arm (95% CI 5.3-7.1). In addition, there was a statistically significant improvement in DFS in the preoperative radiation therapy arm (HR 0.76, 95% CI 0.62-0.94, p=0.013), however OS did not differ significantly between the two groups. This study further confirmed the value of preoperative radiation therapy.
Preferred techniques/regimens
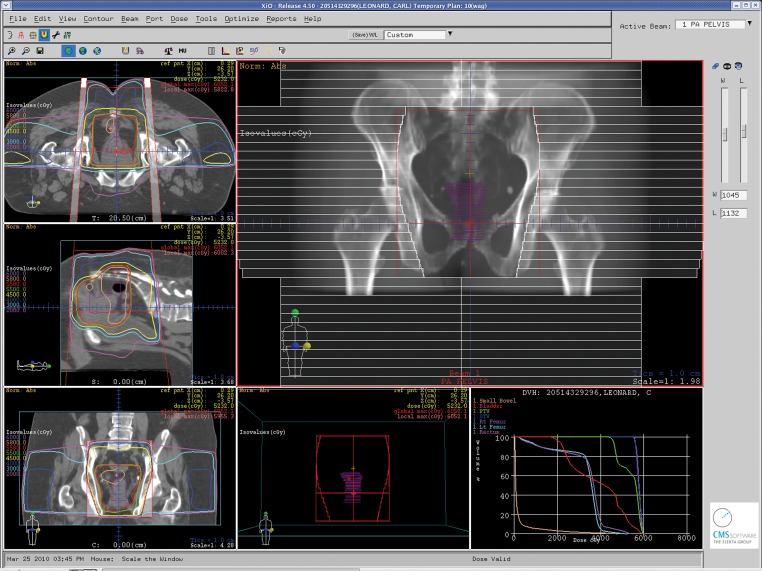
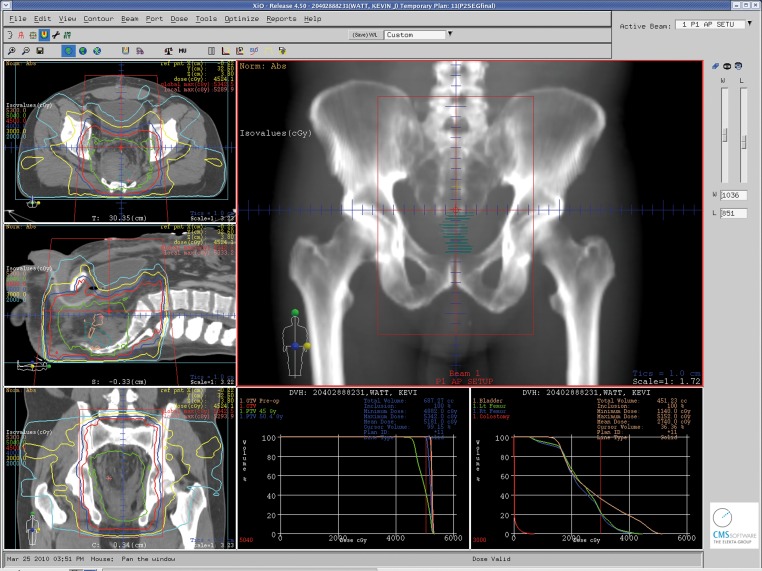
In the United States, it is recommended that patients staged with resectable stage II or III rectal cancer should be treated initially with preoperative CMT unless there are medical contraindications (4). Radiation therapy should employ multiple treatment portals and the treatment volume should include the tumor with margin, along with the internal iliac and presacral lymph nodes (as well as the external iliac lymph nodes with T4 disease) (Figures 1 and 2). Recommended treatment dose is 45-50 Gy in standard fractionation (1.8-2.0 Gy/fraction). Concurrent 5-FU by continuous infusion is preferred, as outlined in the German Rectal Cancer Trial (43). Definitive surgery should then take place 4-6 weeks after completion of CMT. Postoperative systemic therapy should then be initiated approximately 4 weeks after resection, with a goal of approximately 6 months total of perioperative systemic therapy (combined preoperative CMT and postoperative chemotherapy). Postoperatively, chemotherapy should be 5-FU based, with emerging rectal studies and extrapolation from adjuvant colon cancer studies suggesting potential merits to the use of capecitabine or FOLFOX in the place of adjuvant 5-FU (45),(46). For patients thought to have stage I disease on preoperative staging who are subsequently upstaged upon final pathologic staging after surgery to stage II/III disease, it is recommended that they be assessed for adjuvant treatment. The recommended strategy in this scenario is a “sandwich” approach with adjuvant 5-FU based chemotherapy followed by CMT followed by additional 5-FU based chemotherapy with approximately a total of 6 months of systemic therapy (4).
Figure 1. A conformal 3-dimensional radiation treatment plan with sagittal, coronal and axial views through the treatment isocenter along with a view of a posterior-anterior (PA) treatment portal for a patient with stage III rectal cancer undergoing neoadjuvant combined modality therapy. Isodose lines representing radiation dose to the “at-risk” regional lymph nodes and pelvic tissues along with higher doses to the areas of gross disease are demonstrated.
Figure 2. An intensity-modulated radiation therapy (IMRT) treatment plan with sagittal, coronal and axial views through the treatment isocenter for a patient with stage III rectal cancer undergoing neoadjuvant combined modality therapy. Isodose lines representing radiation dose to the “at-risk” regional lymph nodes and pelvic tissues along with higher doses to the areas of gross disease are demonstrated.
Follow-up
Randomized studies have demonstrated therapeutic benefits to a proactive intensive post-treatment surveillance program in patients with stage II/III disease (4),(47)-(49). For patients who have completed definitive trimodality therapy, follow-up including history and physical exam and CEA level should be performed every 3-6 months for 2 years and then every 6 months up to 5 years. A colonoscopy is recommended 1 year after resection, again at 3 years postoperatively, and every 5 years thereafter (assuming no suspicious findings are found in the interim). A CT scan of the chest/abdomen/pelvis is recommended on a yearly basis for 3-5 years after definitive treatment. In addition, patients are recommended to undergo proctoscopy every 6 months for the first 5 years after treatment in order to evaluate for recurrences at the anastomosis.
Conclulsion
In patients with stage II and III rectal cancer, both local and distant recurrences are of concern following definitive surgical resection despite advances in surgical technique. Adjuvant therapies such as radiation to the tumor/tumor bed and regional lymph nodes and 5-FU-based systemic therapies have helped to reduce these recurrences. The current recommendation in the United States is for preoperative CMT with surgery several weeks after CMT is completed to maximize treatment response, followed by approximately 4 months of adjuvant 5-FU-based systemic therapy. In Europe, the benefits of neoadjuvant radiation therapy (both short-course and a protracted course) have been shown in randomized phase III trials, but the role of concurrent chemotherapy remains a question of debate. The importance pre-therapy staging is stressed as sequencing of therapy appears to significantly impact outcome. In addition, close follow-up in the post-treatment setting appears of great importance both in terms of managing treatment-related side-effects and for early recurrence detection.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Greene FL, Page D, Fleming I, Fritz A. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. [Google Scholar]
- 3.Skibber JM, Hoff PM, Minsky BD. Cancer of the Rectum. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology, 6th edition. Philadelphia: Lippincott, Williams and Wilkens; 2001. pp. 1271–1318. [Google Scholar]
- 4.National Comprehensive Cancer Network (2010) Rectal Cancer, version 1.2010. Available online: http://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf.
- 5.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773–83. doi: 10.1148/radiol.2323031368. [DOI] [PubMed] [Google Scholar]
- 6.Kane JM, 3rd, Petrelli NJ. Controversies in the Surgical Management of Rectal Cancer. Semin Radiat Oncol. 2003;13:403–18. doi: 10.1016/j.semradonc.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465–72. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 8.Douglass HO, Jr, Moertel CG, Mayer RJ, Thomas PR, Lindblad AS, Mittleman A, et al. Survival after postoperative combination treatment of rectal cancer [letter] N Engl J Med. 1986;315:1294–5. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–9. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–7. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 11.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–60. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 12.Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335–45. [PubMed] [Google Scholar]
- 13.Heald RJ, Husband EM, Ryall RDH. The mesorectum in rectal cancer surgery-the clue to pelvic recurrence. Br J Surg. 1982;69:613–6. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 14.Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Lancet. 2000;356:93–6. doi: 10.1016/s0140-6736(00)02469-7. [DOI] [PubMed] [Google Scholar]
- 15.Arbman G, Nilsson E, Hallböök O, Sjödahl R. Local recurrence following total mesorectal excision for rectal cancer. Br J Surg. 1996;83:375–9. doi: 10.1002/bjs.1800830326. [DOI] [PubMed] [Google Scholar]
- 16.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–11. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 17.Hall NR, Finan PJ, al-Jaberi T, Tsang CS, Brown SR, Dixon MF, et al. Circumferential margin involvement after mesorectal excision of rectal cancer with curative intent: Predictor of survival but not local recurrence. Dis Colon Rectum. 1998;41:979–83. doi: 10.1007/BF02237384. [DOI] [PubMed] [Google Scholar]
- 18.Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: Not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–7. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Marr R, Birbeck K, Garvican J, Macklin CP, Tiffin NJ, Parsons WJ, et al. The modern abdominoperineal excision: the next challenge after total mesorectal excision. Ann Surg. 2005;242:74–82. doi: 10.1097/01.sla.0000167926.60908.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pahlman L, Glimelius B. Radiotherapy additional to surgery in the management of primary rectal carcinoma. Acta Chir Scand. 1990;156:475–85. [PubMed] [Google Scholar]
- 21.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. New Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 22.van de Velde CJH. Preoperative radiotherapy and TME-surgery for rectal cancer: detailed analysis in relation to quality control in a randomized trail. Pros ASCO. 2002;21:127a. [Google Scholar]
- 23.Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer. A meta-analysis. JAMA. 2000;284:1008–15. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 24.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 22 randomised trials involving 8507 patients. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 25.Yang G, Wagner TD, Thomas CR. Multimodality approaches for rectal cancer. Curr Probl Cancer. 2004;28:316–42. doi: 10.1016/j.currproblcancer.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Minsky BD. Adjuvant therapy for rectal cancer-a good first step[letter] New Engl J Med. 1997;14:1016–7. doi: 10.1056/NEJM199704033361410. [DOI] [PubMed] [Google Scholar]
- 27.Rödel C, Sauer R. Perioperative Radiotherapy and Concurrent Radiochemotherapy in Rectal Cancer. Semin Surg Oncol. 2001;20:3–12. doi: 10.1002/ssu.1011. [DOI] [PubMed] [Google Scholar]
- 28.Holm T, Singnomklao T, Rutqvist LE, Cedermark B. Adjuvant preoperative radiotherapy in patients with rectal carcinoma. Adverse effects during long term follow-up of two randomized trials. Cancer. 1996;78:968–76. doi: 10.1002/(SICI)1097-0142(19960901)78:5<968::AID-CNCR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Holm T, Rutqvist LE, Johansson H, Cedermark B. Postoperative mortality in rectal cancer treated with or without preoperative radiotherapy: causes and risk factors. Br J Surg. 1996;83:964–8. doi: 10.1002/bjs.1800830725. [DOI] [PubMed] [Google Scholar]
- 30.Marijnen CA, Kapiteijn E, van de Velde CJ, Martijn H, Steup WH, Wiggers T, et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: Report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–25. doi: 10.1200/JCO.2002.20.3.817. [DOI] [PubMed] [Google Scholar]
- 31.Frykholm GJ, Sintorn K, Montelius A, Jung B, Påhlman L, Glimelius B. Acute Lumbosacral plexopathy after radiation therapy in rectal carcinoma. Radiother Oncol. 1996;38:121–30. doi: 10.1016/0167-8140(95)01665-1. [DOI] [PubMed] [Google Scholar]
- 32.Willett CG, Czito BG. Chemoradiotherapy in gastrointestinal malignancies. Clin Oncol (R Coll Radiol) 2009;21:543–56. doi: 10.1016/j.clon.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–50. [PubMed] [Google Scholar]
- 34.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 35.Gastrointestinal Tumor Study Group. Radiation therapy and 5-FU with or without semustine for the treatment of patients with surgical adjuvant adenocarcinoma of the rectum. J Clin Oncol. 1992;10:549–57. doi: 10.1200/JCO.1992.10.4.549. [DOI] [PubMed] [Google Scholar]
- 36.O'Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–7. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 37.Wolmark N, Wieand HS, Hyams DM, Colangelo L, Dimitrov NV, Romond EH, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Nat Can Inst. 2000;92:388–96. doi: 10.1093/jnci/92.5.388. [DOI] [PubMed] [Google Scholar]
- 38.Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–5. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 39.Bosset JF, Calais G, Mineur L. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results of EORTC 22921. J Clin Oncol. 2005;23:5620–7. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 40.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 41.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 42.Roh MS, Petrelli N, Weiand H. Phase III randomized trial of preoperative versus postoperative multimodality therapy in patients with carcinoma of Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J the rectum (NSABP R-03) Pros ASCO. 2001;20:123a. [Google Scholar]
- 43.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 44.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–20. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–16. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 46.Twelves C, Wong A, Nowacki MP, Abt M Burris H 3rd, Carrato A, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 47.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care's gastrointestinal cancer disease site group. J Clin Oncol. 2004;22:3395–407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 48.Renehan AG, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Desch CE Benson AB 3rd, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, et al.; American Society of Clinical Oncology. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–9. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]