Abstract
Objective
Since the anti-tumor activity of 5-fluorouracil (5-FU) is due to induction of apoptosis, we assessed the value of expression of key apoptotic molecules (Bax, Bcl-2 and p53) in predicting the efficacy of 5-FU therapy for colorectal adenocarcinomas (CRCs).
Methods
Archival tissues of CRCs from 56 patients who received a complete regimen of 5-FU-based chemotherapy after surgery, and 56 patients matched for age, gender, ethnicity, tumor stage, tumor location, and tumor differentiation who had undergone only surgery (without any pre- or post-surgery therapy), were evaluated for immunophenotypic expression of Bax, Bcl-2, and p53. Also, these CRCs were evaluated for Bax mutations. The predictive capacity or prognostic value of these markers was assessed by estimating overall survival.
Results
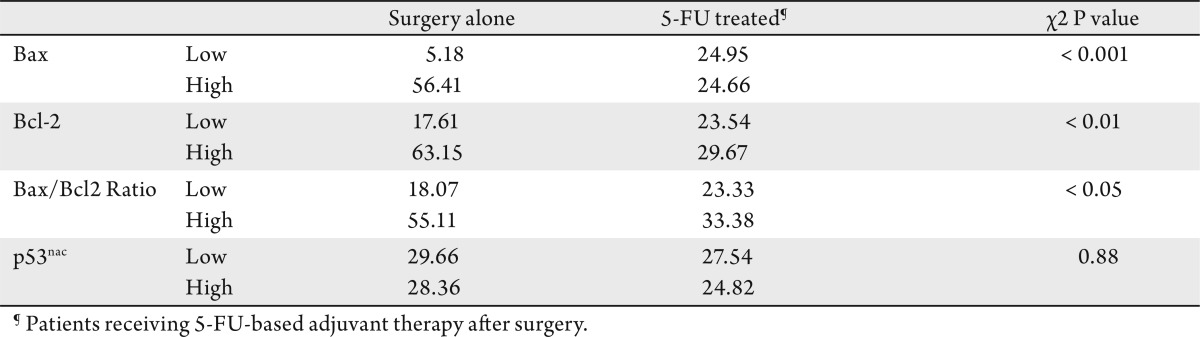
The majority of low Bax expressing CRCs have exhibited mutations at the G (8) tract. There was no significant difference in overall survival rates between the categories of surgery alone and 5-FU-treated patients. However, a better survival was observed for patients who received chemotherapy when their CRCs had low Bax/Bcl2 ratio (HR, 1.55; 95% CI: 1.46-31.00). Patients who received surgery alone and whose CRCs lacked Bax expression had 5.33 times higher mortality than those with high Bax expression (95% CI: 1.78-15.94), when controlled for tumor stage and other confounders. Bcl-2 and nuclear p53 accumulation had no predictive value in either patient group.
Conclusion
These findings are the first to demonstrate that high Bax expression is a good prognosticator for patients who underwent surgery alone, and that patient with low Bax/Bcl-2 expression ratio benefit from 5-FU-based adjuvant therapies.
Keywords: colorectal adenocarcinoma, predictive marker, Bax, 5-Flurouracil
Introduction
5-Fluorouracil (5-FU) remains the most commonly used chemotherapeutic agent for the treatment of colorectal cancers (CRCs). Nevertheless, more than 40 years of 5-FU usage has not yielded responses greater than 35-40% (1)-(5), neither has it decreased the rates of recurrence (6),(7). Therefore, novel strategies are required to predict response to treatment. Although several molecular markers have prognostic value for CRCs (8)-(15) their predictive value in assessing treatment response remains controversial(7),(16)-(18). In addition to selecting the best chemotherapeutic tools, a new challenge is to identify genetic and/or molecular markers that can be used as predictors of response to treatment.
As demonstrated for cultured cells, p53-dependent apoptosis modulates the cytotoxic effect of chemotherapeutic agents; cells with functional p53 or wild-type p53 (wt-p53) are more sensitive, and cells with mutated or lack of p53 are more resistant (19),(20). Lenz et al demonstrated a better rate of response to 5-FU for patients whose tumors were wild-type for p53 than those whose tumors had overexpressed or mutated p53 (21). In contrast, Allegra et al found that overexpressed p53 correlated with a better response to treatment (22),(23), and Elsaleh et al (24) could not find any relationship between p53 status and 5-FU response or survival of patients with colon or rectal tumors. Thus, data relating to the predictive value of p53 in CRCs is contradictory and inconclusive.
Apoptosis is a complex process that proceeds through two pathways. The extrinsic pathway is based on cell surface receptors and cytoplasmic proteins. The intrinsic pathway occurs in the mitochondria, where the balance of pro-and anti-apoptotic proteins is largely regulated by the members of the Bcl-2 family. p53 has been described as a main modulator of apoptosis in both pathways (25). The anti-tumor activity of 5-FU has been related to its capacity to induce apoptosis by damaging the DNA and/or by altering the expression profiles of pro- and anti-apoptotic molecules (26)-(28). Chemo-resistance may depend on the function and relationship between pro-and anti-apoptotic proteins (29),(30).
The balance between anti-apoptotic (e.g., Bcl-2) and pro-apoptotic proteins (e.g., Bax) in a cell determines its susceptibility to apoptosis after 5-FU treatment(31). Since the checkpoint is controlled by the ratio between promoters and inhibitors of apoptosis (i.e., the ratio of Bax to Bcl2) and p53 (26)-(28), their concomitant expression should be considered together in assessing their clinical significance. In the current report, as a proof of concept, we evaluated the predictive and prognostic usefulness of these markers in two groups of CRC patients, one treated with surgery alone and the second treated with surgery and 5-FU-based adjuvant chemotherapy.
Patients and methods
Patients
The institutional review board of the University of Alabama at Birmingham (UAB) approved these experiments, and the Bioethics Committee reviewed the proposed effort. From the UAB Hospital, we collected data for 650 patients who were diagnosed and underwent surgery for primary colorectal adenocarcinoma with curative intent between 1987 and 1993. Use of patients from this period maximized post-surgery follow-up, because 70% of the patients (78 of 112) had either stage II or III CRCs (Table 1).
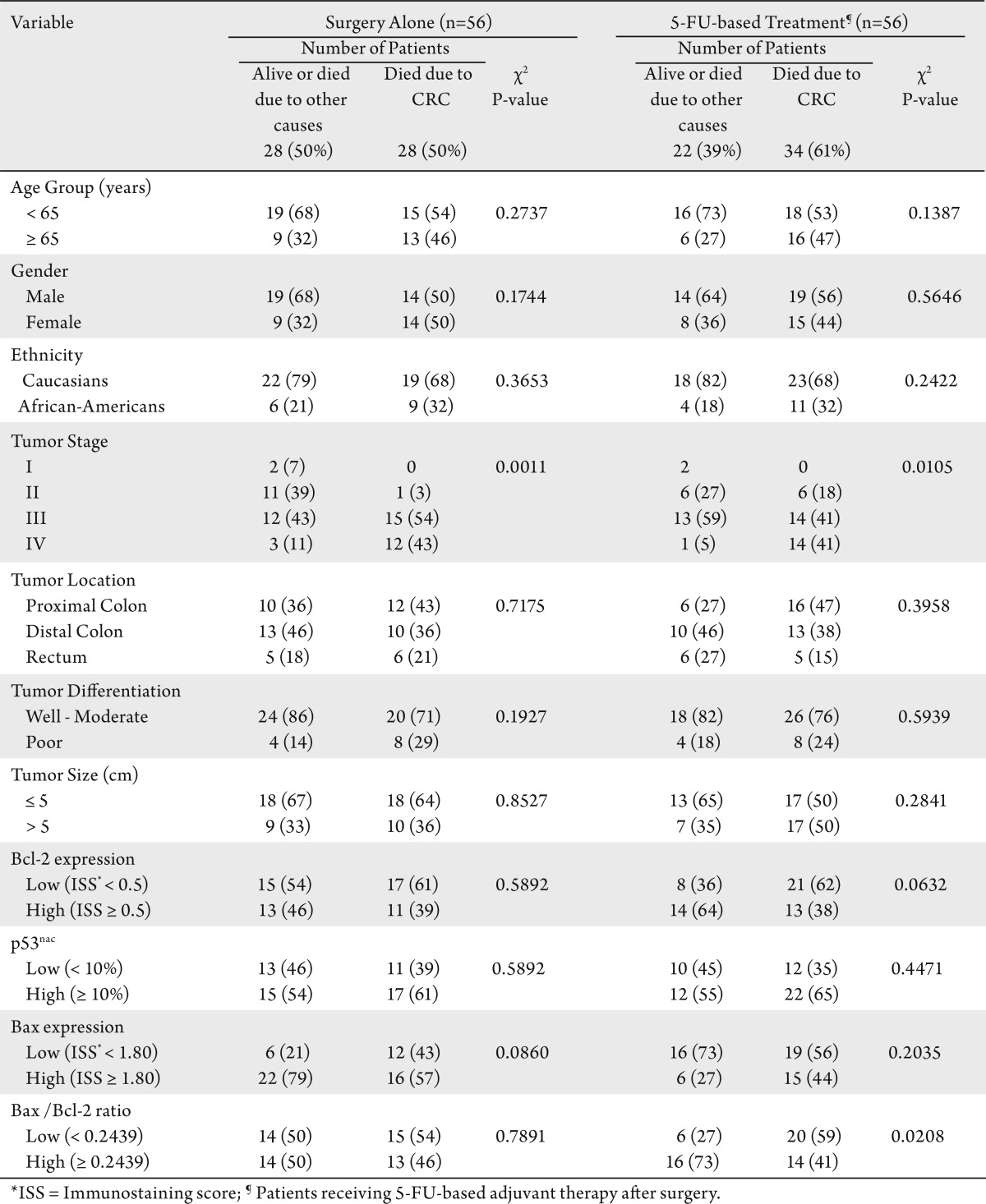
Table 1. Correlations between the vital status and the characteristics of treated and untreated patients.
Patient eligibility criteria
During our initial selection process, patients who received radiation and/or any chemotherapy before surgery were excluded. We included only those patients who completed at least 3 months of post-surgical adjuvant chemotherapy and for whom there was complete information on dosage and duration of treatment. With these criteria and the availability of paraffin blocks, the final group consisted of 56 patients who had surgery plus 5-FU-based adjuvant therapy.
Treatment
Details of the chemotherapy were as follows: Twenty eight patients received 5-FU alone, 12 patients received 5-FU plus levamisole (LV), 10 patients received 5-FU plus leucovorin (LC), 4 patients received 5 -FU plus doxorubicin (5-FUDR), 1 patient received 5-FU/1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea) (CCNU), and 1 patient received 5-FU/LV/LC. The control group of 56 patients, who were matched for age; gender; ethnicity; and tumor stage, location, and histologic differentiation, and who had undergone only curative resection without adjuvant therapy after surgery were selected randomly from the initial patient pool. In the surgery-alone group, patients with stage III or IV CRCs did not receive adjuvant therapy for various clinical and personal reasons but had undergone surgery with a palliative intent. The final study sample consisted of 112 patients; their characteristics are given in Table 1.
Pathological evaluations
The surgical pathology reports were reviewed by three investigators (CS-C, NCJ & CKS), and a pathologist (NCJ) individually reviewed slides stained with hematoxylin and eosin for the degree of histologic differentiation and re-graded lesions as well, moderate, poor or undifferentiated (32),(33). Well and moderately differentiated tumors were pooled into a low-grade group, and poor and undifferentiated tumors into a high-grade group (34). Pathologic staging was performed according to the criteria of the American Joint Commission on Cancer (35). The International Classification of Diseases for Oncology codes were used to specify the anatomic location of the tumor (32). The tumor was considered mucinous if ≥ 50% demonstrated mucinous histology (32). The anatomic sub-sites were the proximal colon, the distal colon, and the rectum. Three-dimensional tumor size was determined; the largest dimension was used for statistical purposes.
Patient demographics and follow-up information
Patient demographics, along with clinical and follow-up information, were retrieved retrospectively from medical records, physician charts, and pathology reports and from the UAB tumor registry. Patients were followed, either by their physician or by personnel associated with the tumor registry, until their death or the date of the last documented contact. Through telephone and mail contacts, these personnel ascertained outcome (mortality) information directly from patients (or relatives) and physicians. This information was validated by examination of the state death registry. Demographic data, including patient age at diagnosis, gender, race/ethnicity, date of surgery, date of the last follow-up (if alive), date of recurrence (if any) and date of death, were collected. Collection of follow-up information, performed every six months, ended in April 2010. Laboratory investigators (VRK & CS-C) were blinded to the outcome information until completion of the assays.
Mutational analysis
Earlier studies have reported a decreased expression of Bax in CRCs which exhibited mutations in the poly G(8) region of the bax gene (36),(37). Therefore, in this study, we also analyzed the genomic DNA samples extracted from CRCs and their corresponding normal tissues to assess the expression status of Bax in relation to the bax mutational status.
Genomic DNA was extracted from tissue sections (10-µm thick) of primary CRCs and LoVo cell line as described (38). The 94-base-pair region encompassing the (G) 8 tract in the bax coding sequence was amplified by PCR on the CRCs, with carboxyfluorescein (6FAM)-labeled 5’atccaggatcgagcagggcga-3’ sense primer and 5’cactcgctcagcttcttggtggac-3’ antisense primer. PCR was accomplished in a 25-µL reaction volume containing approximately 100 ng of genomic DNA, a 200-µmol/L concentration of dNTPs (Invitrogen, Carlsbad, CA), and 0.5 U of Platinum Taq DNA polymerase (Invitrogen). Amplification consisted of a 15-min denaturation step at 95°C, followed by 36 cycles of 30 sec at 95°C, 30 sec at 50°C, and 30 sec at 72°C and a final extension step of 5 min at 72°C. Appropriate dilutions of f luorescent PCR products were mixed with formamide and carboxy-X-rhodamine-labeled molecular weight standards (GeneScan-500 ROX, Applied Biosystems, Foster City, CA), heat denatured, and run in a 50-cm capillary array containing GS Performance Optimized Polymer6 (Applied Biosystems), at a voltage of 15kV on the ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). The profiles of PCR products were analyzed by use of GeneScan 3.1 software (Applied Biosystems). Numerous normal DNA samples were used to establish the normal peak size and the profile pattern of the bax gene fragment. All PCRs with abnormal profiles were repeated twice, independently, to confirm the presence of mutations.
Immunohistochemistry
Formalin-fixed, paraffin-embedded archival tissues were collected from the surgical pathology division of the UAB Hospital. From the blocks, tissue sections (5-µm thick), representative of normal mucosa and invasive adenocarcinomas were cut 1 to 2 days before staining to avoid potential problems in antigen recognition due to storage of cut sections on glass slides(39),(40). Sections were de-paraffinized in xylene and rehydrated in graded alcohols. For antigen retrieval of Bax and Bcl-2, the slides were microwave boiled in citrate buffer (10 mmol/L, pH 6.0) for 7 min. For p53, antigen retrieval is not required (8),(41),(42). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 5 min. Non-specific binding of the primary antibodies was blocked by incubating the slides in 3% goat serum at room temperature for 1 hr in humidity chambers with the primary mouse monoclonal antibodies for Bax (Clone B9, Santa Cruz Biotechnology Inc, CA, USA) (1:200), Bcl-2 (Clone 124, Roche Diagnostic corporation, Indianapolis, IN, USA) (1:60) and p53 (Clone BP53, BioGenex, San Ramon, CA, USA) (1:80). A biotin-streptavidin horseradish peroxidase detection kit was used as the secondary detection system (BioGenex). The biotinylated goat anti-mouse secondary and avidin-horseradish peroxidase label were each applied for 10 min. The antigen-antibody complex was recognized by incubating with the chromogen, diamino-benzidine, for 7 min. The slides were counterstained with hematoxylin for 1 min. Known positive controls were included in each staining run; negative controls were obtained by omitting the primary antibody. Slides were then dehydrated in graded alcohols, cleared in 3 xylene baths, and mounted with Permount™ mounting media. As we reported earlier (43), these antigens are stable in paraffin blocks.
Staining evaluation
Stained slides were evaluated under a light microscope, and the staining was scored semi-quantitatively by CS-C, NCJ and UM, CKS together to limit the bias; if there was a disagreement in their scores, they reached to a consensus before proceeding. Observers were blinded for the clinicopathologic data and the treatment status. Phenotypic expression of Bax and Bcl-2 was present in the cell cytoplasm and accumulation of p53 in the nucleus (p53nac). As described earlier (8),(9),(12),(13), the percentage of positive cells and staining intensity were taken into consideration for estimation of the final immunostaining score (ISS). The intensity of staining of individual cells was scored on a scale of 0 (no staining) to + 4.0 (strong staining). In addition, each reviewer estimated the proportion of cells stained at each intensity level. The percentage of cells and the corresponding intensity were then multiplied to obtain the ISS. For each case, the final ISS was the average of the values estimated by these three investigators.
Cutoff values
Molecular marker expression was dichotomized into high expressors and low expressors, based on the cut-off values discussed below. For Bax expression, the median ISS (1.8) of tumor tissues was taken as the cut-off value; i.e., the tumors expressing ≥ 1.80 were considered as “high expressors” (equivalent to > +1 of routine immunohistochemistry, IHC, scoring in the diagnostic pathology setting) and those CRCs with ISS < 1.80 as “low expressors” (≤ +1). For Bax expression, similar low and high expressor categories of IHC scoring have been described by others(27),(44),(45). For Bcl-2 expression, based on prior studies by us and others (8), we chose 0.5 ISS as the cut-off value. We considered only tumor cells with distinct nuclear immunostaining for p53 as positive and considered the tumor positive only if there was ≥10% positivity of all malignant cells in a tissue section, as described earlier (9). We chose this cutoff because, at this value, there was the highest concordance between immunohistochemical detection of p53nac and point mutations of the p53 gene detected by single-strand confirmation polymorphism and DNA sequencing analyses. At this cutoff value, IHC detects 95% of point mutations in the p53 gene (42). The cut-off value for Bax/Bcl-2 ratio was based on their levels of expression in benign colonic epithelium. We used the ISS values of Bax and Bcl-2 to determine the Bax/Bcl-2 ratio of each case, then a median value of 0.25 was obtained. This 0.25 value was used as a cut-off for Bax/Bcl-2 ratio to dichotomize CRCs into groups of “high” and “low” ratios.
Statistical analysis
Correlations between biomarkers and clinical response (overall survival) were evaluated by chi-square tests. The type-I error rate of each test was controlled at <0.05. All analyses were performed with SAS statistical software, version 9.0 (46). Kaplan-Meier curves and log-rank tests were used to assess the effect of the selected biomarkers in univariate analyses (47). Overall survival was estimated as the number of months from surgery to the date of death or last contact. Patients who were alive at last contact and those who died due to a cause other than colorectal cancer were “right censored.” Only those deaths due to CRCs were considered as events. Multivariate Cox proportional hazards tests (48) were utilized to identify the independent prognostic value of molecules indicators of survival, after controlling for patient age, gender, race, tumor location, tumor size, tumor stage, tumor grade, and the three molecular markers, Bax, Bcl-2, and p53nac. Models were built separately for each patient group (the group of patients who received chemotherapy after surgery and the group who underwent surgery without any pre- or post-surgery chemo- or radiation therapy).
Results
Demographic and clinicopathologic characteristics of the patient population
Table 1 shows the patient distribution; their demographic, clinicopathological and molecular characteristics; and their correlation with survival. For both treatment groups, there were similar distributions of patient age, gender, ethnicity, tumor stage, tumor location, tumor size, and tumor grade, in terms of deaths due to CRC. The median follow-up period of the complete study population of 112 patients was 9.31 years (range <1 – >20 years).
Survival analysis based on treatment
Univariate Kaplan-Meier survival analysis demonstrated no significant differences in overall survival rates between the surgery-alone and the 5-FU-treated patient groups (log rank, P=0.71) (data not shown).
Bax (G) 8 mutation frequency and its relation to clinicopathologic features
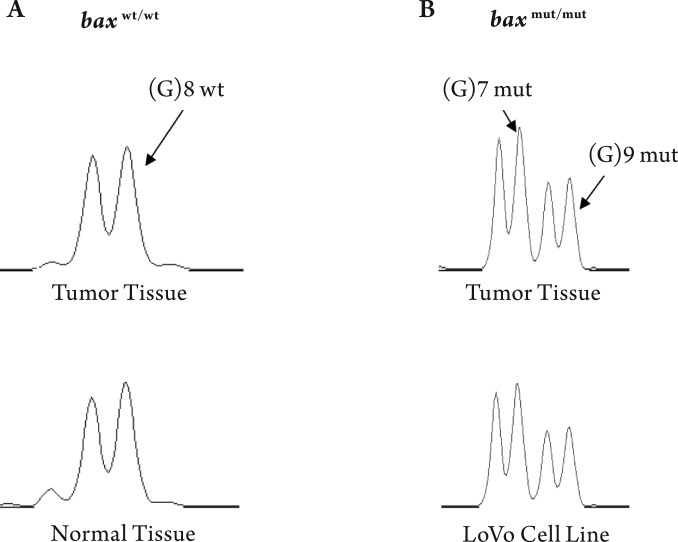
We analyzed for the presence of mutations in the (G) 8 tract of the Bax gene in a human CRC cell line (LoVo) and in 83 CRCs. The LoVo cells displayed a bi-allelic Bax (G) 8 frame-shift mutation; this status was used as a reference in CRCs for Bax mutations (Fig 1). In our analysis, 23 of 83 (28%) CRCs demonstrated biallelic Bax (G) 8 frame-shift mutations. The majority of CRCs with mutations at the G (8) tract also had low Bax expressing (20 of 23, 87%). CRCs that displayed these mutations were significantly higher for male patients (17 of 23, 74%) and distal tumors (18 of 23, 79%). However, there was no association between the presence of Bax (G) 8 mutations with age, race/ethnicity, depth of wall infiltration, tumor grade, tumor stage, lymph node invasion, or presence of distant metastasis (data not shown). Since the number of CRCs with Bax mutations is small, we have not further analyzed the mutational data to assess correlation between Bax mutations and patient survival in the surgery alone and surgery and 5-FU therapy patient groups separately.
Figure 1. Mutational analysis at 94-base-pair region encompassing the (G) 8 tract in the Bax coding sequence in colorectal adenocarcinoma, adjacent benign epithelium and in LoVo cell line. The CRC and corresponding normal tissue demonstrated lack of Bax (G) 8 frame-shift mutation (Panel-A). CRCs and the LoVo cells displayed a biallelic Bax(G) 8 frame-shift mutation (Panel-B).
Bax immunophenotypic expression analysis
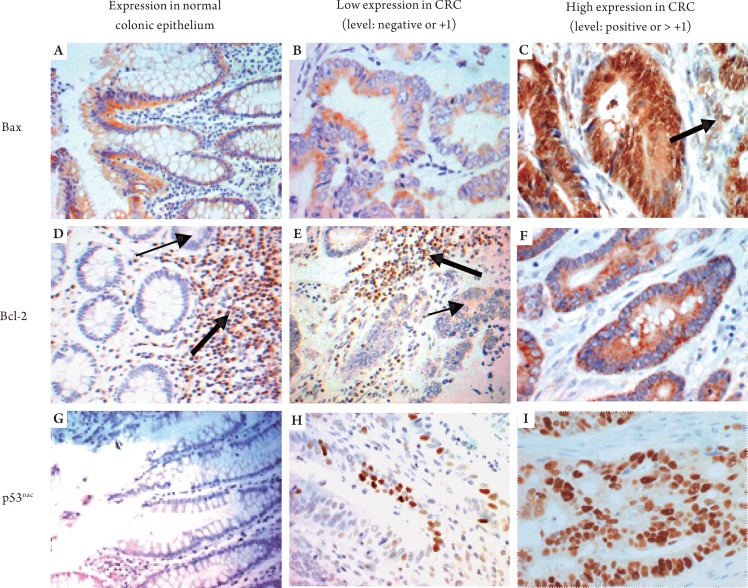
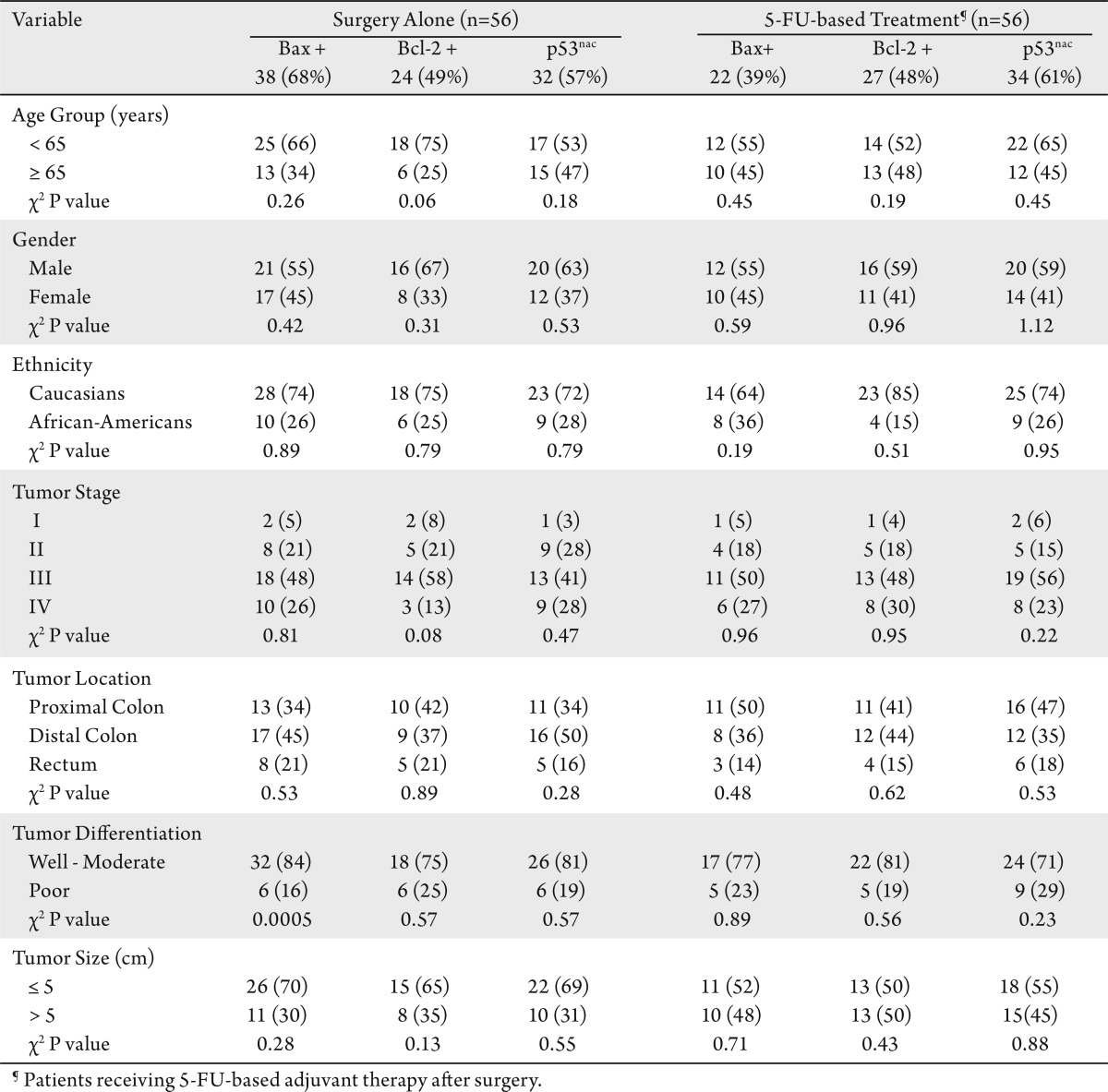
Immunoreactivity for Bax was observed in the cytoplasm. In most CRCs, the Bax staining pattern was homogenous, ranging from low to high levels. In 11% of CRCs (12 cases), however, there was intratumoral heterogeneity. A low level of Bax expression was observed consistently in benign colonic epithelium, lymphocytes, and endothelial cells (Fig 2A-C). The presence of staining in intra-tumoral lymphocytes was used as an internal positive control. Of the CRCs, 54% (60 of 112) had high levels of Bax expression (22 of the 5-FU treated group and 38 of the surgery-alone group). Twelve of 28 surgery-alone patients (43%) with low Bax expression died due to CRCs; 19 of 34 5-FU treated patients (56%) with low Bax expression died due to CRCs (Table 1). There was no association between Bax expression and p53nac in either patient group (data not shown). CRCs with negative or low Bax immunostaining were significantly associated with CRCs that demonstrated frame-shift mutations at the Bax (G) 8 tract (20 of 23, 87%) as compared to CRCs without this mutation (25 of 60, 41%) (data not shown). In addition, most CRCs with poor differentiation had low Bax expression in the surgery-alone group (χ2, P= 0.0005) (Table 2).
Figure 2. Examples of immunohistochemical expression of Bax, Bcl-2, and p53nac in colorectal adenocarcinomas and adjacent benign epithelium. Examples of immunostaining of the adjacent benign colorectal epithelium are presented for Bax expression (Panel-A, x20), Bcl-2 expression (Panel-D, x20) and p53 nuclear accumulation (Panel-G, x20). Examples of immunostaining in the malignant regions of CRCs are shown for Bax expression (low level of expression in Panel-B, x40 and high level of expression Panel-C, x40), Bcl-2 expression (low level in Panel-E, x40 and high level in Panel-F, x40) and p53 nuclear accumulation (low level in Panel-H, x40 and high level in Panel-I, x40). Panel-C shows strong expression of Bax in stromal cells (thick arrow). Panels-D and E show strong Bcl-2 staining in lymphocytes (thick arrows) (internal positive controls) and weak staining in the basal colonic crypts (thin arrow in Panel-D) and in the malignant glands (thin arrow in Panel-E).
Table 2. Correlations between expression of Bax, Bcl-2 and p53nac and the characteristics of treated and untreated patients.
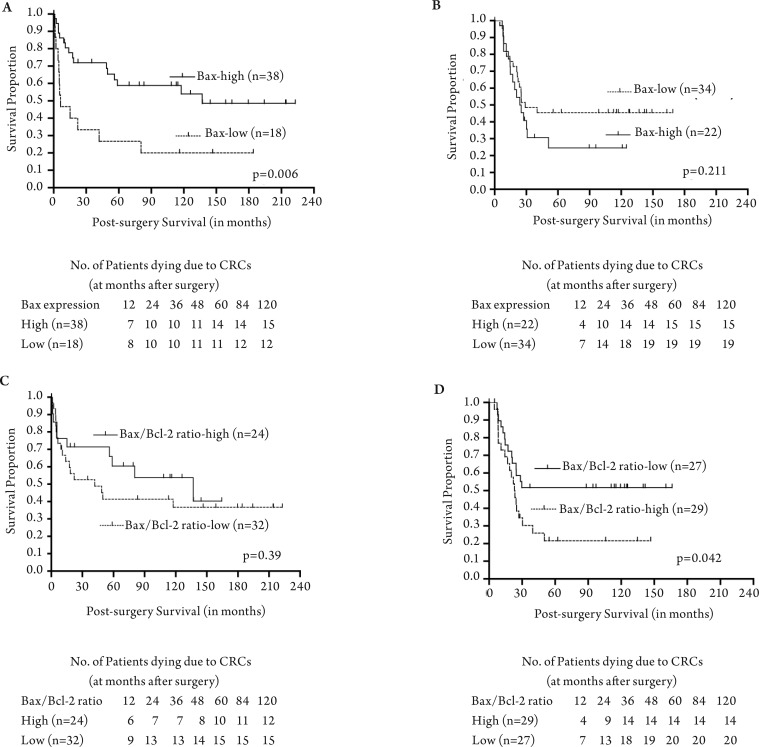
The median survival of the 5-FU treated group of patients with low Bax expression was 25 months relative to 5 months for surgery-alone patients with low Bax expression (Table 3). The median survival for 5-FU treated patients with high Bax expression was 25 months relative to 56 months for surgery-alone patients with high Bax expression (Table 3). Kaplan-Meier analyses demonstrated a significant association between high Bax expression and better patient survival in the surgery-alone group (log rank P=0.006) (Fig 3A). Although there was no significant association between Bax expression status and patient survival in the 5-FU treated group, patients with decreased Bax expression had improved survival (overall log rank P=0.211) (Fig 3B).
Table 3. Median survival (in months) of patient groups based on the status of expression of Bax, Bcl-2, and p53nac.
Figure 3. Correlation of Bax and Bcl-2 expression with overall survival of colorectal cancer patients undergoing surgery alone or treated with 5-FU-based adjuvant therapy after surgery. The overall survival of patients with high Bax expression was compared to patients with low Bax expression within the surgery alone patient group (Panel-A) and within the surgery plus 5-FU-based adjuvant therapy patient group (Panel-B). Similar survival comparisons based on Bax/Bcl2 expression ratio are shown for the surgery-alone group (Panel-C) and in the surgery plus 5-FU-based adjuvant therapy group (Panel-D). The long-rank P values of Kaplan-Meier analyses of overall survival were compared.
Bcl-2 immunophenotypic expression analysis
Immunoreactivity for Bcl-2 was localized in the cytoplasm; overall, the staining was homogenous. The staining in intra-tumoral lymphocytes was used as an internal control (Fig 2D-F). Of the patients, 46% had high levels of Bcl-2 expression (27 5-FU-treated patients and 24 surgery-alone patients). There were no significant differences in the incidence of deaths due to CRCs in the Bcl-2 low and high expressors of among the 5-FU-treated or surgery-alone patients (Table 1). However, the median survival was higher (63.15 months) for surgery-alone patients with high levels of Bcl-2 expression as compared to those with low expression (17.61 months). There was no significant difference in the median survival of 5-FU treated patients with low or high Bcl-2 expression (Table 3).
Univariate Kaplan-Meier survival analysis demonstrated no statistically significant differences in survival of patients with or without Bcl-2 expression in the surgery-alone group (overall log rank P=0.431) or the 5-FU-treated group (overall log rank P=0.112) (data not shown).
For each patient, the ratio of Bax/Bcl2 expression was determined and correlated with patient survival separately for each treatment category. The ratio of Bax/Bcl2 expression was not correlated with patient survival in surgery alone category (Fig 3C). In contrast, the ratio of Bax/Bcl2 expression significantly correlated with patient survival, indicating that those patients with a high Bax/Bcl2 ratio value would not benefit from 5-FU treatment; however, those patients with low Bax/Bcl2 ratios were more likely to have better survival when treated with 5-FU based therapies (Fig 3D).
Analysis of nuclear accumulation of p53
Expression of p53nac was generally homogenous; however, p53 staining was observed in the cytoplasm of malignant cells in 7% of cases (8 of 112) (Fig 2G-I). The cytoplasmic staining was not further analyzed. High levels of p53nac were found in tumors of 59% of patients (33 5-FU-treated and 32 surgery-alone). For both categories of patients, there were no significant differences in the incidence of deaths due to CRCs (Table 1), in the median survival (Table 3), or survival rates (data not shown) in relation to p53nac (Table 1).
Multivariate survival analyses
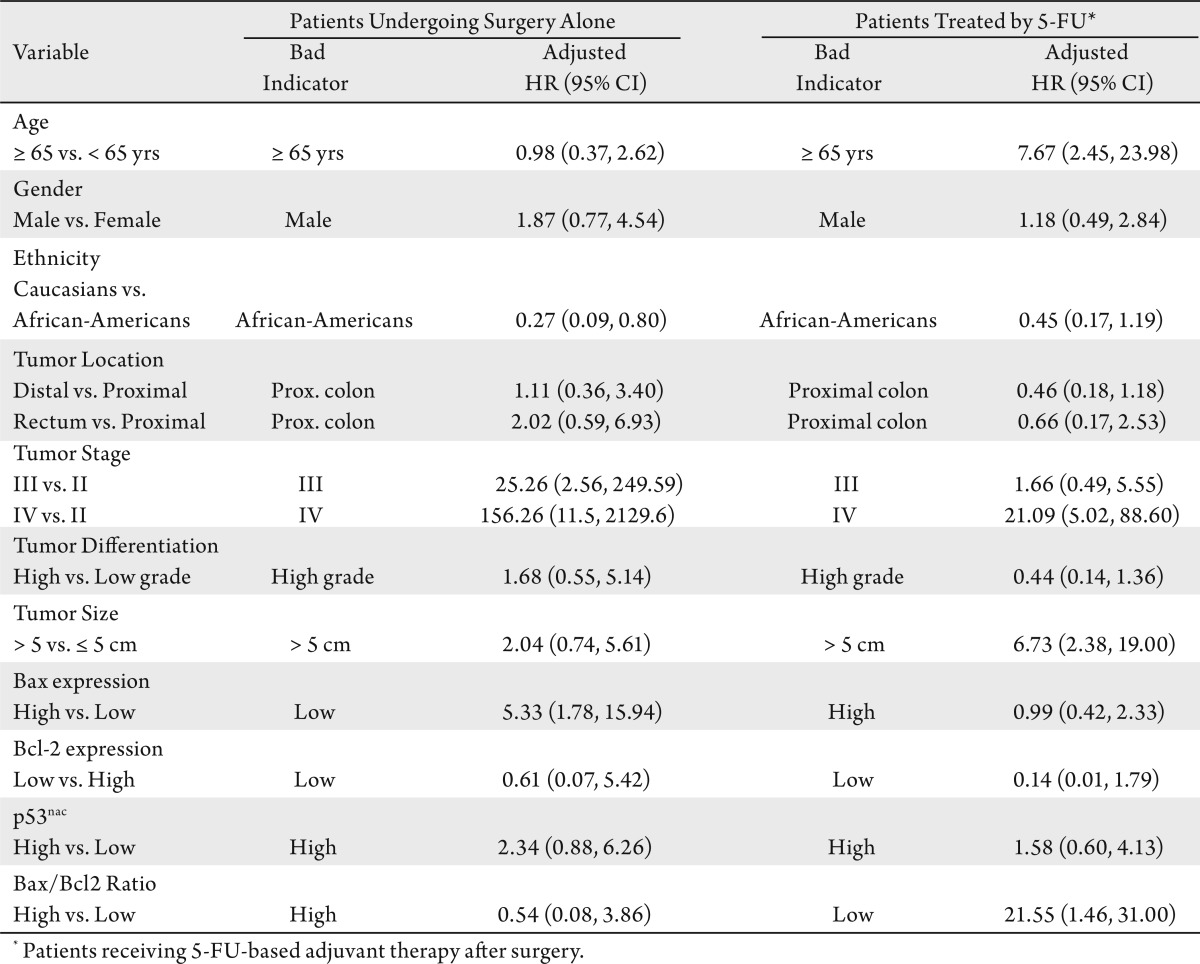
As determined by multivariate analyses of Cox proportional hazards, surgery-alone patients with low Bax expression had 5.33 times higher mortality compared to those with high Bax expression (HR: 5.33; CI: 1.78-15.94) when adjusted for demographic and clinicopathological variables and for expression Bcl-2, the Bax/Bcl2 ratio, and p53nac (Table 4). 5-FU treated patients with low Bax/Bcl2 ratios had 21.55 times higher mortality compared to those with high Bax/Bcl2 ratios (HR: 21.55; CI: 1.46-31.00), when adjusted for other variables (Table 4). Bcl-2 and p53nac, however, were not independent prognostic indicators of survival in either group of patients (Table 4).
Table 4. Multivariate Cox regression analyses to assess the prognostic significance of clinicopathological and molecular features based on treatment status on overall survival.
Discussion
The current study, we analyzed the predictive value of Bax, Bcl-2, and p53nac and determined their association with survival in CRC patients who received 5-FU-based adjuvant treatment after surgery and in CRC patients matched for age, gender, ethnicity, tumor stage, tumor location, and tumor differentiation who underwent surgery alone with curative or palliative intent. These analyses demonstrate a better survival of patients who received chemotherapy when their CRCs lacked Bax expression. In contrast, patients with CRCs that exhibited high Bax expression had worse survival when they received 5-FU-based adjuvant chemotherapy. Furthermore, multivariate Cox regression analysis showed that surgically treated patients with low levels (or lack) of Bax expression had 5.33 times higher mortality than those with high Bax expression, after adjusting for confounding variables, including tumor stage. Bcl-2 or p53nac had no predictive value in either group of patients. These findings are the first to indicate that patients with CRCs that lack or express low levels of Bax, but not those with high expression, benefit from 5-FU-based adjuvant therapies. Analysis of a large sample set, however, could provide more definitive information.
Although the current evaluation was performed in a retrospective setting, and the sample was small, the inclusion and exclusion criteria and the sample matching method, described in the Material and Methods section, minimizes the risk of error and provides strength to the findings. By including only those patients who completed at least 3 months of treatment when on continuous infusion regimens or 6 months when on bolus regimens, and excluding all patients who received any kind of treatment prior to surgery, we lowered the potential errors from using a population from different protocols and different physicians.
Although several studies have been performed to identify potential predictive markers of 5-FU for CRC treatment, the results are inconclusive (16)-(18),(49). 5-FU and other chemotherapeutic agents may cause death of cancer cells by inducing apoptosis. Since apoptosis can be initiated either in the mitochondria by activation of the caspases cascade or by the induction of p53 and apoptotic molecules such as Bax and Bcl-2, we assessed the prognostic and predictive value of expression of Bax and Bcl-2 and p53nac.
Relative to p53, Bax is downstream and can act synergistically with p53, but it does not completely depend on p53 to function in apoptosis (27),(28). Furthermore, the efficacy of Bax in predicting response or resistance to chemotherapy and apoptosis is tissue-specific (28). In agreement with previous studies (28),(50) the current investigation demonstrated that Bax expression in CRCs is not associated with the status of p53nac; however, Bax expression has both predictive and prognostic value. The findings that the patients with CRCs expressing high levels of Bax had a better survival than those with low Bax expression, particularly in patients who have undergone surgery alone, are consistent with several other earlier studies of CRCs (27),(51)-(53) and other human malignancies (44),(54).
Although it was not significant, the predictive role of Bax expression was evident in our observation that patients with low Bax expression who received 5-FU-based adjuvant therapy had a longer survival than those patients with high Bax expression, showing that patients with low Bax expression have an apparent benefit from 5-FU-based adjuvant therapy. An earlier analysis involving patients with advanced CRCs (recurrent or metastatic) who received methotrexate plus 5-FU demonstrated a similar trend in an association between Bax expression and overall survival, as the median survival for low expressors of Bax was 9 months compared to 14 months for high expressors (55). For a group of patients subjected to preoperative radiochemotherapy for locally advanced rectal carcinoma, however, there was no correlation between the level of Bax expression and tumor recurrence (56).
Contrary to our findings, results of studies performed in vitro demonstrate that CRC cell lines with high Bax expression responded well to long-term 5-FU exposure, which induced apoptosis (57),(58). Additionally, studies performed in vitro have indicated that antioxidants, such as N-acetylcysteine and vitamin E, are required to augment Bax expression to elicit 5-FU-induced apoptosis (59). Nevertheless, there were no such findings in clinical studies or in experimental studies performed in vivo. Based on our findings, however, the low levels of Bax may exert less intrinsic resistance to the complex cascade of intracellular signals of apoptotic pathways triggered by chemotherapeutic agents. Thus, there are apparently distinct mechanisms of Bax involvement in the manifestation of apoptosis.
Molecular markers have different functional roles, similar to the Bax expression observed here. A recent investigation by Tsuji et al (60) demonstrated that high expression of dihydropyrimidine dehydrogenase (DPD) in Stage II and III CRCs was an effective indicator of oral 5-FU-based adjuvant therapy; however, low expression of tumor DPD predicted poor survival for patients undergoing surgery alone. The prognostic value of high Bax expression observed for the surgery-alone group might be useful for a sub-set of Stage I and Stage II patients; in contrast, the predictive value of Bax expression might be useful in predicting the efficacy of 5-FU-based therapy, particularly for patients with advanced stage disease (Stage III or IV), who routinely receive 5 -FU-based adjuvant therapy. Larger studies determining the clinical usefulness of Bax expression in CRCs according to pathologic stage may confirm these findings.
In the current investigation, increased Bcl-2 expression in CRCs was not predictive of 5 -FU-based adjuvant therapy; however, increased Bcl-2 expression was an indicator of prolonged survival for patients who had surgery alone. The prognostic value of Bcl-2 expression in CRCs has been demonstrated (8),(61). The association between increased Bcl-2 expression and patient overall survival was stronger in early-stage CRCs (62)-(64) and for CRCs located in the distal colorectum (11). Similar to our findings, other studies demonstrated that, for patients receiving 5-FU-based chemotherapy, Bcl-2 expression did not influence response to chemotherapy and did not affect overall survival(55),(65),(66). Our multivariate survival analysis, however, demonstrated a better survival of patients whose tumors had low a Bax/Bcl-2 ratio (i.e., Bax was low) and who received 5-FU-based adjuvant chemotherapy. Furthermore, the expression of these two apoptotic markers was not associated with p53nac. Similar to our findings, Mirjolet et al (67) and Violette et al (58) demonstrated, by experiments performed in vitro, that 5-FU sensitivity is independent of p53. The current findings support the premise that patients with CRCs expressing high levels of Bax should not be considered for 5-FU-based adjuvant chemotherapy. These results indicate that the balance between pro-apoptotic and anti-apoptotic markers has a function in the response to therapy. Nevertheless, large prospective studies are required to provide further information useful for making therapeutic decisions.
p53 has been considered to be a prognostic and predictive marker, and it has been established as an important prognostic indicator, specifically for non-Hispanic Caucasian patients with tumors located in the proximal colon (9). As anticipated, p53nac was not useful in predicting the overall survival of patients receiving surgery alone, because these two cohorts consist of tumors from all anatomic locations of the colorectum, and from African Americans and non -Hispanic Caucasians.
The current report demonstrates that p53nac is not useful in predicting the response to 5-FU-based adjuvant therapy. Several studies have shown that p53 has a function in chemotherapy-induced apoptosis and is a predictor of 5-FU-based adjuvant therapy response in CRCs (68); others did not find such an association (58),(67),(69). These conflicting findings may be due to the admixture of patient populations for ethnicity, tumor stage, or tumor location, as has been observed in the evaluation of p53nac for its prognostic value (9). Other reasons for these conflicting results could be the technical variations in detecting p53nac, including the antigen enhancement methods and antibodies used or the choice of cut-off values considered for tumor positivity for abnormal p53 expression (8). The predictive capacity of p53 in CRCs remains controversial.
Findings of the current investigation show that, for the surgery-alone group, high Bax expression is associated with better survival. Although statistically not significant, low Bax expression in the 5-FU-based adjuvant chemotherapy group was associated with improved survival. Further, these data reveal that patients with low Bax/Bcl2 expression ratios would benefit from 5-FU-based adjuvant therapy. Findings from the present proof-of-principle studies provide evidence that phenotypic expression of Bax and Bcl-2 predict the response to 5-FU-based adjuvant therapy in CRCs. Future prospective studies will assess the clinical utility of these markers.
Acknowledgments
We thank Dr. Donald Hill, Division of Preventive Medicine, University of Alabama at Birmingham, for his critical comments.
Footnotes
This work was supported in part by grants from the National Institute of Health/National Cancer Institute to Dr. U Manne (U54-CA118948, R03 CA139629, and R01-CA98932-S1).
References
- 1.Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull. 2002;64:1–25. doi: 10.1093/bmb/64.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Sun W, Haller DG. Chemotherapy for colorectal cancer. Hematol Oncol Clin North Am. 2002;16:969–94. doi: 10.1016/s0889-8588(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Dicato M, Wils J, Cunningham D, Diaz-Rubio E, Glimelius B, et al. Adjuvant treatment of colorectal cancer (current expert opinion derived from the Third International Conference: Perspectives in Colorectal Cancer, Dublin, 2001) Eur J Cancer. 2002;38:1429–36. doi: 10.1016/s0959-8049(02)00122-3. [DOI] [PubMed] [Google Scholar]
- 4.Conley BA, Kaplan RS, Arbuck SG. National cancer institute clinical trials program in colorectal cancer. Cancer Chemother Pharmacol. 1998;42:S75–9. doi: 10.1007/s002800051084. [DOI] [PubMed] [Google Scholar]
- 5.Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000;321:531–5. doi: 10.1136/bmj.321.7260.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ragnhammar P, Hafstrom L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282–308. doi: 10.1080/02841860151116367. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Jatkoe T, Zhang Y, Mutch MG, Talantov D, Jiang J, et al. Gene expression profiles and molecular markers to predict recurrence of Dukes' B colon cancer. J Clin Oncol. 2004;22:1564–71. doi: 10.1200/JCO.2004.08.186. [DOI] [PubMed] [Google Scholar]
- 8.Manne U, Myers RB, Moron C, Poczatek RB, Dillard S, Weiss H, et al. Prognostic significance of Bcl -2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int J Cancer. 1997;74:346–58. doi: 10.1002/(sici)1097-0215(19970620)74:3<346::aid-ijc19>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Manne U, Weiss HL, Myers RB, Danner OK, Moron C, Srivastava S, et al. Nuclear accumulation of p53 in colorectal adenocarcinoma: prognostic importance differs with race and location of the tumor. Cancer. 1998;83:2456–67. doi: 10.1002/(sici)1097-0142(19981215)83:12<2456::aid-cncr8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 10.Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000;6:4017–25. [PubMed] [Google Scholar]
- 11.Manne U, Weiss HL, Grizzle WE. Bcl-2 expression is associated with improved prognosis in patients with distal colorectal adenocarcinomas. Int J Cancer. 2000;89:423–30. doi: 10.1002/1097-0215(20000920)89:5<423::aid-ijc5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Manne U, Jhala NC, Jones J, Weiss HL, Chatla C, Meleth S, et al. Prognostic significance of p27(kip-1) expression in colorectal adenocarcinomas is associated with tumor stage. Clin Cancer Res. 2004;10:1743–52. doi: 10.1158/1078-0432.ccr-03-0037. [DOI] [PubMed] [Google Scholar]
- 13.Manne U, Gary BD, Oelschlager DK, Weiss HL, Frost AR, Grizzle WE. Altered subcellular localization of suppressin, a novel inhibitor of cell-cycle entry, is an independent prognostic factor in colorectal adenocarcinomas. Clin Cancer Res. 2001;7:3495–503. [PubMed] [Google Scholar]
- 14.Grizzle WE, Manne U, Jhala N, Weiss H. The molecular characterization of colorectal neoplasia in translational research. Arch Path & Lab Med. 2001;125:91–8. doi: 10.5858/2001-125-0091-MCOCNI. [DOI] [PubMed] [Google Scholar]
- 15.Nehls O, Okech T, Hsieh CJ, Enzinger T, Sarbia M, Borchard F, et al. Studies on p53, BAX and Bcl-2 protein expression and microsatellite instability in stage III (UICC) colon cancer treated by adjuvant chemotherapy: major prognostic impact of proapoptotic BAX. Br J Cancer. 2007;96:1409–18. doi: 10.1038/sj.bjc.6603728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maughan NJ, Quirke P. Pathology--a molecular prognostic approach. Br Med Bull. 2002;64:59–74. doi: 10.1093/bmb/64.1.59. [DOI] [PubMed] [Google Scholar]
- 17.Duffy MJ, van Dalen A, Haglund C, Hansson L, Klapdor R, Lamerz R, et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur J Cancer. 2003;39:718–27. doi: 10.1016/s0959-8049(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 18.Bast RC, Jr, Ravdin P, Hayes DF, Bates S, Fritsche H, Jr, Jessup JM, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–78. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 19.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–6. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 20.Yang B, Eshleman JR, Berger NA, Markowitz SD. Wild-type p53 protein potentiates cytotoxicity of therapeutic agents in human colon cancer cells. Clin Cancer Res. 1996;2:1649–57. [PubMed] [Google Scholar]
- 21.Lenz HJ, Hayashi K, Salonga D, Danenberg KD, Danenberg PV, Metzger R, et al. p53 point mutations and thymidylate synthase messenger RNA levels in disseminated colorectal cancer: an analysis of response and survival. Clin Cancer Res. 1998;4:1243–50. [PubMed] [Google Scholar]
- 22.Allegra CJ, Parr AL, Wold LE, Mahoney MR, Sargent DJ, Johnston P, et al. Investigation of the prognostic and predictive value of thymidylate synthase, p53, and Ki-67 in patients with locally advanced colon cancer. J Clin Oncol. 2002;20:1735–43. doi: 10.1200/JCO.2002.07.080. [DOI] [PubMed] [Google Scholar]
- 23.Allegra CJ, Paik S, Colangelo LH, Parr AL, Kirsch I, Kim G, et al. Prognostic value of thymidylate synthase, Ki-67, and p53 in patients with Dukes’ B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2003;21:241–50. doi: 10.1200/JCO.2003.05.044. [DOI] [PubMed] [Google Scholar]
- 24.Elsaleh H, Powell B, Soontrapornchai P, Joseph D, Goria F, Spry N, et al. p53 gene mutation, microsatellite instability and adjuvant chemotherapy: impact on survival of 388 patients with Dukes’ C colon carcinoma. Oncology. 2000;58:52–9. doi: 10.1159/000012079. [DOI] [PubMed] [Google Scholar]
- 25.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 26.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74:957–67. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 27.Sturm I, Köhne CH, Wolff G, Petrowsky H, Hillebrand T, Hauptmann S, et al. Analysis of the p53/BAX pathway in colorectal cancer: low BAX is a negative prognostic factor in patients with resected liver metastases. J Clin Oncol. 1999;17:1364–74. doi: 10.1200/JCO.1999.17.5.1364. [DOI] [PubMed] [Google Scholar]
- 28.McCurrach ME, Connor TM, Knudson CM, Korsmeyer SJ, Lowe SW. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc Natl Acad Sci U S A. 1997;94:2345–9. doi: 10.1073/pnas.94.6.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chresta CM, Masters JR, Hickman JA. Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high Bax:Bcl-2 ratio. Cancer Res. 1996;56:1834–41. [PubMed] [Google Scholar]
- 30.Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995;11:1217–28. [PubMed] [Google Scholar]
- 31.Koshiji M, Adachi Y, Taketani S, Takeuchi K, Hioki K, Ikehara S. Mechanisms underlying apoptosis induced by combination of 5-fluorouracil and interferon-gamma. Biochem Biophys Res Commun. 1997;240:376–81. doi: 10.1006/bbrc.1997.7657. [DOI] [PubMed] [Google Scholar]
- 32.WHO. International classification of diseases for oncology. In:1990; Geneva: World Health Organization. 1990 [Google Scholar]
- 33.Purdie CA, Piris J. Histopathological grade, mucinous differentiation and DNA ploidy in relation to prognosis in colorectal carcinoma. Histopathology. 2000;36:121–6. [PubMed] [Google Scholar]
- 34.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–94. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 35.Green FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. New York: Springer-Verlag; 2006. AJCC cancer staging handbook: From the AJCC cancer staging manual. 6th ed. [Google Scholar]
- 36.Jansson A, Sun XF. Bax expression decreases significantly from primary tumor to metastasis in colorectal cancer. J Clin Oncol. 2002;20:811–6. doi: 10.1200/JCO.2002.20.3.811. [DOI] [PubMed] [Google Scholar]
- 37.Sturm I, Köhne CH, Wolff G, Petrowsky H, Hillebrand T, Hauptmann S, et al. Analysis of the p53/BAX pathway in colorectal cancer: low BAX is a negative prognostic factor in patients with resected liver metastases. J Clin Oncol. 1999;17:1364–74. doi: 10.1200/JCO.1999.17.5.1364. [DOI] [PubMed] [Google Scholar]
- 38.Fredricks DN, Relman DA. Paraffin removal from tissue sections for digestion and PCR analysis. Biotechniques. 1999;26:198–200. doi: 10.2144/99262bm04. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1996;88:1054–9. doi: 10.1093/jnci/88.15.1054. [DOI] [PubMed] [Google Scholar]
- 40.Prioleau J, Schnitt SJ. p53 antigen loss in stored paraffin slides. N Engl J Med. 1995;332:1521–2. doi: 10.1056/NEJM199506013322217. [DOI] [PubMed] [Google Scholar]
- 41.Baas IO, van den Berg FM, Mulder JW, Clement MJ, Slebos RJ, Hamilton SR, et al. Potential false-positive results with antigen enhancement for immunohistochemistry of the p53 gene product in colorectal neoplasms. J Pathol. 1996;178:264–7. doi: 10.1002/(SICI)1096-9896(199603)178:3<264::AID-PATH485>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 42.Grizzle WE, Myers RB, Manne U, Srivastava S. Immunohistochemical evaluation of biomarkers in prostatic and colorectal neoplasia. In: Hanausek M, Walaszek Z, editors. John Walker's methods in molecular medicine-tumor marker protocols. Totowa: Humana Press; 1998. pp. 143–60. [Google Scholar]
- 43.Manne U, Myers RB, Srivastava S, Grizzle WE. Re: loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1997;89:585–6. doi: 10.1093/jnci/89.8.585. [DOI] [PubMed] [Google Scholar]
- 44.Krajewski S, Blomqvist C, Franssila K, Krajewska M, Wasenius VM, Niskanen E, et al. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 1995;55:4471–8. [PubMed] [Google Scholar]
- 45.Miquel C, Borrini F, Grandjouan S, Aupérin A, Viguier J, Velasco V, et al. Role of bax Mutations in Apoptosis in Colorectal Cancers With Microsatellite Instability. Am J Clin Pathol. 2005;123:1–9. doi: 10.1309/JQ2X-3RV3-L8F9-TGYW. [DOI] [PubMed] [Google Scholar]
- 46.Allison P. Cary, NC: SAS Institute Inc; 1995. Survival analysis using the SAS system: A practical guide. [Google Scholar]
- 47.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–48. [Google Scholar]
- 48.Cox DR. Regression models and life tables. J Roy Stat Soc. 1972;34:187–220. [Google Scholar]
- 49.Allen WL, Johnston PG. Role of genomic markers in colorectal cancer treatment. J Clin Oncol. 2005;23:4545–52. doi: 10.1200/JCO.2005.19.752. [DOI] [PubMed] [Google Scholar]
- 50.De Angelis PM, Stokke T, Thorstensen L, Lothe RA, Clausen OP. Apoptosis and expression of Bax, Bcl-x, and Bcl-2 apoptotic regulatory proteins in colorectal carcinomas, and association with p53 genotype/phenotype. Mol Pathol. 1998;51:254–61. doi: 10.1136/mp.51.5.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogura E, Senzaki H, Yamamoto D, Yoshida R, Takada H, Hioki K, et al. Prognostic significance of Bcl-2, Bcl-xL/S, Bax and Bak expressions in colorectal carcinomas. Oncol Rep. 1999;6:365–9. doi: 10.3892/or.6.2.365. [DOI] [PubMed] [Google Scholar]
- 52.Schelwies K, Sturm I, Grabowski P, Scherübl H, Schindler I, Hermann S, et al. Analysis of p53/BAX in primary colorectal carcinoma: low BAX protein expression is a negative prognostic factor in UICC stage III tumors. Int J Cancer. 2002;99:589–96. doi: 10.1002/ijc.10380. [DOI] [PubMed] [Google Scholar]
- 53.Nehls O, Hass HG, Okech T, Zenner S, Hsieh CJ, Sarbia M, et al. Prognostic implications of BAX protein expression and microsatellite instability in all non-metastatic stages of primary colon cancer treated by surgery alone. Int J Colorectal Dis. 2009;24:655–63. doi: 10.1007/s00384-009-0635-0. [DOI] [PubMed] [Google Scholar]
- 54.Sturm I, Petrowsky H, Volz R, Lorenz M, Radetzki S, Hillebrand T, et al. Analysis of p53/BAX/p16(ink4a/CDKN2) in esophageal squamous cell carcinoma: high BAX and p16(ink4a/CDKN2) identifies patients with good prognosis. J Clin Oncol. 2001;19:2272–81. doi: 10.1200/JCO.2001.19.8.2272. [DOI] [PubMed] [Google Scholar]
- 55.Paradiso A, Simone G, Lena MD, Leone B, Vallejo C, Lacava J, et al. Expression of apoptosis-related markers and clinical outcome in patients with advanced colorectal cancer. Br J Cancer. 2001;84:651–8. doi: 10.1054/bjoc.2000.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tannapfel A, Nusslein S, Fietkau R, Katalinic A, Kockerling F, Wittekind C. Apoptosis, proliferation, bax, bcl-2 and p53 status prior to and after preoperative radiochemotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 1998;41:585–91. doi: 10.1016/s0360-3016(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi T, Sawa H, Morikawa J, Zhang W, Shiku H. Bax induction activates apoptotic cascade via mitochondrial cytochrome c release and Bax overexpression enhances apoptosis induced by chemotherapeutic agents in DLD-1 colon cancer cells. Jpn J Cancer Res. 2000;91:1264–8. doi: 10.1111/j.1349-7006.2000.tb00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Violette S, Poulain L, Dussaulx E, Pepin D, Faussat AM, Chambaz J, et al. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int J Cancer. 2002;98:498–504. doi: 10.1002/ijc.10146. [DOI] [PubMed] [Google Scholar]
- 59.Adeyemo D, Imtiaz F, Toffa S, Lowdell M, Wickremasinghe RG, Winslet M. Antioxidants enhance the susceptibility of colon carcinoma cells to 5-fluorouracil by augmenting the induction of the bax protein. Cancer Lett. 2001;164:77–84. doi: 10.1016/s0304-3835(00)00720-5. [DOI] [PubMed] [Google Scholar]
- 60.Tsuji T, Sawai T, Takeshita H, Nakagoe T, Hidaka S Atsushi Nanashima, et al. Tumor dihydropyrimidine dehydrogenase in stage II and III colorectal cancer: low level expression is a beneficial marker in oral-adjuvant chemotherapy, but is also a predictor for poor prognosis in patients treated with curative surgery alone. Cancer Lett. 2004;204:97–104. doi: 10.1016/j.canlet.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Sinicrope FA, Hart J, Michelassi F, Lee JJ. Prognostic value of bcl-2 oncoprotein expression in stage II colon carcinoma. Clin Cancer Res. 1995;1:1103–10. [PubMed] [Google Scholar]
- 62.Krajewska M, Kim H, Kim C, Kang H, Welsh K, Matsuzawa S, et al. Analysis of apoptosis protein expression in early-stage colorectal cancer suggests opportunities for new prognostic biomarkers. Clin Cancer Res. 2005;11:5451–61. doi: 10.1158/1078-0432.CCR-05-0094. [DOI] [PubMed] [Google Scholar]
- 63.Meterissian SH, Kontogiannea M, Al-Sowaidi M, Linjawi A, Halwani F, Jamison B, et al. Bcl-2 is a useful prognostic marker in Dukes' B colon cancer. Ann Surg Oncol. 2001;8:533–7. doi: 10.1007/s10434-001-0533-3. [DOI] [PubMed] [Google Scholar]
- 64.Chatla C, Jhala NC, Katkoori VR, Alexander D, Meleth S, Grizzle WE, et al. Recurrence and survival predictive value of phenotypic expression of Bcl-2 varies with tumor stage of colorectal adenocarcinoma. Dis Markers. 2005;21:1–10. doi: 10.3233/cbm-2005-14-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosati G, Chiacchio R, Reggiardo G, De Sanctis D, Manzione L. Thymidylate synthase expression, p53, bcl-2, Ki-67 and p27 in colorectal cancer: relationships with tumor recurrence and survival. Tumour Biol. 2004;25:258–63. doi: 10.1159/000081389. [DOI] [PubMed] [Google Scholar]
- 66.Schneider HJ, Sampson SA, Cunningham D, Norman AR, Andreyev HJ, Tilsed JV, et al. Bcl-2 expression and response to chemotherapy in colorectal adenocarcinomas. Br J Cancer. 1997;75:427–31. doi: 10.1038/bjc.1997.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mirjolet JF, Barberi-Heyob M, Didelot C, Peyrat JP, Abecassis J, Millon R, et al. Bcl-2/Bax protein ratio predicts 5-fluorouracil sensitivity independently of p53 status. Br J Cancer. 2000;83:1380–6. doi: 10.1054/bjoc.2000.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang R, Wang JY, Fan CW, Tsao KC, Chen HH, Wu CM, et al. p53 is an independent pre-treatment markers for long-term survival in stage II and III colorectal cancers: an analysis of interaction between genetic markers and fluorouracil-based adjuvant therapy. Cancer Lett. 2004;210:101–9. doi: 10.1016/j.canlet.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 69.Ahnen DJ, Feigl P, Quan G, Fenoglio-Preiser C, Lovato LC Bunn PA Jr, et al. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res. 1998;58:1149–58. [PubMed] [Google Scholar]