Abstract
Background
Two previous first-line studies showed an improved trend in response rate (RR) and progression free survival (PFS) in metastatic colorectal cancer (CRC) patients with KRAS mutation. Others have reported a worsened outlook for metastatic CRC patients with KRAS mutation and a higher likelihood of metastatic disease to the lungs. In this study, we aimed to address the impact of KRAS on the pattern of metastatic disease at presentation and on RR and PFS with first-line 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy.
Methods
Patients with CRC who underwent KRAS testing using DxS assay at Roswell Park Cancer Institute (RPCI) were identified. Patients with metastatic CRC treated with first-line FOLFOX +/- bevacizumab were assessed for response and survival using RECIST 1.1 guidelines. A two-sided Fisher's exact test was used to determine the statistical significance.
Results
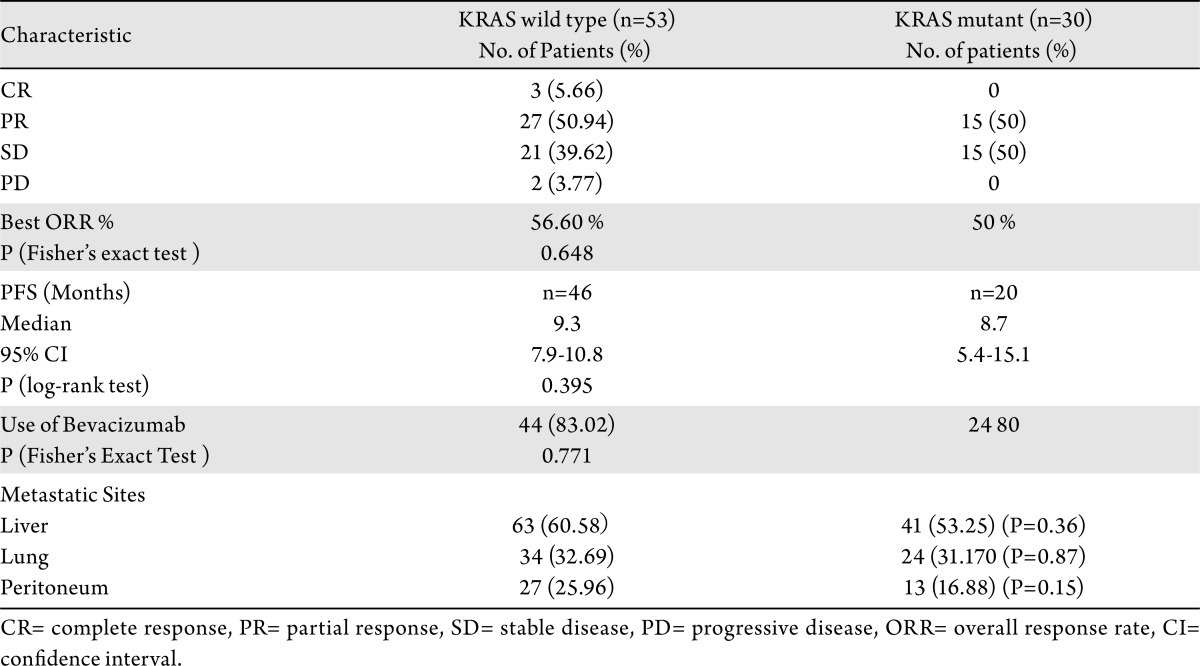
181 patients with metastatic CRC and KRAS testing were identified. 83/181 patients were treated with FOLFOX (+/- bevacizumab) in the first-line setting at RPCI and were evaluable as per study guidelines. KRAS mutation (MT) occurred in 40.31% cases. There was no difference in organ-metastases distribution, RR (56.60% in KRAS wild-type (WT) and 50% in KRAS mutant) or PFS (9.3 months KRAS WT and 8.7 months in KRAS MT) based on KRAS status.
Conclusion
In this single institute study, our findings do not support any predictive role for KRAS-MT in terms of response to FOLFOX first-line chemotherapy, or in terms of sites of metastatic disease at mCRC presentation.
Keywords: metastatic colorectal cancer, KRAS, FOLFOX, cetuximab
Introduction
The treatment of metastatic colorectal cancer (mCRC) has made significant progress in the past decade, including the introduction of agents targeting epidermal growth factor receptor (EGFR). The therapeutic success of monoclonal antibodies against EGFR (cetuximab and panitumumab) in treating patients with mCRC highlights the importance of counteracting the EGFR pathway to control advanced disease (1). In unselected patient populations, response to anti-EGFR treatment has been modest, which prompted investigators to identify biomarkers that predict increased likelihood of response in a subpopulation. Among a number of potential biomarkers studied, mutational activation of RAS oncogenes has emerged as the most important factor for determining non-responsiveness to EGFR inhibitors.
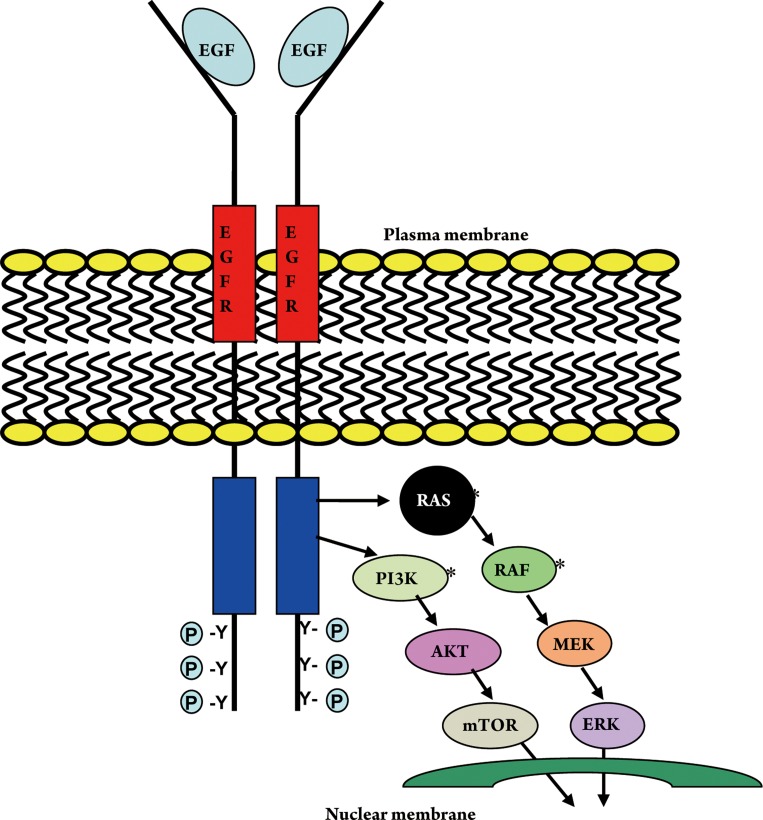
KRAS is a protein which in humans is encoded by the KRAS gene and functions as an essential component of the EGFR signaling cascade. Activating mutations in KRAS gene cause constitutively active Ras GTPase, which leads to over-activation of downstream Raf/Erk/Map kinase and other signaling pathways, resulting in cell transformation and tumorigenesis (Fig 1) (2),(3). KRAS mutations are present in approximately 30% to 50% of colon cancer specimens (4). Fearon and Vogelstein established a stepwise hypothesis for colorectal cancer tumorigenesis and delineated the importance of mutation in RAS gene as an initiating event in the formation of malignant tumor (5).
Figure 1. Epidermal growth factor receptor signal transduction pathway. * Common sites of mutation in colorectal cancer.
Preclinical studies have suggested that constitutively activated mutant KRAS can promote tumor invasion and metastasis by stimulating matrix metalloproteases, cysteine proteases, serine proteases, and urokinase plasminogen activator that facilitate migration through the basement membrane (6),(7),(8). Despite such findings the role of KRAS mutation in prognosis of mCRC patients is not clear. The RASCAL study, which was the largest study designed to analyze the prognostic value of KRAS status showed that a glycine-to-valine mutation in codon 12 increased the likelihood of disease relapse and a lower overall survival (OS) (9). Multiple other studies with smaller sample size did not demonstrate any impact of KRAS mutations on survival (10),(11),(12). Even in the updated RASCAL II study, the evidence of a statistically significant worse clinical outcome was limited to stage III disease and was not confirmed for other stages (13). These results are limited by their retrospective nature and lack of adequate power to detect significant differences.
The relationship between KRAS status of primary tumor and stage at diagnosis as well as pattern of spread is also not clear. Samowitz et al. reported that codon 12 mutations in KRAS gene were found to be much more common in proximal tumors and were associated with advance stage at presentation (14). Bazan and colleagues showed that codon 12 mutation in tumor was associated with mucinous histology and mutation in codon 13 was associated with advanced Duke stage (15). In a retrospective study KRAS mutation of the primary tumor was also associated with higher incidence of metastatic disease to lungs (16). Analysis of KRAS and BRAF mutation status in PETACC-3, an adjuvant trial with 3,278 patients with stage II to III colon cancer revealed that incidence of either mutation was not significantly different according to tumor stage. KRAS mutation was associated with grade of the tumor, while BRAF mutation was associated with right-sided tumors, older age, female gender, high grade, and MSI-high tumors. KRAS mutations were not prognostically related to relapse-free survival (RFS) or OS whereas BRAF mutation was not prognostic for RFS, but was for OS, particularly in patients with microsatellite instability-low (MSI-L) and stable (MSI-S) tumors (17).
Multiple studies have demonstrated an association between KRAS mutational status in the primary tumor and resistance to EGFR inhibitors (cetuximab and panitumumab) in patients with mCRC (18),(19). Recently based on convincing data, National Comprehensive Cancer Network (NCCN) has also made recommendation that patients with known KRAS mutations should not be treated with EGFR inhibitors (20).
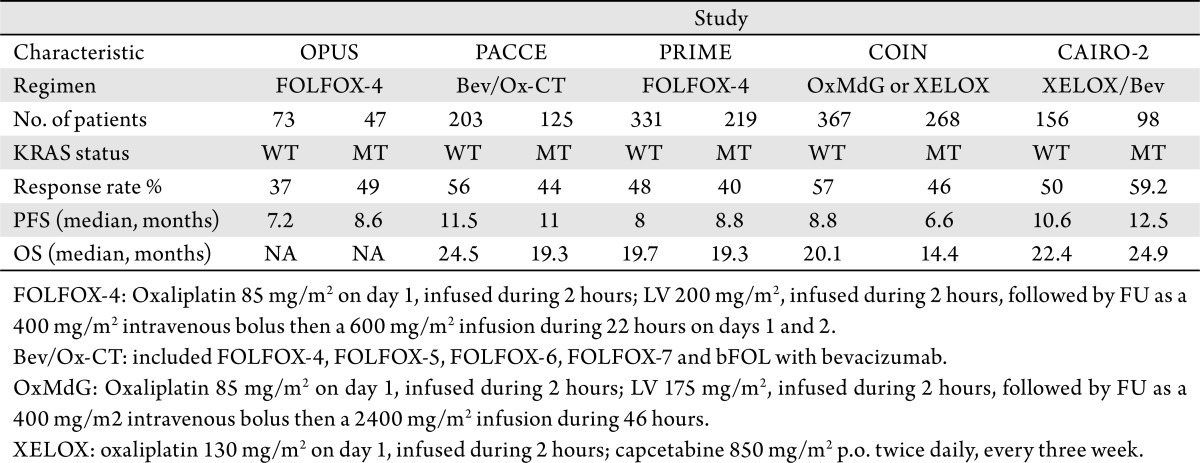
Although there is robust data regarding the association of WT KRAS status and response to EGFR inhibitors, the relationship between KRAS MT and response to first line oxaliplatin based chemotherapy without anti-EGFR antibodies is conflicting. Two previous first-line studies showed an improved trend in response rate (RR) and progression free survival (PFS) in mCRC patients with KRAS MT, who were treated with first line chemotherapy regimen including oxaliplatin without cetuximab or panitumumab while others have reported a worsened outlook for patients with KRAS MT who were treated similarly (Table 1) (21),(22).
Table 1. Efficacy data of selected prospective studies in patients with known KRAS status.
In this study, we aimed to address the impact of KRAS on the pattern of metastatic disease at presentation and on clinical outcome with first line FOLFOX chemotherapy.
Patients and methods
Study endpoints
The primary endpoint of this study was to compare the progression free survival of KRAS WT and KRAS MT CRC patients treated with first-line FOLFOX (with or without bevacizumab) chemotherapy. Secondary endpoints included overall survival, response rate, and pattern of metastatic disease in the KRAS WT and MT populations.
Patient population
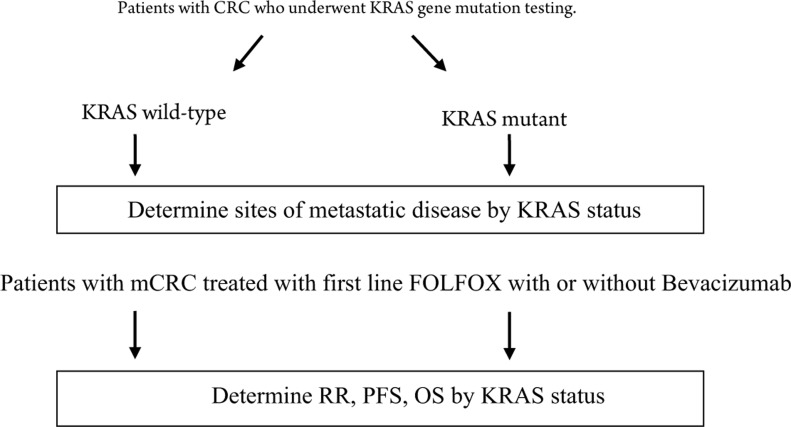
All patients with metastatic colorectal cancer with a known KRAS status and who were treated at Roswell Park Cancer Institute (RPCI) with first-line FOLFOX or FOLFOX plus bevacizumab were eligible for this study (Fig 2). Most of these patients were treated at our institute. Patients who received first line chemotherapy at a community hospital were included in the study only if their imaging studies were available for response evaluation.
Figure 2. Study scheme for assessment of outcome based on KRAS status in patients treated with first-line FOLFOX with or without Bevacizumab. CRC= colorectal cancer, mCRC= metastatic colorectal cancer, FOLFOX= Folinic acid, Fluorouracil, Oxaliplatin.
Treatment plan
First line chemotherapy consisted of oxaliplatin 85mg/m2 infused over 2 hours; bevacizumab 5mg/kg intravenous (I.V) over 10 minutes; leucovorin (LV) 400mg/m2 infused during 2 hours, followed by fluorouracil (FU) as a 400mg/m2 I.V. bolus on day 1 then a 2.4 grams/m2 continuous infusion over 46 hours on a 14-day treatment cycle. Patients receiving bevacizumab were dosed at 5mg/Kg every 2 weeks on day 1 of FOLFOX.
Efficacy assessment
CT images for all the patients were reviewed by the investigators for evaluation of response. Response was assessed according to revised RECIST (version 1.1) criteria for response evaluation in solid tumors (23). Change in tumor burden was classified as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). Patients with CR or PR were included in overall response rate (ORR). Patients had imaging studies every 4 cycles of chemotherapy. Best response after the start of chemotherapy was considered for ORR.
Statistical analysis
Response to treatment according to the mutational status was evaluated using the Fisher’s exact test. Progression free survival (PFS) was defined as the time from initiation of FOLFOX (with or without bevacizumab) until first evidence of radiographic progression or death, whichever occurred earlier. Overall survival (OS) was calculated as the period from the beginning of treatment to death or the last follow-up at which point data were censored. OS and PFS were estimated with the Kaplan-Meier algorithm. A P-value P≤0.05 was considered to indicate statistical significance. Test for differences in survival distributions was done using the log-rank test. Statistical analysis and plots were done using SAS, version 9.1, statistical software (SAS Institute Inc., Cary, NC).
KRAS mutation detection
Tumor DNA was isolated from formaldehyde-fixed paraffin-embedded tissues and screened for the presence of KRAS codon 12 and 13 mutations using a DxS K-RAS mutation test kit (DxS Ltd). This assay detects 7 KRAS mutations in codons 12 and 13 using qualitative real-time PCR assay combining Scorpions® and ARMS® (allele-specific PCR) technologies. Detectable mutations are; 1. Gly12Asp (GGT>GAT) 2. Gly12Ala (GGT>GCT) 3. Gly12Val (GGT>GTT) 4. Gly12Ser (GGT>AGT) 5. Gly12Arg (GGT>CGT) 6. Gly12Cys (GGT>TGT) 7. Gly13Asp (GGC>GAC). The method used in this kit is highly sensitive and depending on the total amount of DNA present, can detect approximately 1% of mutant in a background of wild-type genomic DNA.
Results
Patient demographics
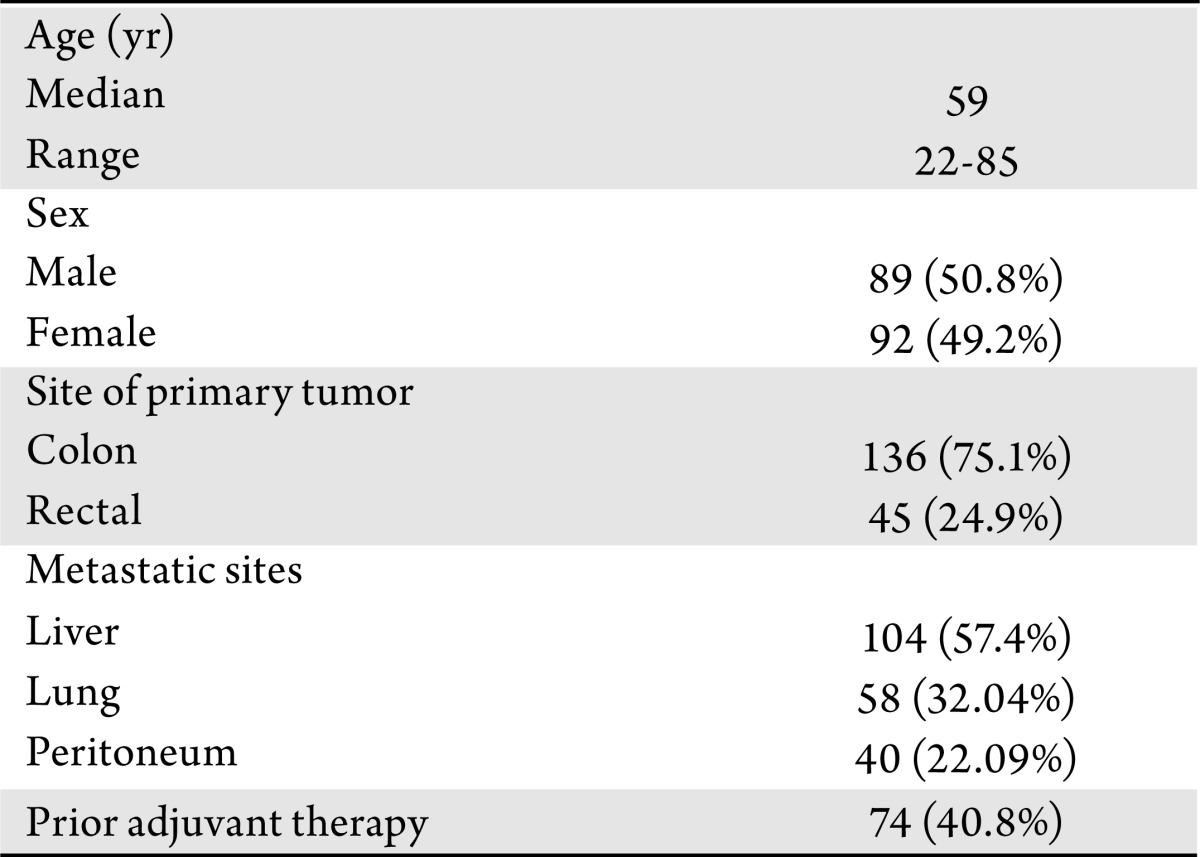
We identified 191 patients with CRC who underwent KRAS gene mutation testing using DxS assay at Roswell Park Cancer Institute (RPCI). KRAS DsX assays were performed between June 2008 and May 2009. 181/191 patients had confirmed metastatic disease and were included to assess the impact of KRAS status on the pattern of metastatic disease. The sites of metastastic disease at presentation were classified into 3 main categories: liver, lung, and peritoneum. Only 83 of the 181 patients received first-line FOLFOX or FOLFOX plus bevacizumab chemotherapy at RPCI and were subject to efficacy analysis. Baseline patient characteristics are summarized in Table 2.
Table 2. Baseline characteristics of the study patients (n=181).
KRAS mutation status and pattern of metastasis
Mutations in the KRAS were detected in 77 (40.31%) tumors with the following distributions: codon 13 mutations (Aspartate) 9.09%, codon 12 mutations (Aspartate) 46.75%, (Valine) 19.48%, and (Alanine) 7.79%. The most common site of metastasis was the liver, followed by lung, and peritoneum. The pattern of metastases was not significantly different between KRAS WT and MT patients (Table 3). Liver metastases occurred in 63/104 (60.6%) of KRAS WT and 41/77 (53.3%) of KRAS MT (P=0.36). Lung metastases occurred in 34/104 (32.7%) of KRAS WT and 24/77 (31.2%) of KRAS MT (P=0.87). Peritoneal metastases occurred in 27/104 (26%) of KRAS WT and 13/77 (16.9%) of KRAS MT (P=0.15).
Table 3. Pattern of metastatic disease and clinical outcome based on KRAS status.
KRAS mutations and outcome with first-line FOLFOX +/- bevacizumab
Out of 181 patients with metastatic disease, 83 received first line FOLFOX (+/- bevacizumab) chemotherapy at RPCI and were evaluable for response. Among the response-evaluable patients, 44/53 (83.02%) and 24/30 (80%) received bevacizumab in combination with FOLFOX in the KRAS WT and MT populations, respectively (P= 0.771).
The best overall response rate was 56.60% (27/53 PR and 3/53 CR) in KRAS WT and 50% (15/30 PR) in KRAS mutant patients (P=0.64). None of the patient with KRAS mutation had CR. Twenty one patients (39.6%) had stable disease in KRAS WT and 15 (50%) in KRAS mutant patients (Table 3).
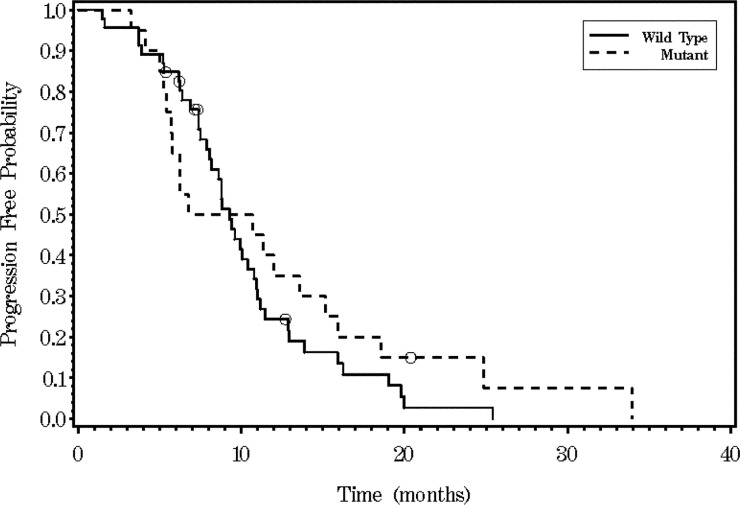
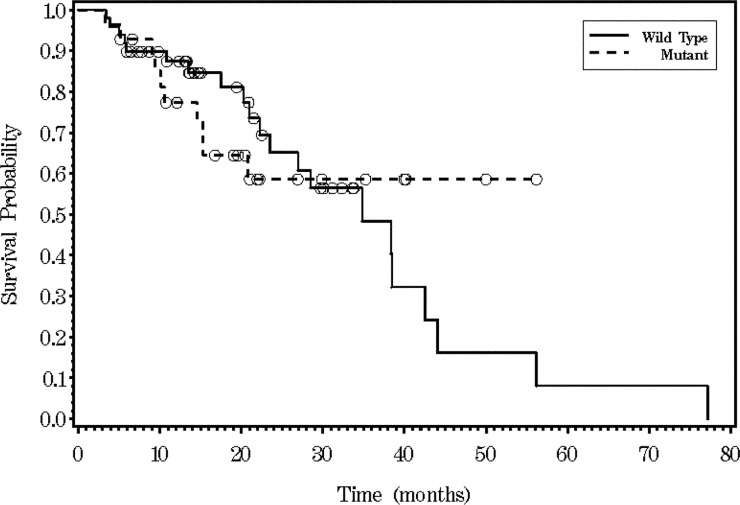
The median PFS was 9.3 months (95% CI, 7.85 to 10.78) in KRAS WT and 8.7 months (95% CI, 5.42 to 15.18) in KRAS MT populations (P=0.395, log-rank test) (Fig 3). Patients with resection of metastatic disease after first-line FOLFOX (+/- bevacizumab) were not included for estimation of PFS. Seven patients in KRAS MT population and four patients in KRAS WT population had resection of metastatic disease after first line chemotherapy. Median OS was 34.8 months (95% CI, 23.5-42.5) in KRAS WT and not achieved in MT patients (Fig 4).
Figure 3. Kaplan-Meier survival analysis for progression-free survival time according to KRAS status (P=0.3954).
Figure 4. Kaplan-Meier survival analysis for overall survival (OS) time according to KRAS status (P=0.7407). Median OS was not achieved.
Discussion
Several studies have reported that WT KRAS status of tumor is predictive of response to addition of EGFR inhibitors (cetuximab or panitumumab) in chemotherapy regimens involving oxaliplatin (FOLFOX or XELOX) (21),(24). Although the combination of EGFR inhibitors with first-line FOLFOX or XELOX significantly enhanced the clinical outcome in patients with WT KRAS tumors in several studies, the effect of KRAS status on patients receiving FOLFOX alone or FOLFOX plus bevacizumab remains uncertain. Table 1 summarizes effect of KRAS mutation on clinical outcome of patients treated with FOLFOX or XELOX in various studies. In the phase II OPUS (Oxaliplatin and Cetuximab in First-Line Treatment of metastatic CRC) study, patients with KRAS mutation had a trend to a better response rate and PFS when treated with FOLFOX-4 alone when compared to patients with WT KRAS. Similarly, the phase III CAIRO2 (capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer) study showed a trend towards an improvement in response rate and PFS in patients with KRAS mutation treated with capecitabine/oxaliplatin plus bevacizumab compared to patients with WT KRAS. In contrast, no trends in improved outcome were noted in the PACCE (Panitumumab Advanced Colorectal Cancer Evaluation), PRIME (Panitumumab Randomized Trial In Combination With Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy) or COIN (Continuous Chemotherapy Plus Cetuximab or Intermittent Chemotherapy with Standard Continuous Palliative Combination Chemotherapy with Oxaliplatin and a Fluoropyrimidine in First Line Treatment of Metastatic Colorectal Cancer) studies when comparing KRAS MT or WT patients receiving non-EGFR inhibitor-containing oxaliplatin-based therapy.
Interestingly in OPUS and CAIRO2 studies patients with KRAS mutation who received cetuximab in combination with FOLFOX or XELOX had significantly worse response rate and survival compared to similar group who received only FOLFOX or XELOX. These findings raised the concern that the addition of EGFR inhibitors to FOLFOX or XELOX could impair the efficacy of oxaliplatin component of the combined regimen in patients with KRAS mutation.
In this study, we did not find any advantages to tumors with KRAS MT in terms of response or progression free survival with FOLFOX-based chemotherapy. In our study, patients with KRAS mutation had response rate of 50% with FOLFOX ± bevacizumab which was not significantly different than that of patients with KRAS WT (56.6%). These response rates are comparable to other studies utilizing FOLFOX and bevacizumab as first line chemotherapy in metastatic CRC patients. Both treatment groups were well balanced in terms of bevacizumab use (83.02% in KRAS WT type and 80% in KRAS MT) making bevacizumab an unlikely confounder on the impact of KRAS on outcome. William et al. have shown that benefit derived from addition of bevacizumab to chemotherapy in patients with mCRC is not affected by their KRAS status (25).
In this study we also examined if KRAS status of tumor was predictive of certain pattern of metastasis in patients with metastatic CRC. Incidence of KRAS mutation in our study was similar to other large studies (13). Cejas et al. reported that tumors with KRAS mutation had higher propensity to metastasize to lungs (16). We did not confirm this finding in our study as tumors with KRAS wild type or mutant status had similar propensity to metastasize to liver, lung or peritoneum. In the RASCAL study it was suggested that individual mutations may have different impact on tumor biology as glycine to valine mutation on codon 12 of the KRAS gene had significant association with more aggressive biological behavior and worse outcome. The incidence of predominant mutations (Glycine to Aspartate and Glycine to valine on codon 12) in our study was similar to the study by Cejas et al. making it an unlikely explanation for different results.
In summary, our single institution experience does not support any predictive role for KRAS-MT in terms of response to FOLFOX first-line chemotherapy (in the absence of anti-EGFR inhibitors), or in terms of sites of metastatic disease at presentation.
Footnotes
No potential conflict of interest.
References
- 1.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 2.Wood KW, Sarnecki C, Roberts TM, Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992;68:1041–50. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- 3.Leevers SJ, Marshall CJ. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992;11:569–74. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, et al. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–7. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 5.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Itoh F, Senota A, Adachi Y, Yoshimoto M, Endoh T, et al. Expression of matrix metalloproteinase matrilysin (MMP-7) was induced by activated Ki-ras via AP-1 activation in SW1417 colon cancer cells. J Clin Lab Anal. 1995;9:297–301. doi: 10.1002/jcla.1860090504. [DOI] [PubMed] [Google Scholar]
- 7.Jankun J, Maher VM, McCormick JJ. Malignant transformation of human fibroblasts correlates with increased activity of receptor-bound plasminogen activator. Cancer Res. 1991;51:1221–6. [PubMed] [Google Scholar]
- 8.Buo L, Meling GI, Karlsrud TS, Johansen HT, Aasen AO. Antigen levels of urokinase plasminogen activator and its receptor at the tumor-host interface of colorectal adenocarcinomas are related to tumor aggressiveness. Hum Pathol. 1995;26:1133–8. doi: 10.1016/0046-8177(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 9.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst. 1998;90:675–84. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 10.Tortola S, Marcuello E, Gonzalez I, Reyes G, Arribas R, Aiza G, et al. p53 and K-ras gene mutations correlate with tumor aggressiveness but are not of routine prognostic value in colorectal cancer. J Clin Oncol. 1999;17:1375–81. doi: 10.1200/JCO.1999.17.5.1375. [DOI] [PubMed] [Google Scholar]
- 11.Bell SM, Scott N, Cross D, Sagar P, Lewis FA, Blair GE, et al. Prognostic value of p53 overexpression and c-Ki-ras gene mutations in colorectal cancer. Gastroenterology. 1993;104:57–64. doi: 10.1016/0016-5085(93)90835-z. [DOI] [PubMed] [Google Scholar]
- 12.Dix BR, Robbins P, Soong R, Jenner D, House AK, Iacopetta BJ. The common molecular genetic alterations in Dukes' B and C colorectal carcinomas are not short-term prognostic indicators of survival. Int J Cancer. 1994;59:747–51. doi: 10.1002/ijc.2910590606. [DOI] [PubMed] [Google Scholar]
- 13.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br J Cancer. 2001;85:692–6. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193–7. [PubMed] [Google Scholar]
- 15.Bazan V, Migliavacca M, Zanna I, Tubiolo C, Grassi N, Latteri MA, et al. Specific codon 13 K-ras mutations are predictive of clinical outcome in colorectal cancer patients, whereas codon 12 K-ras mutations are associated with mucinous histotype. Ann Oncol. 2002;13:1438–46. doi: 10.1093/annonc/mdf226. [DOI] [PubMed] [Google Scholar]
- 16.Cejas P, Lopez-Gomez M, Aguayo C, Madero R, de Castro Carpeno J, Belda-Iniesta C, et al. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PLoS One. 2009;4:e8199. doi: 10.1371/journal.pone.0008199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 18.Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 19.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 20.Sharma N, He Q, Sharma RP. Sphingosine kinase activity confers resistance to apoptosis by fumonisin B1 in human embryonic kidney (HEK-293) cells. Chem Biol Interact. 2004;151:33–42. doi: 10.1016/j.cbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 22.Sharma N, Suzuki H, He Q, Sharma RP. Tumor necrosis factor alpha-mediated activation of c-Jun NH(2)-terminal kinase as a mechanism for fumonisin B(1) induced apoptosis in murine primary hepatocytes. J Biochem Mol Toxicol. 2005;19:359–67. doi: 10.1002/jbt.20102. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–72. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 25.Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J Natl Cancer Inst. 2005;97:981–9. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]