Abstract
Chronic, antibiotic-resistant pneumonia, sometimes with concurrent polyarthritis, occurs in feedlot cattle in western Canada. The prevalence of Mycoplasma bovis, bovine viral diarrhea virus, and Haemophilus somnus was determined by using immunohistochemical staining of lung and heart tissue from 2 groups of animals with this history. Mycoplasma bovis antigen was present in 44/48 cases submitted between 1995 and 1998 (retrospective group) and 15/16 of cases from 1999 (prospective group), and was associated with pulmonary necrosis. Bovine viral diarrhea virus antigen was present in association with microscopic vascular lesions in 31/48 retrospective and 9/16 of prospective cases. Types Ib and II bovine viral diarrhea virus were isolated from 4/16 prospective cases. Haemophilus somnus antigen was present in heart, lung, or both of 15/48 retrospective and 8/16 prospective cases. The results suggest that there may be synergism between bovine viral diarrhea virus and M. bovis in this pneumonia with arthritis syndrome.
Introduction
Bovine respiratory diseases are the most significant cause of mortality and elimination of calves from feedlots, and have great economic impact on the cattle industry (1). A recent study of feedlots in western Canada has shown an increase in cases of antibiotic-resistant pneumonia and fibrinous polyarthritis in which Mycoplasma bovis and bovine viral diarrhea virus (BVDV) infection were frequently detected (2). Animals with M. bovis infection have chronic bronchopneumonia with necrosis and bronchiectasis, which is sometimes associated with fibrinous to fibrous pleuritis. Pulmonary lesions resemble those described in M. bovis infection in younger calves (3,4,5,6,7).
These animals are chronically ill and fail to thrive, so they are eliminated from the herd because of lack of appropriate weight gain and failure to respond to treatment. The major organism recovered from the lungs, joints, or both is M. bovis, either alone or in combination with other mycoplasmas, including M. arginini, M. bovirhinis, and M. bovigenitalium.
The role of acute BVDV infection in bovine respiratory tract disease is not clear; variable results have been obtained through experimental reproduction of respiratory disease in calves with different strains of BVDV (8). Certain strains can cause primary respiratory disease and mild respiratory disorder in calves (9,10). However, a number of studies have demonstrated a synergistic role of BVDV in bovine respiratory disease by increasing pathogenicity of both viral and bacterial concomitant infection; this has been attributed to immunosuppressive effects of BVDV on the host (11,12,13,14). There is evidence that cattle with persistent and primary postnatal infections with BVDV undergo immunosuppression, which increases the susceptibility of these animals to secondary infection (15).
We conducted retrospective and prospective studies, using histopathologic and immunohistochemical (IHC) examinations to determine the frequency of occurrence and distribution of M. bovis, BVDV, and Haemophilus somnus in the lung and heart of feedlot animals that were submitted for necropsy to the Western College of Veterinary Medicine (WCVM), University of Saskatchewan, and Prairie Diagnostic Services (PDS) because of chronic, unresponsive, necrotizing bronchopneumonia.
Materials and methods
Retrospective study
The case histories of 187 cases of feedlot cattle in which a final diagnosis of mycoplasma pneumonia had been made in the diagnostic laboratory of the Department of Veterinary Pathology at the WCVM between January 1, 1995, and December 30, 1998, were reviewed. The cattle were from large feedlots in Alberta (n = 5), Saskatchewan (n = 5), and Manitoba (n = 1), as well as from a few smaller feedlots in Saskatchewan. Forty-eight of 187 cases were selected for further study on the basis of availability of paraffin-embedded heart and lung samples. Multiple sections of heart and lung, stained with hematoxylin and eosin (H&E), were examined histologically for the presence of myocardial and pneumonic lesions, with careful attention being given to vascular lesions. Multiple (1 to 3) sections of heart and lung from each animal, with or without specific lesions, were then examined after IHC staining by an avidin-biotin immunoperoxidase technique (16) for BVDV, H. somnus, and M. bovis antigens. Duplicate sections of each block were tested for each antigen. In this method, after the antigen had been retrieved with protease XIV (Sigma, St Louis, Missouri, USA), the tissues were exposed to primary antibodies and incubated overnight at 4°C. The primary antibody used for detecting M. bovis was a polyclonal rabbit antiserum (provided by Dr. J. Tulley, National Institute of Allergy and Infectious diseases, Fredricton, Maryland, USA) at dilutions of 1:1000 and 1:2000. For H. somnus, a rabbit polyclonal antiserum (provided by the Veterinary Infectious Diseases Organization, Saskatoon, Saskatchewan), diluted at 1:2000 and 1:4000, was used; and for BVDV, a cell culture supernatant of monoclonal antibody 15C5 (provided by Cornell University, Ithaca, New York, USA) was used at dilutions of 1:100 and 1:200. A biotinylated goat anti-rabbit (H. somnus and M. bovis stain) or horse anti-mouse serum (BVDV stain) (Vector, Burlingame, California, USA) was used as the secondary antibody. Staining was completed by using peroxidase-labelled avidin-biotin complex (Vectastain, ABC Elite; Vector). Diaminobenzidine was used as the chromogen substrate. Positive control sections from samples confirmed positive for BVDV by virus isolation were stained concurrently with test tissues. Stained tissues were scored as either positive or negative for the presence of each antigen. A case was scored as positive if staining, consistent with the antigen, was detected in 1 or more blocks. No negative control was used in this study.
Prospective study
Feedlot animals with gross pulmonary lesions suggestive of M. bovis pneumonia (3,4), with or without joint lesions, were selected from among submissions for necropsy to the PDS, Saskatoon laboratory, during 1999. Lungs of these animals showed lesions of bronchopneumonia with marked consolidation of pulmonary tissues, raised firm nodules on the pleural surface of the lung, and often fibrinous to fibrous pleuritis. On the cut surface, numerous 0.5- to 3.0-cm diameter cavities filled with yellow caseous necrotic material were present throughout the affected lung. Most of the animals originated from the “chronic pen” at 2 large feedlots near Saskatoon, which are serviced by WCVM field service clinicians. The submissions consisted of either entire animals or parts of carcasses, which included tongue, glottis and epiglottis, trachea, oesophagus, lungs, heart, and adjacent lymph nodes, multiple pieces of ileum, spleen, and, occasionally, skin, collected in the field by clinicians. A complete necropsy was performed on intact carcasses. Respiratory tracts were examined grossly for severity, distribution, and type of lesions. At least 8 samples of lung, from all lobes, were collected whether lesions were observed or not. The heart was examined for abnormalities, and sections of the left and right ventricular walls, the interventricular septum, and the right and left atria were collected. The ileum from 11 animals was examined for lesions on the mucosa, serosa, and Peyer's patches. At least 2 cross sections of ileum were collected from these animals for histopathologic examination. Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 4–5 mm, stained with H&E, and examined using light microscopy. Immunohistochemical staining of heart and lung samples was as described for the retrospective cases. It was also applied to the ileum, spleen, and skin, if available.
Three samples of fresh tissue from affected areas of lungs from each animal were submitted to the diagnostic bacteriology laboratory of PDS. In cases with multiple lobes of the lung involved, the 3 samples were collected from 3 different affected lung lobes. If only a single lobe was involved, samples were collected from different areas of the affected lobe. In animals with other lesions, such as arthritis or myocarditis, samples from these areas were also submitted for culture. Culture for bacteria was carried out using standard laboratory methods, including inoculation on blood agar, chocolate agar, and MacConkey agar. A standard method was used for culturing mycoplasmas (17). Mycoplasmas were identified by using a fluorescent antibody (FA) and antisera to M. bovis, M. arginini, M. bovirhinis, and M. bovigenitalium (17).
Samples of lung, heart, and ileum were submitted to the PDS virology laboratory for testing by FA and for virus isolation of BVDV, as previously described (18), with slight modifications. Virus isolation was done by inoculating tissue homogenates onto fetal bovine tracheal (FBTr) cells. The cultures were repassed onto Mardin Darby bovine kidney cells and FBTr cells. Presence of BVDV antigen was confirmed by FA staining on cell smears of 2nd passage cultures, using a pool of monoclonal antibodies 15C5 and 20.10.6 raised against BVDV (provided by Cornell University). Bovine viral diarrhea virus antigen was detected by FA in frozen sections of fresh tissue by using the same pool of monoclonal antibodies. The genotype of isolated BVDVs was identified by polymerase chain reaction (PCR) (19).
Results
Retrospective study
Mycoplasma bovis antigen was found in the lung of 44/48 (91%) of the retrospective cases by IHC staining (Table 1). Microscopic lesions in the lungs of the 48 animals were characterized by severe, necrotizing, suppurative bronchopneumonia with bronchiectasis and abscessation. In 5 animals, there was sequestration of necrotic lung tissue. Pulmonary lesions were classified as acute, subacute, or chronic, based on histologic features, type of inflammatory reaction, and indicators of chronicity, such as fibrosis, in order to distinguish different stages of disease progression in pulmonary tissue. All stages were seen with variable severity in lungs of all cases.
Table 1.
In acute or early lesions, there was a small amount of hypereosinophilic exudate within the bronchioles and terminal airways, but the epithelial lining was intact. The exudate was composed of degenerate neutrophils, macrophages, proteinaceous material, and necrotic debris; it stained positively for M. bovis antigen. Peribronchiolar mononuclear cell infiltration of affected airways was variable.
In subacute lesions, there was focal to multifocal loss of bronchiolar epithelium. The bronchiolar lumen was dilated and filled with large amounts of hypereosinophilic necrotic exudate. Mycoplasma bovis antigen was detected mainly in the necrotic exudate and at the periphery of the lumen. In many animals, affected airways were surrounded by a zone of lymphocytes and plasma cells or by large multifocal lymphoid aggregations.
In chronic lesions, there were multifocal, well- circumscribed foci of coagulative or liquefactive necrosis, approximately 1 to 3 mm in diameter, with eosinophilic debris containing ghost-like outlines of cells surrounded by a thin zone of degenerate neutrophils; cellular debris; and pyknotic nuclei, and a wider area of plasma cells and macrophages mixed with fibroblasts. Mycoplasma bovis antigen was present at the margins of the necrotic foci in association with the zone of necrotic debris extending into the mononuclear cell layer.
Bacterial culture was not performed on 24 cases, either because some cases were submitted as formalin-fixed tissues or because mycoplasmal culture was not performed. Mycoplasma bovis was isolated from samples from 8 of the 9 animals cultured specifically for mycoplasmas. Mycoplasma arginini and M. bovigenitalium were each isolated from a single animal. Pasteurella multocida was isolated from 5 lungs, Mannheimia haemolytica from 4 lungs, Arcanobacterium pyogenes from 6 lungs, and H. somnus from 1 lung.
Bovine viral diarrhea virus antigen was detected in the heart of 26/48 (54%) and lungs of 21/48 (44%) animals. In total, 31/48 (64%) animals had BVDV antigen in one or both tissues. Antigen was located in small- to medium-sized arteries, predominantly in damaged areas of vessels. Vascular lesions varied from necrotizing fibrinoid vasculitis, with focal to multifocal eosinophilic amorphous material within tunica media associated with cellular debris and pyknotic nuclei, to necrotizing lymphocytic vasculitis, with infiltration of lymphocytes within vessel walls and scattered cellular debris in the tunica media and tunica intima (Figure 1). Lymphoplasmacytic perivasculitis and proliferative reparative changes in the tunica media and tunica intima, characterized by mild proliferation of tunica media and intima, were features of the affected vessels. Vascular lesions were observed in the heart of 35/48 (73%) and lung of 26/48 (54%) animals. In none of the damaged vessels was there recognizable endothelial injury or thrombosis. Haemophilus somnus antigen was present in the myocardium of 2 animals and in necrotic lung tissue of 13 other animals, but it was not associated with vascular lesions in any of the animals.
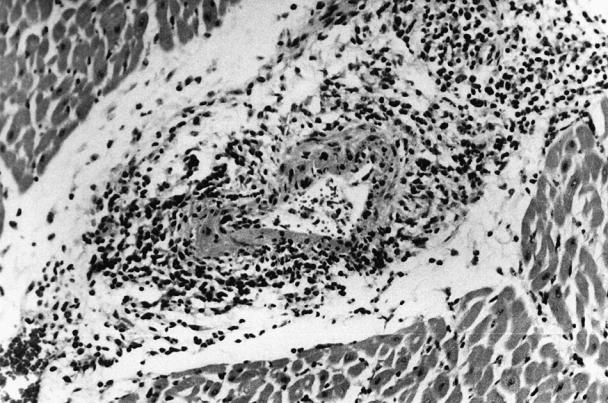
Figure 1. Myocardial artery with necrotizing vasculitis from a calf with Mycoplasma bovis pneumonia. Bovine viral diarrhea virus antigen was demonstrated by immunohistochemical staining.
Prospective study
All 16 animals had moderate to severe bronchopneumonia with fibrinous to fibrous pleuritis. The affected cranioventral areas of the lungs were markedly consolidated with raised, firm nodules on the pleural surface (Figure 2). On the cut surface, cavities that were 0.5 to 3.0 cm in diameter and filled with pale yellow necrotic materials were distributed throughout the parenchyma (Figure 3). Five animals had moderate to severe fibrinonecrotizing polyarthritis involving stifle, tarsus, carpus, shoulder, and, in one case, the coxofemoral joints. Fibrinonecrotizing tenosynovitis involving mainly tendon of the long digital extensor muscle was observed in 4 animals. One animal had a large, 1.0 × 2.5 cm diameter area of myocardial necrosis and sequestration in the left ventricular papillary muscle. Other grossly visible lesions included focal necrotizing tracheitis in 2 animals, necrotizing laryngitis in 2 animals, and deep focal necrotizing dermatitis and panniculitis in the carpal area of 1 animal.
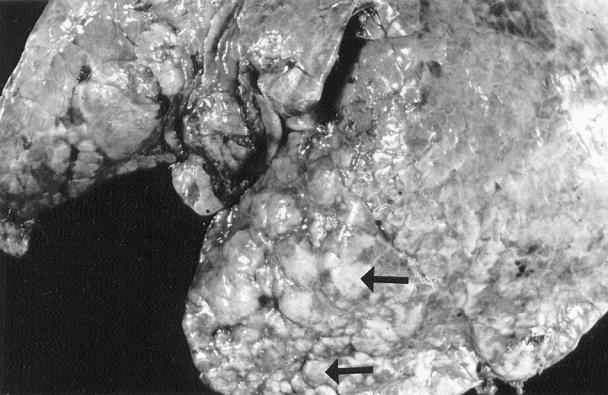
Figure 2. Cranial lung lobe of a calf affected by Mycoplasma bovis pneumonia. Note variably sized, raised nodules on the lung surface (arrows).
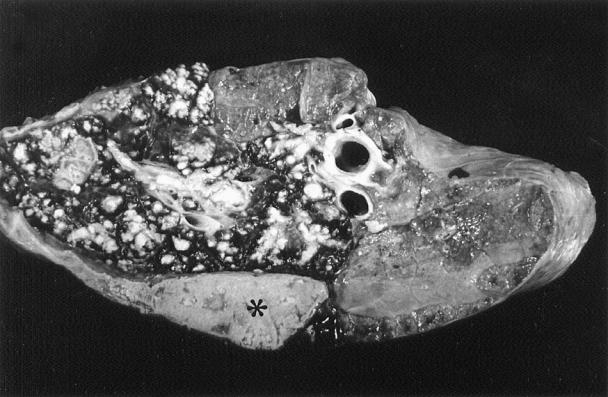
Figure 3. Lung section with multiple foci of caseous necrosis and a large area of coagulation necrosis (asterisk) as a result of Mycoplasma bovis infection.
Mycoplasma bovis antigen was detected by IHC staining in lung from 15/16 (94%) animals (Table 2). The staining pattern was similar to that described in the retrospective study. Mycoplasma spp. were cultured from the lungs of 15/16 animals. From 6 animals, both M. bovis and M. arginini were recovered; from 2, M. bovis, M. arginini, and M. bovirhinis were isolated; from 6, only M. bovis was recovered; and from 2, either M. arginini or M. bovirhinis was isolated. Mycoplasma spp., predominantly M. bovis, were recovered from 4 of 5 affected joints. No mycoplasmas were isolated from the lung or joints of 1 animal with severe polyarthritis and a large focal area of myocardial necrosis; however, M. bovis was cultured from the area of myocardial sequestration and H. somnus was isolated from joints. There were no areas of necrosis in the lung, and M. bovis antigen was not detected in the lung of this animal. Lesions in the lungs of the 15 animals with cultures that were positive for mycoplasmas were similar to those previously described in the retrospective cases and included the acute, subacute, and chronic stages. Pasteurella multocida was isolated from the lungs of 6 animals; H. somnus was recovered from the lungs of 3 animals and the joint of 1 animal.
Table 2.
Microscopic vascular lesions associated with BVDV were found in the heart of 10 (63%) and the lung of 9 (56%) animals. In 2 animals (calves #7 and 12), severe, fibrinoid vasculitis associated with BVDV was observed in multiple organs, including the heart, lung, spleen, kidney, liver, and lymph nodes. One animal (calf #14), had fibrinoid necrotizing vasculitis confined to the submucosa of the ileum, and in 5 animals (calves #5, 7, 9, 11, 12), there was lymphoplasmacytic myocarditis, in addition to the pulmonary and cardiac vascular lesions. Vascular lesions were not detected in 5 animals (calves # 6, 10, 13, 14, 15). One animal (calf #13), had severe mineralization of medium and large arteries in the lung and spleen, without any associated inflammation. Depletion of lymphoid cells in Peyer's patches was present in all 11 animals in which the ileum was examined. Bovine viral diarrhea virus antigen was detected in tissues of 9/16 (56%) animals. Antigen was found in the heart of 8 (50%) and lungs of 6 (38%) animals. In 7 animals with vascular lesions, BVDV antigen was present in the wall of damaged vessels. Immunohistochemical staining of the ileum was positive for BVDV antigen in 5/11 (45%) animals, with a small amount of staining noted in the mucosa and Peyer's patches. Bovine viral diarrhea virus was detected in 3 animals by both FA and virus isolation; BVDV was recovered from another animal by virus isolation, while the FA test was negative. Two of the 4 isolates were identified by PCR as type Ib and 2 were type II BVDV.
Haemophilus somnus antigen was present in the heart of 1 animal (calf #7) within necrotic myofibers and in the lungs of 8 animals. Staining was located in the center of the necrotic areas in the lungs and was not associated with vascular lesions.
Discussion
This study demonstrated that mycoplasmal infection, particularly with M. bovis, is common in chronic pneumonia of feedlot cattle in western Canada, and that the organism can frequently be isolated from the pneumonic lungs. Mycoplasma bovis antigen was detected by IHC staining in the lungs of > 90% of both groups of cattle studied. The lesions were similar to those described in younger calves infected with M. bovis (3,4,5,6,7). Our results suggest that M. bovis should be considered as a principal pathogen in chronic unresponsive pneumonia of feedlot cattle. In a previous study, mycoplasmas were recovered from about 90% of feedlot cattle with fatal pneumonia (20), with M. bovis being the major isolate. In another study, mycoplasmas were isolated from almost 90% of lungs; M. dispar, M. bovis, M. arginini, M. bovirhinis, and ureaplasmas were isolated in different proportions (21). We did not isolate M. dispar or ureaplasma; however, these are fastidious microorganisms and are difficult to isolate under routine conditions. The animals in our study were selected from the “chronic pen” and had had extensive antibiotic treatment before death, which likely affected the result of bacterial culture. In addition to the characteristic pneumonic lesions, M. bovis was detected by culture or IHC staining in a variety of other lesions, such as necrotizing mycocarditis, necrotizing tracheitis, and panniculitis. Some of these lesions have not previously been associated with M. bovis.
The severity of M. bovis infection might be influenced by factors such as virulence of the mycoplasmal strains, changes in immune status of the host caused by other agents, or stressors exacerbating the pathogenicity of M. bovis. Mycoplasma bovis may become pathogenic after tissue trauma or other physiological stress (22). Decreased host immune response with M. bovis infection has also been described (22,23).
While the exact role of BVDV in chronic diseases of feedlot animals is unknown, the presence of BVDV antigen in 64% of retrospective and 56% of prospective cases and the isolation of the virus from 4/16 prospective cases suggests that there may be synergism between BVDV and M. bovis. The frequency of occurrence of persistent infection with BVDV in cattle entering a feedlot in western Canada was reported to be < 0.1% (24); these animals may provide a source of virus for primary infection of other animals.
We theorize that many animals in our study had a primary BVDV infection and were not persistently infected animals. The pattern of IHC staining in all but 1 of the cases was primarily in smooth muscle of small- to medium-sized arteries and in the Peyer's patches of the ileum, with no or very little evidence of antigen in the mucosal epithelium or glands. This pattern of viral antigen distribution is suggestive of primary BVDV infection and has been reported in experimental primary infection (25). Seroconversion to BVDV has been reported to increase the risk of respiratory disease in feedlot cattle (26). It has been suggested that cattle with primary infection undergo immunosuppression (15), which might predispose to secondary infection with bacteria such as M. bovis.
The vascular lesions seen in this study were in the tunica intima and tunica media of arteries and did not involve the endothelial lining of the vessels. Presence of vascular lesions in a significant number of the animals with positive IHC staining for BVDV suggests that these vascular lesions are associated with BVDV infection. Vascular lesions have been reported in cattle with mucosal disease (27,28) and have also been attributed to BVDV (29). Our study suggests that in a majority of calves, primary BVDV infection is responsible for vascular lesions. These vascular lesions could be used as a diagnostic indicator for the presence of BVDV infection. Among the tissues examined, vascular lesions were found more often in the heart than in other tissues. Collection of multiple sections of heart for histologic examination should be encouraged during postmortem examination of cattle suspected of having BVDV infection.
The reason for the lack of a consistent correlation between the presence of vascular lesions and presence of BVDV antigen is not clear. During this study and in other cases (unpublished observations), even in animals that have BVDV antigen, not all individual histologic sections have vascular lesions or are positive for BVDV antigen. Other factors, such as the stage of viral infection or immune status of the animal, as well as unknown mechanisms associated with BVDV vasculitis, might also play a role. In order to increase the chance of detecting vascular lesions, it is recommended that multiple sections of heart and lung be examined.
Cardiac lesions in feedlot cattle are usually associated with H. somnus infection (30,31), but we did not find H. somnus antigen in association with cardiac vascular lesions. In the current study, H. somnus antigen was present in 15 (31%) animals in the retrospective study and in 9 (56%) animals in the prospective study. Haemophilus somnus was not a common bacterial isolate from affected lungs. The role of H. somnus in chronic pneumonia is unclear, and it was not associated with vascular lesions. Antibiotic treatment probably negatively affected the likelihood of isolation of this and other bacteria from the lungs.
Footnotes
Acknowledgment
The authors thank Dr. Colleen Pollock for her valuable information. CVJ
Address correspondence and reprint requests to Dr. Farshid Shahriar, e-mail: farshid.shahriar@usask.ca
This study was supported by a grant from the Saskatchewan Beef Development Fund.
References
- 1.Griffin D. Economic impact associated with respiratory disease in beef cattle. Vet Clin North Am Food Anim Pract 1997;13:367–377. [DOI] [PubMed]
- 2.Haines DM, Martin MK, Clark EG, Kee Jim G. The immunohistochemical detection of Mycoplasma bovis and bovine virus diarrhea virus in tissues of feedlot cattle with chronic unresponsive repiratory disease and/or arthritis. Can Vet J 2001;42:857–860. [PMC free article] [PubMed]
- 3.Adegboye DS, Halbur PG, Cavanaugh DL, et al. Immunohistochemical and pathological study of Mycoplasma bovis-associated lung abscesses in calves. J Vet Diagn Invest 1995;7: 333–337. [DOI] [PubMed]
- 4.Adegboye DS, Halbur PG, Nutsch RG, Kadlec RG, Rosenbusch RF. Mycoplasma bovis-associated pneumonia and arthritis complicated with pyogranulomatous tenosynovitis in calves. J Am Vet Med Assoc 1996;209:647–649. [PubMed]
- 5.Gourlay RN, Thomas LH, Wyld SG. Increased severity of calf pneumonia associated with the appearance of Mycoplasma bovis in a rearing herd. Vet Rec 1989;124:420–422. [DOI] [PubMed]
- 6.Rodriguez F, Bryson DG, Ball HJ, Forster F. Pathological and immunohistochemical studies of natural and experimental Mycoplasma bovis pneumonia in calves. J Comp Pathol 1996; 115:151–162. [DOI] [PubMed]
- 7.Thomas LH, Howard CJ, Stott EJ, Parsons KR. Mycoplasma bovis infection in gnotobiotic calves and combined infection with respiratory syncytial virus. Vet Pathol 1986;23:571–578. [DOI] [PubMed]
- 8.Baker JC. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim Pract 1995;11:425–445. [DOI] [PubMed]
- 9.Archambault D, Beliveau C, Couture Y, Carman S. Clinical response and immunomodulation following experimental challenge of calves with type 2 noncytopathogenic bovine viral diarrhea virus. Vet Res 2000;31:215–27. [DOI] [PubMed]
- 10.Baule C, Kulcsar G, Belak K, et al. Pathogenesis of primary respiratory disease induced by isolates from a new genetic cluster of bovine viral diarrhea virus type I. J Clin Microbiol 2001;39:146–53. [DOI] [PMC free article] [PubMed]
- 11.Potgieter LND, McCracken MD, Hopkins FM, Walker RD, Guy JS. Experimental production of bovine respiratory tract disease with bovine viral diarrhea virus. Am J Vet Res 1984;45: 1582–1585. [PubMed]
- 12.Potgieter LND. Bovine respiratory tract disease caused by bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract 1997; 13:471–481. [DOI] [PubMed]
- 13.Richer L, Marios P, Lamontagne L. Association of bovine viral diarrhea virus with multiple viral infections in bovine respiratory disease outbreaks. Can Vet J 1988;29:713–717. [PMC free article] [PubMed]
- 14.Stott EJ, Thomas LH, Collins AP, et al. A survey of virus infections of the respiratory tract of cattle and their association with disease. J Hyg 1980;85:257–270. [DOI] [PMC free article] [PubMed]
- 15.Potgieter LND. Immunology of bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract 1995;11:501–520. [DOI] [PubMed]
- 16.Haines DM, Chelack BJ. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Invest 1991;3:101–112. [DOI] [PubMed]
- 17.Whitford HW. Isolation of mycoplasmas from clinical specimens. In: Howard WW, Ricardo FR, H.L. L, eds. Mycoplasmosis in Animals: Laboratory Diagnosis. 1st ed. Iowa, Ames: Iowa State Univ Pr, 1994.
- 18.Ellis JA, Martin K, Norman GR, Haines DM. Comparison of detection methods for bovine viral diarrhea virus in bovine abortions and neonatal death. J Vet Diagn Invest 1995;7:433–436. [DOI] [PubMed]
- 19.Ridpath JF, Bolin SR. Differentiation of types 1a, 1b and 2 bovine viral diarrhoea virus (BVDV) by PCR. Mol Cell Probes 1998;12: 101–106. [DOI] [PubMed]
- 20.Hjerpe CA. The role of mycoplasma in bovine respiratory disease. Vet Med Small Anim Clin 1980;75:297–298. [PubMed]
- 21.Muenster OA, Ose EE, Matsuoka T. The incidence of Mycoplasma dispar, ureaplasma and conventional Mycoplasma in the pneumonic calf lung. Can J Comp Med 1979;43:392–398. [PMC free article] [PubMed]
- 22.Boothby JT, Jasper DE, Zinkl JG, Thomas CB, Dellinger JD. Prevalence of mycoplasmas and immune responses to Mycoplasma bovis in feedlot calves. Am J Vet Res 1983;44:831–838. [PubMed]
- 23.Bennett RH, Jasper DE. Immunosuppression of humoral and cell-mediated responses in calves associated with inoculation of Mycoplasma bovis. Am J Vet Res 1977;38:1731–1738. [PubMed]
- 24.Taylor LF, Donkersgoed JV, Dubovi EJ, et al. The prevalence of bovine viral diarrhea virus infection in a population of feedlot calves in western Canada. Can J Vet Res 1995;59:87–93. [PMC free article] [PubMed]
- 25.Ellis JA, West KH, Cortese VS, et al. Lesions and distribution of viral antigen following an experimental infection of young seronegative calves with virulent bovine virus diarrhea virus-type II. Can J Vet Res 1998;62:161–169. [PMC free article] [PubMed]
- 26.Martin SW, Bateman KG, Shewen PE, Rosendal S, Bohac JG, Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res 1990;54: 337–342. [PMC free article] [PubMed]
- 27.Barker IK, Van Dreumel AA, Palmer N. The alimentary system. In: Jubb KVF, Kennedy PC, Palmer N, eds. Pathology of Domestic Animals. 4th ed. vol. 2. San Diego: Academic Pr, 1994:149–158.
- 28.Mills JHL, Luginbuhl RE, Nielsen SW. Transmission of bovine mucosal disease using virus recovered from urine. Res Vet Sci 1968;9:500–505. [PubMed]
- 29.Van Vleet FJ, Ferrans VJ. Cardiovascular system. In: McGavan MD, Carlton WW, Zachary JF, eds. Thopmson's Special Veterinary Pathology. 3rd ed. Missouri: Mosby, 2001:197–221.
- 30.Donkersgoed JV, Janzen ED, Harland RJ. Epidemiological features of calf mortality due to hemophilosis in a large feedlot. Can Vet J 1990;31:821–825. [PMC free article] [PubMed]
- 31.Donkersgoed JV, Janzen ED, Potter AA, Harland RJ. The occurrence of Haemophilus somnus in feedlot calves and its control by postarrival prophylactic mass medication. Can Vet J 1994;35: 573–580. [PMC free article] [PubMed]