Obesity, a condition characterised by chronic hyperinsulinemia, is a firmly established risk factor for incident colon cancer (1). With plausible biological explanations, consistency of association, long durations between anthropometric measurements (typically body mass index, BMI) and cancer occurrence, and dose-effect with increasing BMI, these associations are probably causal (2). Given this association, it is tempting to extrapolate that increased body adiposity, and the inevitable concomitant increased ‘insulin milieu’ at a tissue level, obesity may also be associated with adverse treatment outcome, including resistance to chemotherapies. Where BMI is determined at baseline in prospective cohorts, obesity is certainly associated with increased colon cancer-related mortality (3), but it is unclear at what steps on the cancer pathway, excess adiposity exerts its influence. In patients with the diagnosis of colon cancer undergoing 5-fluorouracilbased adjuvant chemotherapy, pooled analysis from seven randomized trials (n=4381) suggests that obesity is an independent prognosticator (4). However, there are caveats – thus, among men with colon cancer, the significant increased hazard ratio for overall survival is limited to patients with BMI values greater than 35 kg/m(2); while in women, reduced overall survival is limited to patients with BMI values between 30 and 35 kg/m(2). The influence of obesity in the setting of chemotherapy for metastatic colon cancer has been less extensively studied – although one small non-randomized French series suggests that surrogates of adiposity are not associated with oncological outcome where conventional combined chemotherapy is administered in metastatic colorectal cancer, but increased visceral fat may predict for a reduced response to bevacizumab-based therapies in the metastatic setting (5).
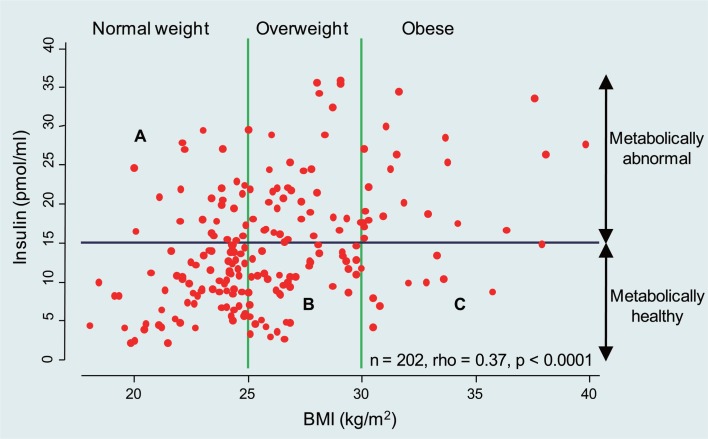
In this issue of the journal, the study reported by Chen and colleagues (6) adds an interesting new dimension. Using the HT29 colon cancer cell line, the authors show that the addition of high-dose insulin in the presence of oxaliplatin was associated with Akt activation and chemoresistance, effects which were reversed by the use of a PI3K inhibitor. The reductionist approach and simplicity of the preclinical experiments renders these data preliminary but certainly thought provoking. Furthermore, given the mixed clinical observations summarized in the opening paragraph, the reader may well ask, are these findings clinically relevant? The answer is simple at one level – obesity is a heterogeneous condition – and complex at many more levels. It is well known that serum insulin levels increase with increasing BMI, but despite this good correlation, as shown in Figure 1, there is wide variability. Increasingly, the metabolic literature recognizes that obesity may be dichotomized into metabolically benign and malign states defined by criteria of insulin resistance, subclinical inflammation and dyslipidemia. Based on recent NHANES data, 23.5% of normal-weight US adults are metabolically abnormal, whereas 51.3% of overweight adults and 31.7% of obese adults are metabolically healthy (7). High circulating levels of insulin may prevail in both normal weight and obese individuals and in turn, as depicted by Chen and colleagues (6), insulin may be pro-tumorigenic either directly via the insulin receptor and insulin-like growth factor I receptor (IGF-IR), or indirectly through changes in the IGF-binding protein balance favoring IGF-IR activation. When one takes these into consideration, it is perhaps not surprising that BMI and other anthropometric surrogates may not be ideal predictors of cancer treatment and outcome. Further complexity is gleamed by the recent recognition that the metabolically abnormal status of an individual is more strongly driven by fatty liver changes (non-alcoholic steatohepatitis, NASH) rather than by, as conventionally believed, visceral (central) fat (8).
Fig 1. Serum insulin levels increase with increasing BMI. Results for fasting samples.
Combinational oxaliplatin is now widely used in the treatment of metastatic colorectal cancer, and in many cases, the metastatic disease occurs in the liver. Initial responses are good (greater than 50%) but the development of chemoresistance is almost inevitable. Pulling together the various new insights into insulin resistance and the importance of fat distribution in the liver, the clinical importance of the ‘insulin milieu’ and chemotherapy becomes clearer. Excess liver fat (present in 20 to 25 % of the population) is an important driver of insulin resistance and hyperinsulinemia; while at the same time, NASH may be associated with a peri-tumor environment rich in pro-inflammatory factors and cytokines, favoring tumour progression (9).
The present study has defined the need for additional exploration of the role of insulin and chemoresistance in colon cancer. However, going forward, a number of critical issues will need to be addressed if answers are to be found. These include: a) consideration of the insulin concentrations examined, typical molar concentrations for in vitro experimentation range between 15 and 40 nM, corresponding to the supra-physiological ranges depicted in Figure 1, rather than 1000 nM used in the current study; and b) the in vitro models employed, as chemoresistance in in vitro models generally take several weeks to develop. Other colon cancer lines and chemotherapy agents need to be explored.
Evaluating the effects of chronic insulin administration on the PI3K/Akt pathway does indeed seem to be a worthy pursuit. However, the cellular actions of insulin are likely to be pleotropic and the endpoints of the PI3K/Akt pathway extend beyond cell growth and apoptosis. Furthermore, small-molecule inhibitors used to assess the physiological roles of these enzymes should be cautiously interpreted, and specifically for PI3K inhibition, PI-103 is now the recommended in vitro tool, with superior specificity over LY294002 (as used in the present study), and rapamycin, a specific inhibitor of TORC1, should be used in parallel to check whether any observed effects of PI-103 result from TORC1 inhibition (10). Finally, baseline mutational profiling of the cell lines of interest should be considered. HT29 cells are PI3K mutant, and as PIK3CA mutations lead to increased basal phosphatidylinositol-3-kinase activity, it is tempting to speculate that insulin signalling is constitutive in these cells. However, our laboratory has shown no distinguishing differences in cell growth properties among cells carrying PIK3CA mutations from a panel of commonly used colon cancer cell lines under basal culture conditions (11), but others have shown that mutational activation of the PI3K/Akt pathway may be essential for cellular growth under adverse conditions, and for invasion (12).
The paper of Chen and colleagues is timely, highlighting the many complexities and challenges facing investigators attempting to link clinical observations with biological mechanisms in the field of obesity and cancer. To better understand these complexities, there is a need for multi-disciplinary expertise to translate pre-clinical findings into meaningful clinical benefit for our patients with colorectal cancers.
Footnotes
AGR has served on the advisory board of and received research support from Novo Nordisk. CD has no potential conflict of interest.
References
- 1.Renehan A, Tyson M, Egger M, Heller RF, Zwahlen M. Body mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 2.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–16. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 4.Sinicrope FA, Foster NR, Sargent DJ, O'Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–93. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–7. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Qiao L, Huang XF, Katsifis A. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol. 2011;2:27–33. doi: 10.3978/j.issn.2078-6891.2010.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 8.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not v isceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106:15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inf lammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrow CJ, Gray A, Dive C. Comparison of phosphatidylinositol-3-kinase signalling within a panel of human colorectal cancer cell lines with mutant or wild-type PIK3CA. FEBS Lett. 2005;579:5123–8. doi: 10.1016/j.febslet.2005.07.096. [DOI] [PubMed] [Google Scholar]
- 12.Samuels Y, Diaz LA Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]