The GTP-binding proteins known as ADP-ribosylation factors (ARFs) function in cells to regulate membrane traffic and structure. Like all GTPases, ARFs cycle between a GDP-bound, inactive and a GTP-bound, active form. When GTP-bound, ARFs alter membrane lipid composition, assemble cytosolic coat proteins, and remodel actin associated with various membrane compartments. Although much of the research in the past has focused on the role of ARF1 in membrane trafficking and structure in the endoplasmic reticulum–Golgi system, there is increased interest in functions of ARF1 and the other five mammalian ARFs in the plasma and endosomal membrane systems. This interest has been fueled, in part, by the finding that ARF1 recruits coat proteins onto endosomal membranes and that ARF6 regulates membrane trafficking through endosomes and influences cortical actin at the plasma membrane (PM; refs. 1 and 2). Furthermore, over the years, there has been circumstantial evidence, although no direct proof, that the GTPase cycle of ARF could be coordinated by signaling from the PM. Several years ago, the growing list of guanine nucleotide exchange factors that catalyze exchange of GTP for GDP and convert ARFs to the active, GTP-bound state dominated the attention of those studying ARF proteins. Attention has now shifted to the function of the GTPase-activating proteins (GAPs), accessory molecules whose activities result in the hydrolysis of ARF-bound GTP and return ARFs to the inactive, GDP-bound state. These interesting multidomain GAPs, many of which have been identified over the past year and found to be peripherally localized, clearly do more than turn off ARF. They provide the link between the ARF GTPase cycle and various signal transduction events in the cell.
ASAP1 is a prototype of the peripheral ARF GAPs, and in this issue of PNAS, Randazzo et al. (3) present compelling evidence that this molecule serves to coordinate cell signaling, the actin cytoskeleton, and the ARF nucleotide cycle. These authors previously identified ASAP1 through biochemical purification of the ARF GAP activity and also independently in a two-hybrid screen for proteins that interact with the SH3 domain of the tyrosine kinase src (4). In this issue, they show that endogenous ASAP1 localizes to focal adhesions, complexes of proteins on the adhesive surface of cells that are sites of coordination and signaling between the extracellular matrix and the actin cytoskeleton (5). ASAP1 moves with focal adhesion proteins, such as vinculin and paxillin, during cell spreading and moves out of focal adhesions and into PM ruffles generated in response to platelet-derived growth factor (PDGF) treatment. The finding of an ARF GAP in focal adhesions may correlate with the observation made 1 year ago by Norman et al. (6) that ARF1-GTP recruits paxillin to focal adhesions.
Randazzo et al. (3) also demonstrate that overexpression of ASAP1 in cells results in a loss of focal adhesions and an inhibition of cell spreading and PDGF-induced ruffling (3). This inhibition by ASAP1 depends on its ARF GAP activity. A point mutation in ASAP1 that abolishes GAP activity reduces ASAP's inhibition of cell spreading and actually stimulates ruffling induced by PDGF (3). The phenotype of GAP overexpression should result in less ARF-GTP being formed and should resemble the phenotype observed by expression of a GTP-binding defective mutant. These mutant ARFs remain GDP-bound and can act to inhibit activation of the endogenous ARF. Intriguingly, expression of such a mutant of ARF6, T27N, inhibits both cell spreading (7) and PM ruffling mediated by Rac (8), a GTPase implicated in PDGF-induced ruffling. ASAP1 can work in vitro as a GAP on ARF1, ARF5, and ARF6, albeit more effectively on ARF1 and ARF5 (3). More studies are needed before we can determine which ARF or ARFs are substrates for ASAP1 in the cell. However, these studies, together with the recent indication that ARF6 affects cortical actin by activation of a polyphosphoinositide 4-phosphate 5-kinase (9), support a role for ARFs and ASAP1 in growth factor-stimulated reorganization of the actin cytoskeleton.
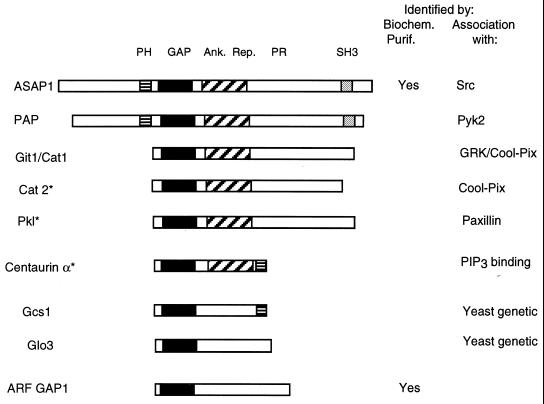
ASAP1 belongs to what we are discovering is a large family of ARF GAP proteins. A schematic representation of the published ARF GAP proteins is depicted in Fig. 1. There are also many related sequences in the database, but these proteins have not been characterized. These proteins share a common GAP domain of 70 amino acids that includes a Zn-finger motif of CXXCX16–17CXXC (where C is cysteine and X is any amino acid) that has been shown to be critical for GAP activity (10). Many of the proteins also have ankyrin repeats, a PH domain, and proline-rich sequences. ASAP1 and a close relative, PAP, also have an SH3 domain. With the exception of Cat2, Pkl, and centaurin-α, which have not been tested, all of the GAPs have ARF GAP activity. However, the ARF specificity in many cases has not been determined. ARF-GAP1, the first to be identified and cloned, regulates GTP hydrolysis on ARF1 and localizes to the Golgi complex (10). The yeast GAPs Gcs1 and Glo3 also seem to function in the early secretory pathway (11). The other mammalian GAPs (ASAP1, PAP, Git1, Cat2, Pkl, and centaurin-α), by contrast, seem to localize and function in the periphery of cells.
Figure 1.
Schematic representation of the ARF GAP proteins. All have the ARF GAP domain (GAP, black) that includes the Zn-finger motif. ASAP1 and PAP also have a pleckstrin homology (PH) domain (PH, horizontal stripes), ankyrin repeats (Ank. Rep., diagonal stripes), a stretch of proline-rich sequences (PR), and an SH3 domain (SH3, gray). There are two forms of PAP, α and β, but one is shown for simplicity. The Git, Cat, and Pkl GAPs have proline-rich sequences, but no PH or SH3 domains. Asterisks denote that GAP activity for these proteins has not been demonstrated. Peripheral GAPs refers to ASAP1, PAP, Git1, Cat2, Pkl, and centaurin-α.
Remarkably, many of these ARF GAPs have been identified through their association with signaling molecules, and this association may provide clues as to their function in cells. ASAP1 interacts with the SH3 domain of src, is phosphorylated by src (4), and colocalizes in focal adhesions with proteins such as paxillin and vinculin (3). Pkl, a putative ARF GAP related to Git1/Cat1 and Cat2, has been shown to bind directly to paxillin through its LD4 motif and to serve as a linker for a set of proteins known to associate with paxillin (12). One of these proteins is Cool/PIX, a Rac and Cdc42 guanine nucleotide exchange factor. Coincidentally, in a two-hybrid screen for proteins that interact with Cool/PIX, Cerione and colleagues (13) identified Cat1 and Cat2 and showed they can be phosphorylated by src and focal adhesion kinase. Thus, four of the ARF GAPs have some association with focal adhesions, raising the question as to how each influences focal adhesion assembly, signaling, turnover, and the role that ARF GAP function plays there.
Two other GAP proteins were identified by association with molecules involved in signaling through heterotrimeric G protein-coupled receptors. PAP, identified in a two-hybrid screen as a binding partner of the nonreceptor tyrosine kinase Pyk2, can be phosphorylated by Pyk2 and by src (14). When overexpressed, PAP is found in the cytosol, at the PM, and associated with the Golgi complex (14). Git1, identical to Cat1, was identified independently as a binding partner of G protein-coupled receptor kinases that are implicated in the down-regulation of receptor signaling by mediating receptor internalization via clathrin-dependent endocytosis (15). Intriguingly, Git1 overexpression inhibits down-regulation of G protein-coupled receptors and the epidermal growth factor receptor and does not inhibit the constitutive internalization of receptors via the clathrin-mediated pathway (16). If this effect of Git1 depends on its GAP activity, it would suggest that an ARF-GTP is required for receptor internalization from the PM.
Studies with these GAPs will advance our understanding of the complexity of ARF function beyond the endoplasmic reticulum–Golgi system. To define the physiological role of the peripheral GAPs in the cell, an important first step will be to determine their precise localization and determine with which ARF they interact. In vitro GAP assays that provide a general test for ARF specificity have been done for ASAP1 and PAP but not the other peripheral GAP proteins. ARF1 and ARF5 are good substrates for ASAP1 and PAP but less so for ARF6 (4, 14). Ultimately, sorting out which GAP works on which ARF will need to be assessed in the context of the cell where compartmentalization of ARFs, guanine nucleotide exchange factors, and GAPs may be important.
ASAP1 and PAP have an interesting dependence on phospholipids that may provide a mechanism for coordinated regulation of the ARF GTPase cycle. Both GAPs show phosphatidylinositol 4,5-bisphosphate (PIP2)-dependence for GAP activity (4, 14) in contrast to ARF GAP1, which is stimulated by diacylglycerols and not PIP2 (17). Randazzo and colleagues (18) recently demonstrated that PIP2 binding to ASAP1 via its PH domain results in a conformational change in the protein that stimulates GAP activity. This observation suggests that, in some instances, PH domains and their polyphosphoinositides may exert functions beyond membrane targeting. Because ARFs have been shown to stimulate polyphosphoinositide 4-phosphate 5-kinase (9), the product, PIP2, could provide an effective feedback mechanism by stimulating ARF GAP activity.
Because many of these peripheral GAP proteins have multiple domains capable of interacting with various signaling and effector molecules, it will be important to assess the requirement for ARF GAP activity for each function and phenotype. For example, Randazzo et al. (3) show that the inhibition of PDGF ruffling observed with ASAP overexpression depends on ARF GAP function, whereas the inhibition of cell spreading is caused only partially by ARF GAP activity (3). The authors accomplish this demonstration by mutating a critical arginine in the ARF GAP domain and show that this mutation results in the loss of GAP activity, although the structure of the protein with this mutation is unchanged (3). These results are consistent with the recently published crystal structure of the ARF GAP domain and ankyrin repeats of PAP (19). The analogous arginine in PAP is surface exposed in the structure and could be part of the ARF interaction site, because mutation of this arginine as well as nearby hydrophobic residues diminishes GAP activity of PAP (19). Because this arginine is conserved in all ARF GAPs, mutation of this arginine will likely render the GAP inactive. Assessment of the functioning of these GAP-negative ARF GAP proteins in cells and in vitro assays will be informative.
Lastly, the multidomain nature of these ARF GAPs suggests that these proteins may serve functions beyond being negative regulators of ARFs and may even be part of ARF effector functions. Indeed, the timing of GAP activity, for example, may be critical for proper ARF functioning as seems to be the case for ARF1 and its GAP at the Golgi complex (20). For the peripheral ARF GAPs discussed herein, the role of phosphorylation by src, focal adhesion kinase, and Pyk2 in GAP activity and localization needs to be assessed. Two-hybrid screens will also be effective at identifying binding partners of the proline-rich and SH3 domains of these GAPs. Another important issue is whether any of the GAPs are associated directly with downstream targets of ARF, such as polyphosphoinositide 4-phosphate 5-kinase, coat proteins, or actin. Gcs1, the yeast homologue of centaurin-α, recently was shown to be involved in the formation of actin structures in yeast and to stimulate actin polymerization in vitro in the absence of ARF (21). The fact that Randazzo et al. (3) also observed an effect of overexpression of GAP-inactive ASAP1 on cell spreading suggests that ASAP1 may have activities in addition to GAP function.
In just 1 year, studies on ARF GAP proteins have pushed forward our understanding of ARF function in the cell. The ability of these GAPs to respond to signals and associate with key regulatory molecules in the cell places them in a position to coordinate ARF activities both spatially and temporally. Defining how this process occurs will be the challenge for future investigations.
Acknowledgments
I thank Fraser Brown, Rob Donaldson, Cathy Jackson, and Ed Korn for comments on the manuscript.
Footnotes
See companion article on page 4011.
References
- 1.Moss J, Vaughan M. J Biol Chem. 1998;273:21431–21434. doi: 10.1074/jbc.273.34.21431. [DOI] [PubMed] [Google Scholar]
- 2.Chavrier P, Goud B. Curr Opin Cell Biol. 1999;11:466–475. doi: 10.1016/S0955-0674(99)80067-2. [DOI] [PubMed] [Google Scholar]
- 3.Randazzo P A, Andrade J, Miura K, Brown M T, Long Y-Q, Stauffer S, Roller P, Cooper J A. Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown M T, Andrade J, Radhakrishna H, Donaldson J G, Cooper J A, Randazzo P A. Mol Cell Biol. 1998;18:7038–7051. doi: 10.1128/mcb.18.12.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge K, Chrzanowska-Wodnicka M. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 6.Norman J C, Jones D, Barry S T, Holt M R, Cockcroft S, Critchley D R. J Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J, Khachikian Z, Radhakrishna H, Donaldson J G. J Cell Sci. 1998;111:2257–2267. doi: 10.1242/jcs.111.15.2257. [DOI] [PubMed] [Google Scholar]
- 8.Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson J G. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- 9.Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris A J, Frohman M A, et al. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 10.Cukierman E, Huber I, Rotman M, Cassel D. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- 11.Poon P P, Cassel D, Spang A, Rotman M, Pick E, Singer R A, Johnston G C. EMBO J. 1999;18:555–564. doi: 10.1093/emboj/18.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner C E, Brown M C, Perrotta J A, Riedy M C, Nikolopoulos S N, McDonald A R, Bagrodia S, Thomas S, Leventhal P S. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagrodia S, Bailey D, Lenard Z, Hart M, Guan J L, Premont R T, Taylor S J, Cerione R A. J Biol Chem. 1999;274:22393–22400. doi: 10.1074/jbc.274.32.22393. [DOI] [PubMed] [Google Scholar]
- 14.Andreev J, Simon J, Sabatini D D, Kam J, Plowman G, Randazzo P A, Schlessinger J. Mol Cell Biol. 1999;19:2338–2350. doi: 10.1128/mcb.19.3.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Premont R T, Claing A, Vitale N, Freeman J L R, Pitcher J A, Patton W A, Moss J, Vaughan M, Lefkowitz R J. Proc Natl Acad Sci USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claing A, Perry S J, Achiriloaie M, Walker J K L, Albanesi J P, Lefkowitz R J, Premont R T. Proc Natl Acad Sci USA. 2000;97:1119–1124. doi: 10.1073/pnas.97.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonny B, Huber I, Paris S, Chabre M, Cassel D. J Biol Chem. 1997;272:30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- 18.Kam J L, Miura K, Jackson T R, Gruschus J, Roller P, Stauffer S, Clark J, Aneja R, Randazzo P A. J Biol Chem. 2000;275:9653–9663. doi: 10.1074/jbc.275.13.9653. [DOI] [PubMed] [Google Scholar]
- 19.Mandiyan V, Andreev J, Schlessinger J, Hubbard S R. EMBO J. 1999;18:6890–6898. doi: 10.1093/emboj/18.24.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoe T, Lee A J, van Donselaar E, Peters P J, Hsu V W. Proc Natl Acad Sci USA. 1998;95:1624–1629. doi: 10.1073/pnas.95.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blader I J, Cope M J, Jackson T R, Profit A A, Greenwood A F, Drubin D G, Prestwich G D, Theibert A B. Mol Biol Cell. 1999;10:581–596. doi: 10.1091/mbc.10.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]