Abstract
Pancreas cancer has a grave prognosis and treatment options remain limited despite advancement in anti-cancer chemotherapeutics. This review provides an overview of the emerging therapies for pancreas cancer, focusing on novel signal transduction inhibitors (insulin-like growth factor receptor, hedgehog/Smo, PI3k/Akt/mTOR) and cytotoxics (nab-paclitaxel) that are currently in clinical development. Despite the impact molecularly targeted agents have on other tumor types, their application without cytotoxics in pancreas cancer remains limited. In addition, recent report of the superiority of an intensive cytotoxic regimen using fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) over gemcitabine reminded us of the importance of cytotoxics in this disease. As such, the future of pancreas cancer therapy may be combination regimens consisting of cytotoxics and molecularly targeted agents.
Keywords: Pancreas cancer, chemotherapy, target therapy
Introduction
Pancreas cancer is a lethal disease with mortality closely mirroring the incidence. Approximately 43,410 new cases will be diagnosed in the United States and 36,800 will die from the disease in 2010 (1). The mortality rate has not improved since the 1970s. A number of genetic mutations, such as KRAS, p16/CDKN2A, TP53, and SMAD4/DPC4, have been linked to aberrant cell proliferation, signaling, and reduced apoptosis in the disease (2). Recent genome-wide analysis showed that the genetic makeup of pancreas cancer is highly complex, with each tumor harboring more than 60 mutations (3). These aberrancies may be broadly categorized into 12 core cell-signaling pathways involved in the initiation and maintenance of malignant phenotype in pancreas tumors. These inter-related pathways function as intracellular ‘highways’, transmitting signals between extracellular events and the nucleus, and are amendable to therapeutic interventions (4).
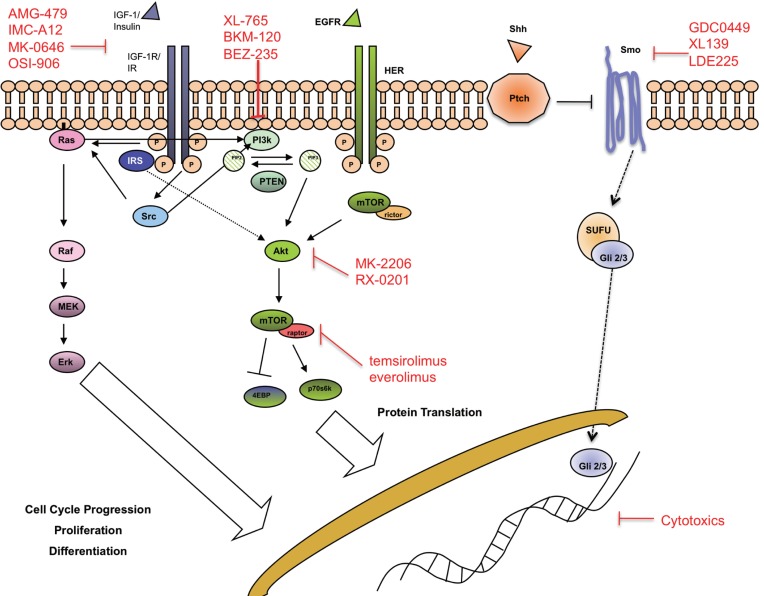
Advancement in molecular biology has increased our understanding of these anomalies and identified a large number of molecular targets, against which a large number of anti-cancer agents had been evaluated during clinical trials. Despite this, erlotinib, a tyrosine kinase inhibitor (TKI) against epidermal growth factor receptor, is the only drug after gemcitabine approved by US Food and Drug Administration for the treatment of advanced pancreas cancer (5). Approaches to target angiogenesis using agents such as bevacizumab and sorafenib have failed to achieve improvement (6)-(9). Reasons for the failure are likely multifactorial, including the wrong target, problems in drug delivery, the existence of resistance or redundant molecular pathways and failure to identify the susceptible molecular phenotype. In this review, we will focus primarily on the classes of targets and corresponding drugs currently in clinical evaluation that may have potential impact on the life of pancreas cancer patients in the near future (Table 1). Agents targeting epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) pathways have been reviewed in detail by other authors and we will discuss them briefly here (Figure 1).
Table 1. Emerging novel therapies in pancreas cancer.
Figure 1. Signaling pathways implicated in pancreas carcinogenesis. Agents against these pathways are under clinical investigation.
Human epidermal growth factor pathway
The human epidermal growth factor receptor pathway family includes EGFR (ErbB-1), HER2/neu (ErbB-2), HER3 (ErbB-3) and Her4 (ErbB-4). EGFR is an attractive target in pancreas cancer due to its frequency, higher grade and that increased expression associated with a worse prognosis (10),(11). In a randomized trial of erlotinib plus gemcitabine versus gemcitabine alone, patients receiving the combination has a statistically significant improvement in overall survival (0.82 HR, 6.24 months vs 5.91 months) (5). However, the improvement is marginal and many oncologists consider the 2 weeks survival improvement unsatisfactory. The inhibitor is being evaluated in the adjuvant setting, and in combination with other targeted agents such as insulin-like growth factor (IGF) pathway targeting drugs.
Cetuximab is a monoclonal antibody (MoAb) against the ligand-binding domain of the EGFR evaluated in combination with gemcitabine in a randomized phase III trial. However, the study failed to demonstrate the superiority of the combination over the gemcitabine control arm (12). Subset analysis showed that tumor EGFR expression does not predict benefit to the cetuximab-containing regimen. A phase II trial with cetuximab +/- gemcitabine and cisplatin showed similar negative results (13). The objective response rate was 17.5% for the combination arm versus 12.2% in control, and median progression-free and overall survivals were 4.2 months vs 3.4 months, and 7.8 months vs 7.5 months respectively.
Anti-angiogenesis
Pancreas cancer was thought to thrive on neovascularization and dependent on a rich blood supply as the tumors grow (14). The importance of vascular endothelial growth factor (VEGF) pathway was shown in preclinical pancreas cancer studies (15). Though the exact mechanism in patients is unclear, anti-angiogenic therapies are thought to interrupt tumor neovascularization and normalize existing inefficient tumor vasculature, thereby enhancing drug delivery and synergize the effects of cytotoxic agents.
Bevacizumab, a MoAb to VEGF ligand was studied in multiple trials. Recently published CALGB 80303 (gemcitabine +/- bevacizumab) treated 535 patients and overall response rates, median OS and PFS were 13%, 5.8 months, and 3.8 months for the gemcitabine/bevacizumab arm and 10%, 5.9 months, and 2.9 months for the gemcitabine/placebo arm, respectively (16). When bevacizumab was evaluated in combination with gemcitabine and erlotinib, the phase III trial failed to demonstrate significant improvement by the bevacizumab-containing arm compared to control (median OS 7.1 months vs 6.2 months respectively) (8). Bevacizumab failed to improve survival when evaluated in combination with gemcitabine and capecitabine in a phase II trial (6). Despite the intial excitement, bevacizumab failed to improve survival in advanced pancreas cancer patients when evaluated in combination with standard of care.
A number of small molecular tyrosine kinase inhibitors against VEGFR2, including sorafenib, sunitinib and vatalatinib, have being evaluated in the disease but none showed positive efficacy signal so far (6)-(9). Combination therapies targeting VEGFRs and other signaling pathways are under investigation.
Insulin-like growth factor pathway
The IGF axis comprises multiple circulating ligands, such as IGF-1, IGF-II and insulin, interacting with membrane bound receptors, such as type I IGF receptor (IGF-1R). The PI3k-Akt pathway is one main downstream mediator of IGF-1R signaling and plays a potentially important role in anticancer drug resistance (17). IGF-1R has been shown in preclinical studies to mediate resistance to EGFR inhibition, and co-targeting of both receptors enhances the abrogation of PI3k-Akt activity and reduces survivin expression (18),(19). Transgeneic mouse models of pancreas cancer expressing high levels of IGF-1R showed increased invasive carcinomas and lymph node metastases (20). Targeting of IGF-1R expression by siRNAs achieved growth inhibition in many gastrointestinal malignancies, suggesting potential importance of the pathway in pancreas cancer (21)-(24). In concert, changing IGF-1R copy number by cDNA plasmid augmented mitogenic response in mouse embryo. Treatments with MoAb seemed to lead to IGF-1R internalization and degradation, and enhanced cytotoxic chemotherapy effects (25). DNA repair pathways are other downstream effectors of IGF-1R axis and provide the rationale for combining IGF-1R inhibitors with cytotoxics (30),(31).
A number of agents targeting IGF-1R, both MoAbs and TKIs, are been evaluated clinically and we are just starting to understand their clinical role and potential mechanisms of resistance to this class of drugs (26).
Anti-IGF-1R monoclonal antibodies
AMG-479 is a fully humanized MoAb that blocks the binding of IGF-I and IGF-II to IGF-1R (IC50 < 0.6 nmol/L), and does not cross-react with the insulin receptor (IR) (27). AMG-479 completely inhibited ligand-induced dimerization and activation of IGF-1R/IGF-1R and IGF-1R/IR in two pancreas cancer cell lines. The antibody reduced IGF-1R-mediated downstream Akt phosphorylation with pro-apoptotic and anti-proliferative effects in the cancer cell lines. The agent demonstrated additive effects with gemcitabine in preclinical studies (27). In a randomized phase II trial, AMG-479 in combination with gemcitabine demonstrated a trend to improvement in median survival when compared to the placebo/gemcitabine control arm (8.7m vs 5.9m; HR 0.67, P=0.12) in previously untreated metastatic pancreas cancer patients. The median PFS was 5.1 months and 2.1 months respectively (HR 0.65, P=0.07). The investigators conclude that there was sufficient efficacy signal to warrant further evaluation in a phase III trial.
IMC-A12 (cixutumumab) (29) and MK-0646 (dalotuzumab) are other anti-IGF-1R MoAb that are being evaluated in untreated metastatic pancreas cancer patients. MK-0646 enhanced gemcitabine induced apoptosis in preclinical studies and is being evaluated clinically. This phase I/II trial is enrolling patients to 3 treatment arms; A: gemcitabine 1000mg/m2 weekly × 3 with MK-0646 weekly × 4, Arm B: gemcitabine + MK-0646 + erlotinib 100mg daily, Arm C: gemcitabine 1000mg/m2 weekly × 3 + erlotinib 100mg daily. MK-0646 achieved 6 partial responses (PR), 1 hepatic complete response (CR) and 8 stable disease (SD) out of 22 patients (32). Grade 3 or dose-limiting toxicities were rare and included hypergylcemia, hepatic transaminitis, and febrile neutropenia. The demonstrated responses confirm the hypothesis of cross-talk between EGFR and IGF axis signaling and the importance of adding cytotoxic therapy.
Small molecule IGF-1R/IR kinase inhibitors
Compensatory activation of IR signaling following inhibition of IGF-1R is emerging as a pathway of resistance to IGF-1R MoAbs. TKIs against IGF axis thus have a theoretical advantage over MoAbs given the IR cross reactivity (33). OSI-906 is a potent and highly selective inhibitor of IGF-1R, with 14 times greater selectivity for IGF-1R over IR.34 OSI-906 alone did not show significant efficacy in pancreas cancer cell lines and was further evaluated in other tumor types preclinically (35). IGF-1R pathway has been reported as potential resistance mechanism to EGFR inhibition and it seems logical to expect increased efficacy when an IGF-1R inhibitor is combined with gemcitabine and erolitinib in pancreas cancer patients. Clinical trials evaluating OSI-906 with gemcitabine and erlotinib combination have yet to be initiated. However, the dosing regimen and toxicity profile of the combination of OSI-906 and erlotinib were reported at 2010 American Society of Clinical Oncology Annual Meeting: OSI-906, administered daily at 50mg and 100mg, combined with erlotinib 100mg daily yielded stable disease in 4 out of 7 (57%) patients, including adrenocortical carcinoma, Ewings sarcoma, chordoma and adenocarcinoma of unknown primary (36). Toxicities included fatigue (31%) gastrointestinal side effects diarrhea (31%) nausea (15%); grade ≥3 hyperglycemia.
Hedgehog/smoothened pathway
Smoothened (Smo) is a transmembrane receptor with seven domains, and the activity is repressed by Patched (Ptch). The repression is relieved when ligands bind to Ptch or when there is activating mutations in Ptch, leading to increased transcription and up-regulation of Gli-1 to 3, thereby modulating cell cycle and adhesion, angiogenesis, and apoptosis. In a comprehensive genomic analysis of pancreas cancers, mutations in at least one Hedgehog (Hh) signaling component has been reported in all samples analyzed, indicating the importance of Hh pathway in pancreas tumorgenesis (3). In addition, Hh signaling may be an important modulator of tumor-stromal interaction in the disease (37),(38). Preclinically, Olive et al. evaluated IP-926, a Smo inhibitor, with gemcitabine which the combination improved survival of tumor-bearing mice and reduced metastasis in a transgenic model (39). The anti-cancer effect seems to be related to a decrease in tumor-associated stromal tissue and improve drug delivery by stimulating VEGF-independent angiogenesis. In this study, the tumor-bearing mice eventually adapted to chronic Smo inhibition and became resistant to the treatment, thus raising the importance in identifying potential resistant mechanisms.
Hh signaling is also implicated as an important mediator of cancer stem cell (CSC) phenotype in pancreas cancer. Several groups have reported on the cellular markers of CSCs in pancreas cancer and the CSCs may be identified by the co-expression of CS133/CXCR4, or CD44/CD24/ESA. Extractions enriched in cancer cells expressing these markers is highly tumorigenic in in vitro and in vivo experiments and re-capitulate the characteristics of parent tumors (40),(41). Analysis of the CSCs found increased activation of Hh signaling and other self-renewal signaling pathways. Mueller et al reported anti-CSC effects when pancreas tumors were treated with a combination of cyclopamine or CUR199691 (Smo inhibitors), rapamycin (mTOR inhibitor) and gemcitabine, and treated tumor-bearing mice survived longer than control (40). This was associated with elimination of CD133-expressing CSCs. As such, approaches targeting CSC signaling pathways are worth exploring clinically.
GDC-0449 (Vismodegib), XL139 (BMS-833923), and LDE225 are oral agents with anti-Smo activities in low nanomolar range, and skin Gli-2 expression has been used a potential pharmacodynamic markers for this class of agents. Known side effects of Hh inhibitors include dysguesia, nausea, muscle spasms, rhabdomyolysis, and alteration in cholesterol biosynthesis. GDC-0449 is furthest in development and clinical trials evaluating the efficacy in combination with gemcitabine and nab-paclitaxel or gemcitabine with and without erlotinib in previously untreated advanced pancreas cancer patients are starting soon (42). The clinical efficacy of Smo inhibitors in pancreas cancer remains unclear from the single-agent phase I trials conducted so far (43),(44). The ability of Hh inhibitors to reduce stromal tissue and enhances the delivery of cytotoxic drugs in preclinical studies may be exploited to enhance the response rate in pancreas cancer patients. Such treatment has the potential of benefiting patients with locally advanced or borderline resectable disease (45).
Potential mechanism of resistance to Smo inhibitors can be learnt from medulloblastoma models, which has been linked to alteration in the binding site of Smo by GDC-0449 (46). For LDE225, resistance may be related to a number of factors including Gli2 chromosomal amplification (a downstream effector of Smo), upregulation of compensatory pathways including PI3K/AKT/mTOR, IGF, and EGFR and, more rarely, point mutations in Smo that led to reactivated Hh signaling and restored tumor growth (47). The resistance may be reversed by co-treatment with agents targeting the PI3K/AKT/mTOR, IGF-axis, or EGFR pathways.
PI3K/AKT/mTOR pathway
The phosphoinositide 3′-kinase (PI3k)/Akt/mammalian target of rapamycin (mTOR) pathway acts as a cellular sensor for nutrients and growth factors, and integrates signals from multiple receptor kinases to regulate cellular growth and metabolism (4). The pathway is regulated by a number of upstream proteins including KRas, which activating mutations are found in the majority of pancreas cancer (48). In addition, Akt2 activation, associated with the development of human cancers, is detected in about half of the tumors (49). PI3K/Akt/mTOR activation was associated with early carcinogenesis and interruption of the pathway achieved anti-proliferation, -survival, -angiogenic and pro-apoptotic effects (50)-(58). Other activating events include PTEN loss and AKT amplification (59)-(61). Activation of this pathway was associated with poor prognosis and contributed to chemoresistance in many cancers (62)-(66). Thus, the PI3k/Akt/mTOR pathway is an attractive pathway to target in pancreas cancer.
mTOR inhibitors
Everolimus 10mg daily was evaluated in 33 metastatic gemcitabine-refractory pancreas cancer patients (67). No objective responses (complete and partial) were reported and 21% had stable disease at the time of first surveillance CT scan. Median PFS and OS were 1.8 and 4.5 months respectively. In two smaller clinical trials, 4 gemcitabine-refractory patients received temsirolimus (CCI-779) and 16 received a combination of everolimus (30mg once weekly) and erlotinib (150 mg daily) (68). The former study with temsirolimus was halted due to toxicities and no objective response was observed, and the median PFS was 19 days and survival 44 days. The everolimus and erlotinib combination was better tolerated, but no response was observed and median PFS and survival was 49 days and 87 days respectively. These trials demonstrate that mTOR inhibition as a single agent is ineffective and combining inhibitors of multiple steps and the role for these inhibitors may lie in combination regimens.
Akt inhibitors
Akt inhibitors are another class of agents that abrogate Akt/mTOR signaling. MK-2206, an allosteric Akt1-3 inhibitor, was evaluated in a phase I trial of 70 patients with advanced cancers (69). Interestingly, tumor shrinkage (23%) was observed in a patient with PTEN-negative pancreas cancer and was associated with a 60% decrease in CA19-9. MK-2206 is being evaluated as weekly (300mg) and every other day (75mg and 90mg) dosing schedules. MK-2206 is also being evaluated in combination with cytotoxic chemo-agents and inhibitors of c-Met and EGFR (70),(71).
RX-0201 is an antisense oligonucleotide against Akt1 mRNA, thereby interrupting the pathway's activation. The anti-sense oligonucleotide demonstrated activity against pancreas cancer cell lines in low nanomolar range, reducing the expression of Akt1 mRNA and protein. In in vivo studies, RX-0201 treatment led to complete response in 2 out of 3 pancreas tumor-bearing mice (72). As such, RX-0201 in combination with gemcitabine is currently being evaluated in a phase II trial for metastatic pancreas cancer patients (73). Given the short half-life typical of anti-sense agents, RX-0201 is being administered by continuous infusion for 14 days of a 21-day cycle and presents a potential obstacle to patient accural. Liposomal formulations are in development (74).
PI3K inhibitors
XL147 and BKM120 are oral class I PI3k inhibitors that are being evaluated in phase I trials, alone and in combination therapies (75)-(77). These trials have focused on lung, colorectal and breast cancers given the higher frequency of pathway aberrations in these tumor types. XL765 is a novel selective inhibitor that interrupts the pathway at various nodes: PI3K, TORC1 and TORC2. The efficacy of such agents in pancreas cancer is to be evaluated (78).
Cytotoxics
Gemcitabine has been the chemotherapy backbone for the treatment of newly diagnosed advanced pancreas cancer (79),(80). Various other cytotoxic drugs had been tested in combination with gemcitabine, including fluoropyrimidines, platinum derivatives, and taxanes (80)-(84). Meta-analysis of various cytotoxic trials over the last one-and-a-half decades suggest improved survival with doublet or triplet gemcitabine-based therapy among patients with good performance status, who can, supposedly, better withstand the toxicities (85).
Final results from the interim analysis of the PRODIDGE 4/ACCORD 11 trial were presented at 2010 European Society for Medical Oncology annual meeting, which randomized 342 patients with previously untreated metastatic pancreas cancer to receiving FOLFIRINOX (oxaliplatin 85 mg/m(2) Day 1 + irinotecan 180 mg/m(2) Day 1 + leucovorin 400 mg/m(2) Day 1 followed by 5-flurouracil 400 mg/m(2) bolus Day 1 and 2,400 mg/m(2) 46 hours continuous infusion biweekly) or gemcitabine alone. The study was stopped on recommendation by the independent monitoring committee during preplanned interim analysis when FOLFIRINIOX was determined to be superior to gemcitabine alone, making the fluoropyrimidine-based regimen first non-gemcitabine based regimen to show significant improvement in overall survival. The objective response rate for FOLFIRINOX, compared to gemcitabine alone, was 31.6% vs 9.4% (P=0.0001), median PFS 6.4 vs 3.3 months (P<0.0001) and median survival 11.1 vs 6.8 months (HR=0.57, 95% CI =0.45-0.73; P<0.001) respectively. However, there were significantly more grade 3 and above toxicities in the FOLFIRINOX arm, including diarrhea, nausea, vomiting, neuropathy, neutropenia, neutropenic fever. Given the higher frequency of clinically significant toxicities, FOLFIRINOX cannot be accepted as the standard first-line treatment for all newly diagnosed advanced pancreas cancer patients. The choice of FOLFIRINOX in advanced patients needs to be personalized according to factors such as performance status, treatment aim, physiological reserve and patient preference, and the role in adjuvant setting is being evaluated.
Nab-paclitaxel (Abraxane®; Abraxis) is a nano-particle preparation in which paclitaxel is bound to albumin as compared to sb-paclitaxel (Taxol®, Bristol Meyers Squibb), which is dissolved in poloxyethylated castor oil (Cremaphor EL®) and ethanol. The absence of castor oil renders nab-paclitaxel clinically advantageous since this avoids the infusion and hypersensitivity reaction characteristics of sb-paclitaxel. In the initial phase I clinical trial of nab-paclitaxel, there was no hypersensitivity reaction typical of sb-paclitaxel and was well tolerated up to 300mg/m2 administered as a 30-minute infusion (86). The recommended dosing for nab-paclitaxel is 260mg/m2 compared to 175 mg/m2 for sb-paclitaxel (87). In a crossover pharmacokinetic study to limit patient variability, nab-pacliataxel had higher peak plasma and unbound concentrations (88). Greater unbound fraction of paclitaxel has been hypothesized to lead to greater efficacy seen in many clinical trials.
One possible mechanism of efficacy by the albumin-bound agent may be related to enhanced tumor uptake through interaction with the SPARC (secreted protein acid rich in cysteine) molecule. The SPARC gene, highly conserved among vertebrates, regulates the assembly, organization, and turnover of the extracellular matrix by binding and modulating the deposition of multiple structural components and attenuating the activity of extracellular proteases. SPARC is expressed in cancer-associated stroma and in malignant cells of some types, affecting tumor development, invasion, metastases, angiogenesis and inflammation. SPARC-induced changes in the tumor microenvironment can suppress or promote progression of different cancers depending on the tissue and cell type. SPARC expression is related to tumor aggressiveness though the exact mechanism is unclear. The molecule regulates the effects of bFGF and VEGF on MAPK signaling and increased expression of SPARC in pancreas tumors has been related to poorer survival (91),(92). Infante et al. characterized SPARC expression in peritumoral fibroblasts and pancreas cells from 299 patients with resectable pancreas cancer. Median survival was halved in patients' tumors that expressed SPARC (15 months vs 30 months) and when cases were controlled for other prognostic factors (tumor size, positive lymph nodes, margin status, tumor grade, and age) the hazard ratio (HR) was significant (HR 1.89; 95% CI, 1.31 to 2.74).
Therapies combining nab-paclitaxel with gemcitabine are under investigation in pancreas cancer given the high expression of SPARC in pancreas cancer. Several studies are underway and preliminary result showed impressive responsive rate and encouraging survival outcome. In a phase I/II trial, 63 previously untreated metastatic patients were treated with nab-paclitaxel and gemcitabine and among the 49 evaluable patients, 1 achieved CR (2%), 12 PRs (24%) and 20 SD (41%) (clinical benefit rate 67%). The response rate and PFS correlated with SPARC expression by immunohistochemistry (89). A single institution retrospective review of this combination in neoadjuvant setting for borderline and unresectable patients confirmed the high response rate (69% PR and 23% SD). About 23% of patients in the study went on to surgical resection with curative intent (90). This regimen is being evaluated in a phase III randomized trial among patients with untreated metastatic pancreas cancer.
Conclusion
Despite advancement in anti-cancer therapeutics, treatment options remain limited and prognosis poor for patients with pancreas cancer. The molecularly targeted agents held significant promise in pancreas cancer for several reasons, including the better-tolerated toxicity profiles and they target known molecular aberrancies. However, strategies to target angiogenesis and EGFR pathways had, in general, not being successful and the underlying reasons remain unclear. Other exciting molecular targets that can be interrupted by clinical grade drugs include the IGF, Hh and PI3k/Akt/mTOR pathways. As these agents complete early phase evaluation, their role in the treatment of pancreas cancer will be evaluated either alone or in combination therapies. Importantly, in-depth correlative studies using patient blood and tumor samples should be incorporated to better select the patient population most likely to benefit from these agents and also, to understand the mechanism of efficacy (or futility).
An important recent development is the demonstration of the superiority of intense cytotoxic regimen (FOLFIRINOX) over gemcitabine alone in previously untreated pancreas cancer patients. Though the regimen can hardly be accepted as the standard for advanced disease due to its significant side effect profile, the trial points to the continual importance of cytotoxic agents in treating the disease. As such, one eagerly awaits the result from the phase III trial of nab-paclitaxel plus gemcitabine versus gemcitabine alone in metastatic pancreas cancer patients given the encouraging result so far.
Footnotes
No potential conflict of interest.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Buchholz M, Gress TM. Molecular changes in pancreatic cancer. Expert Rev Anticancer Ther. 2009;9:1487–97. doi: 10.1586/era.09.107. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–37. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 5.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 6.Javle M, Yu J, Garrett C, Pande A, Kuvshinoff B, Litwin A, et al. Bevacizumab combined with gemcitabine and capecitabine for advanced pancreatic cancer: a phase II study. Br J Cancer. 2009;100:1842–5. doi: 10.1038/sj.bjc.6605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko AH, Venook AP, Bergsland EK, Kelley RK, Korn WM, Dito E, et al. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:1051–7. doi: 10.1007/s00280-010-1257-5. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–7. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 9.Wallace JA, Locker G, Nattam S, Kasza K, Wade-Oliver K, Stadler WM, et al. Sorafenib (S) plus gemcitabine (G) for advanced pancreatic cancer (PC): A phase II trial of the University of Chicago Phase II Consortium [abstract] J Clin Oncol. 2007;s25:4608. [Google Scholar]
- 10.Papageorgio C, Perry MC. Epidermal growth factor receptor-targeted therapy for pancreatic cancer. Cancer Invest. 2007;25:647–57. doi: 10.1080/07357900701522653. [DOI] [PubMed] [Google Scholar]
- 11.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 12.Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ, et al. Phase III study comparing Gemcitabine plus Cetuximab versus Gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-Directed Intergroup Trial S0205. J Clin Oncol. 2010;28:3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cascinu S, Berardi R, Labianca R, Siena S, Falcone A, Aitini E, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]
- 14.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann AC, Mori R, Vallbohmer D, Brabender J, Klein E, Drebber U, et al. High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 2008;10:674–9. doi: 10.1593/neo.08292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedy LK, Donna N, Donna H, Susan S, Deborah S, Herbert H, et al. Gemcitabine plus Bevacizumab compared with Gemcitabine plus Placebo in patients with advanced pancreatic cancer: Phase III trial of the cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;s28:1386. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway--therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 18.Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene. 2007;26:2678–84. doi: 10.1038/sj.onc.1210094. [DOI] [PubMed] [Google Scholar]
- 19.Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- 20.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–53. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Adachi Y, Imsumran A, Yamamoto H, Piao W, Li H, et al. Targeting for insulin-like growth factor-I receptor with short hairpin RNA for human digestive/gastrointestinal cancers. J Gastroenterol. 2010;45:159–70. doi: 10.1007/s00535-009-0151-6. [DOI] [PubMed] [Google Scholar]
- 22.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505–11. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomizawa M, Shinozaki F, Sugiyama T, Yamamoto S, Sueishi M, Yoshida T. Insulin-like growth factor-I receptor in proliferation and motility of pancreatic cancer. World J Gastroenterol. 2010;16:1854–8. doi: 10.3748/wjg.v16.i15.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, et al. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160:90–101. doi: 10.1016/j.jss.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Rubini M, Hongo A, D'Ambrosio C, Baserga R. The IGF-I receptor in mitogenesis and transformation of mouse embryo cells: role of receptor number. Exp Cell Res. 1997;230:284–92. doi: 10.1006/excr.1996.3430. [DOI] [PubMed] [Google Scholar]
- 26.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci U S A. 2010;107:10791–8. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009;8:1095–105. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 28.Kindler HL, Richards DA, Stephenson J, Garbo LE, Rocha Lima CS, Safran H, et al. A placebo-controlled, randomized phase II study of conatumumab (C) or AMG 479 (A) or placebo (P) plus gemcitabine (G) in patients (pts) with metastatic pancreatic cancer (mPC) [abstract] J Clin Oncol. 2010;s28:4035. [Google Scholar]
- 29.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;s13:5549–55. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 30.Héron-Milhavet L, Karas M, Goldsmith CM, Baum BJ, LeRoith D. Insulin-like growth factor-I (IGF-I) receptor activation rescues UV-damaged cells through a p38 signaling pathway. Potential role of the IGF-I receptor in DNA repair. J Biol Chem. 2001;276:18185–92. doi: 10.1074/jbc.M011490200. [DOI] [PubMed] [Google Scholar]
- 31.Trojanek J, Ho T, Del Valle L, Nowicki M, Wang JY, Lassak A, et al. Role of the insulin-like growth factor I/insulin receptor substrate 1 axis in Rad51 trafficking and DNA repair by homologous recombination. Mol Cell Biol. 2003;23:7510–24. doi: 10.1128/MCB.23.21.7510-7524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Javle MMV, Shroff GR, Bhosale RT, Overman P, Weatherly MJ, Wolff J, et al. Phase I/II study of MK-0646, a humanized monoclonal IGF-1R antibody in combination with gemcitabine or gemcitabine plus erlotinib (E) for advanced pancreatic cancer [abstract] J Clin Oncol. 2010;s28:4039. [Google Scholar]
- 33.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor IGF-1R: rationale for cotargeting (IGF-1R) and IR in cancer. Mol Cancer Ther. 2010;9:2652–64. doi: 10.1158/1535-7163.MCT-10-0318. [DOI] [PubMed] [Google Scholar]
- 34.Ji QS, Mulvihill MJ, Rosenfeld-Franklin M, Cooke A, Feng L, Mak G, et al. A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Mol Cancer Ther. 2007;6:2158–67. doi: 10.1158/1535-7163.MCT-07-0070. [DOI] [PubMed] [Google Scholar]
- 35.Flanigan SA, Pitts TM, Eckhardt SG, Tentler JJ, Tan AC, Thorburn A, et al. The IGF-1R/IR Tyrosine Kinase Inhibitor, PQIP, Exhibits Enhanced Anti-Tumor Effects in Combination with Chemotherapy Against Colorectal Cancer Models. Clin Cancer Res 2010 Oct 13. [Epub ahead of print]. doi: 10.1158/1078-0432.CCR-10-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macaulay VM, Middleton MR, Eckhardt SG, Juergens RA, Stephens AW, Poondru S, et al. Phase I study of OSI-906, dual tyrosine kinase inhibitor of insulin-like growth factor-1 receptor (IGF-1R) and insulin receptor (IR) in combination with erlotinib (E) in patients with advanced solid tumors [abstract] J Clin Oncol. 2010;s28:3016. [Google Scholar]
- 37.Hidalgo M, Maitra A. The hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094–6. doi: 10.1056/NEJMcibr0905857. [DOI] [PubMed] [Google Scholar]
- 38.Dosch JS, Pasca di Magliano M, Simeone DM. Pancreatic cancer and hedgehog pathway signaling: new insights. Pancreatology. 2010;10:151–7. doi: 10.1159/000225923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–13. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 41.Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009;568:161–73. doi: 10.1007/978-1-59745-280-9_10. [DOI] [PubMed] [Google Scholar]
- 42.Erlichman C. GDC-0449 and erlotinib hydrochloride with or without gemcitabine hydrochloride in treating patients with metastatic pancreatic cancer or solid tumors that cannot be removed by surgery. ClinicalTrials.gov registration number: NCT00878163. [Google Scholar]
- 43.Siu LL, Papadopoulos K, Alberts SR, Kirchoff-Ross R, Vakkalagadda B, Lang L, et al. A first-in-human, phase I study of an oral hedgehog (HH) pathway antagonist, BMS-833923 (XL139), in subjects with advanced or metastatic solid tumors [abstract] J Clin Oncol. 2010;s28:2501. [Google Scholar]
- 44.Rodon Ahnert J, Baselga J, Tawbi HA, Shou Y, Granvil C, Dey J, et al. A phase I dose-escalation study of LDE225, a smoothened (Smo) antagonist, in patients with advanced solid tumors. [abstract] J Clin Oncol. 2010;s28:2500. [Google Scholar]
- 45.Tuveson D. Hedgehog inhibition for pancreatic ductal adenocarcinoma (PDAC) in the preoperative setting (HIPPoS) ClinicalTrials.gov registration number: NCT01096732. [Google Scholar]
- 46.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53:549–54. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–50. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 50.Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin Cancer Res. 2006;12:679–89. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- 51.Balsara BR, Pei J, Mitsuuchi Y, Page R, Klein-Szanto A, Wang H, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–9. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 52.Fiala ES, Sohn OS, Wang CX, Seibert E, Tsurutani J, Dennis PA, et al. Induction of preneoplastic lung lesions in guinea pigs by cigarette smoke inhalation and their exacerbation by high dietary levels of vitamins C and E. Carcinogenesis. 2005;26:605–12. doi: 10.1093/carcin/bgh341. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Davidson G, Huang Y, Jiang BH, Shi X, Costa M, et al. Nickel compounds act through phosphatidylinositol-3-kinase/Akt-dependent, p70(S6k)-independent pathway to induce hypoxia inducible factor transactivation and Cap43 expression in mouse epidermal Cl41 cells. Cancer Res. 2004;64:94–101. doi: 10.1158/0008-5472.can-03-0737. [DOI] [PubMed] [Google Scholar]
- 54.Segrelles C, Ruiz S, Perez P, Murga C, Santos M, Budunova IV, et al. Functional roles of Akt signaling in mouse skin tumorigenesis. Oncogene. 2002;21:53–64. doi: 10.1038/sj.onc.1205032. [DOI] [PubMed] [Google Scholar]
- 55.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The rapamycin analog CCI-779 is a potent inhibitor of pancreatic cancer cell proliferation. Biochem Biophys Res Commun. 2005;331:295–302. doi: 10.1016/j.bbrc.2005.03.166. [DOI] [PubMed] [Google Scholar]
- 56.Lieberthal W, Fuhro R, Andry CC, Rennke H, Abernathy VE, Koh JS, et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol. 2001;281:F693–706. doi: 10.1152/ajprenal.2001.281.4.F693. [DOI] [PubMed] [Google Scholar]
- 57.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 58.Suhara T, Mano T, Oliveira BE, Walsh K. Phosphatidylinositol 3-kinase/Akt signaling controls endothelial cell sensitivity to Fas-mediated apoptosis via regulation of FLICE-inhibitory protein (FLIP) Circ Res. 2001;89:13–9. doi: 10.1161/hh1301.092506. [DOI] [PubMed] [Google Scholar]
- 59.Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Tanno S, Skele KL, et al. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2002;87:470–6. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]
- 60.Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–6. [PubMed] [Google Scholar]
- 61.Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–41. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473–82. doi: 10.1200/JCO.2005.07.168. [DOI] [PubMed] [Google Scholar]
- 63.Ermoian RP, Furniss CS, Lamborn KR, Basila D, Berger MS, Gottschalk AR, et al. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res. 2002;8:1100–6. [PubMed] [Google Scholar]
- 64.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–6. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 65.Brognard J, Clark AS, Ni Y, Dennis PA. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–97. [PubMed] [Google Scholar]
- 66.Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–17. [PubMed] [Google Scholar]
- 67.Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–8. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, Reddy SA, et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368. doi: 10.1186/1471-2407-10-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yap TA, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Tunariu N, et al. First-in-class phase I trial of a selective Akt inhibitor, MK2206 (MK), evaluating alternate day (QOD) and once weekly (QW) doses in advanced cancer patients (pts) with evidence of target modulation and antitumor activity [abstract] J Clin Oncol. 2010;s28:3009. [Google Scholar]
- 70.Zeneca MaA. A combination therapy study of MK2206 and AZD6244 in patients with advanced solid tumors. ClinicalTrials.gov registration number: NCT01021748. [Google Scholar]
- 71.Merck Sharp, Dohme Corp. A phase I study of MK2206 in combination with standard chemotherapy in participants with locally advanced or metastatic solid tumors. ClinicalTrials.gov registration number: NCT00848718. [Google Scholar]
- 72.Yoon H, Kim DJ, Ahn EH, Gellert GC, Shay JW, Ahn CH, et al. Antitumor activity of a novel antisense oligonucleotide against Akt1. J Cell Biochem. 2009;108:832–8. doi: 10.1002/jcb.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guthrie TH, Oliff I, Cline-Burkhardt V, Marek BJ, Sahoo TP, Kumar L, et al. A safety and efficacy study of RX-0201 plus gemcitabine in metastatic pancreatic cancer. ClinicalTrials.gov registration number: NCT01028495. [Google Scholar]
- 74.Pal SK, Reckamp K, Yu H, Figlin RA. Akt inhibitors in clinical development for the treatment of cancer. Expert Opin Investig Drugs. 2010;19:1355–66. doi: 10.1517/13543784.2010.520701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moldovan C, Soria J, LoRusso P, Guthrie T, Song C, Nguyen LT, et al. A phase I safety and pharmacokinetic (PK) study of the PI3K inhibitor XL147 (SAR245408) in combination with erlotinib in patients (pts) with advanced solid tumors [abstract] J Clin Oncol. 2010;s28:3070. [Google Scholar]
- 76.Edelman G, Bedell C, Shapiro G, Pandya SS, Kwak EL, Scheffold C, et al. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts) with advanced malignancies. J Clin Oncol. 2010;s28:3004. [Google Scholar]
- 77.Baselga J, De Jonge MJ, Rodon J, Burris HA, 3rd, Birle DC, De Buck SS, et al. A first-in-human phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors [abstract] J Clin Oncol. 2010;s28:3003. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 78.Brana I, LoRusso P, Baselga J, Heath EI, Patnaik A, Gendreau S, et al. A phase I dose-escalation study of the safety, pharmacokinetics (PK), and pharmacodynamics of XL765 (SAR245409), a PI3K/TORC1/TORC2 inhibitor administered orally to patients (pts) with advanced malignancies [abstract] J Clin Oncol. 2010;s28:3030. [Google Scholar]
- 79.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 80.Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA 3rd, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–53. doi: 10.1093/oxfordjournals.annonc.a010600. [DOI] [PubMed] [Google Scholar]
- 81.Colucci G, Labianca R, Di Costanzo F, Gebbia V, ìCarten G, Massidda B, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–51. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 82.Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–8. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 83.Conroy T, Desseigne F, Ychou M, Ducreux M, Bouche O, Guimbaud R, et al. Randomized phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O]) versus gemcitabine (G) as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): Preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial [abstract] J Clin Oncol. 2010;s28:4010. [Google Scholar]
- 84.Fine RL, Fogelman DR, Schreibman SM, Desai M, Sherman W, Strauss J, et al. The gemcitabine, docetaxel, and capecitabine (GTX) regimen for metastatic pancreatic cancer: a retrospective analysis. Cancer Chemother Pharmacol. 2008;61:167–75. doi: 10.1007/s00280-007-0473-0. [DOI] [PubMed] [Google Scholar]
- 85.De Jesus-Acosta A, Oliver GR, Flores E, Wilfong LS, Laheru D, Le DT, et al. A multicenter review of gemcitabine, docetaxel, and capecitabine (GTX) in patients with advanced pancreatic adenocarcinoma. J Clin Oncol. 2010;s28:e14580. [Google Scholar]
- 86.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44. [PubMed] [Google Scholar]
- 87.Ibrahim NK, Samuels B, Page R, Doval D, Patel KM, Rao SC, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23:6019–26. doi: 10.1200/JCO.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Gardner ER, Dahut WL, Scripture CD, Jones J, Aragon-Ching JB, Desai N, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14:4200–5. doi: 10.1158/1078-0432.CCR-07-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Hoff DD, Ramanathan R, Borad M, Laheru D, Smith L, Wood T, et al. SPARC correlation with response to gemcitabine (G) plus (nab-p)aclitaxel nab-P in patients with advanced metastatic pancreatic cancer: A phase I/II study [abstract] J Clin Oncol. 2009;27:4525. [Google Scholar]
- 90.Thapaliya P, Kundranda MN, Curtis KK, Callister MD, Ashman JB, Collins J, et al. Gemcitabine and nab-paclitaxel in patients with unresectable/borderline resectable pancreatic cancer [abstract] J Clin Oncol. 2010;s28:e14675. [Google Scholar]
- 91.Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21:55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Prenzel KL, Warnecke-Eberz U, Xi H, Brabender J, Baldus SE, Bollschweiler E, et al. Significant overexpression of SPARC/osteonectin mRNA in pancreatic cancer compared to cancer of the papilla of Vater. Oncol Rep. 2006;15:1397–401. [PubMed] [Google Scholar]