A 74-year old female presented to the emergency room with right lower quadrant abdominal pain, nausea and vomiting for 3 days. Due to a contrast dye allergy, a non-contrast CT scan of the abdomen and pelvis was performed which showed a lesion in the right colon with a dilated cecum and small bowel (Figure 1). Plain X-ray of the chest showed no obvious pathology. An exploratory laparotomy revealed a large obstructing mass at the right colon proximal to the hepatic flexure, with massive lymphadenopathy and abdominal adhesions. Intra-operative palpation and inspection of the liver was unremarkable without evidence of a suspicious mass. The patient underwent right hemicolectomy with anastomosis.
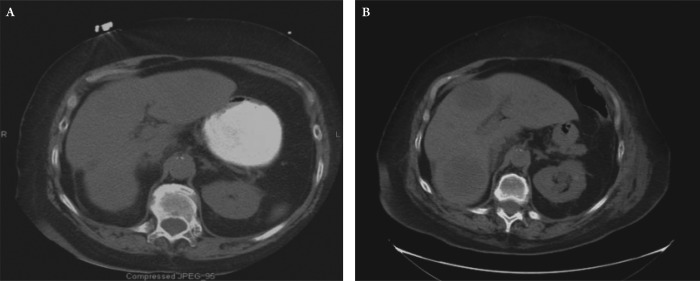
Figure 1. Serial non-contrast CT scans of abdomen. (A) CT performed at the time of initial evaluation at admission showed no visible liver lesions. (B) Subsequent CT taken 10 days later showed multiple large liver lesions.
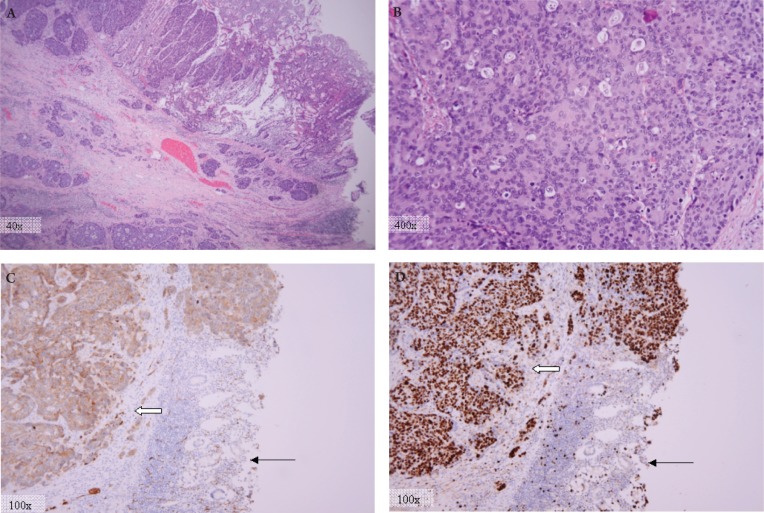
The surgical tissue was reviewed by pathology, and on gross specimen, while the ileocecal valve mucosa contained discontinuous deposits of tumor associated with lymphatic tumor emboli, there was no evidence of distant metastasis. On microscopic evaluation the tumor cells had diffuse architecture with necrosis, ample cytoplasm, and numerous mitotic figures (10-20 mitoses per single high power field). Immunohistochemistry showed diffuse cytoplasmic staining for synaptophysin, as well as positive staining for CD-56, CK-7, Ki-67 (in nearly 90% of the cells) and negative staining for CK-20 consistent with large cell neuroendocrine carcinoma (Figure 2). Of the total lymph nodes present in the surgical specimen 17 out of 24 showed metastatic disease. Thus the final pathology was felt to be most compatible with an aggressive high-grade large cell neuroendocrine carcinoma of the colon.
Figure 2. Pathologic examination with H&E staining and immunohistochemical analysis. (A) H&E stain showing tumor ulcerating though normal surface epithelium. (B) H&E stain at high magnification showing cytological malignant features such as prominent nuclei, vesicularly cleared chromatin, apoptotic figures, and numerous mitotic figures. (pathology photos by Lawrence Matthews M.D.,Ph.D.). Pathology revealed a pT4aN2bMX, LCNEC with diffuse cytoplasmic staining for synaptophysin (C), and 90% Ki-67 staining (D) (thick arrows). Normal tissue is seen at lower right (thin arrows).
On post-operative day number 10, the patient developed bilious drainage from the lower portion of the surgical incision. A non-contrast CT of the abdomen and pelvis showed interval development of approximately 5 hypodense lesions within the liver measuring 4-6 cm in size. Comparison of the post-operative scan to the pre-operative CT indicated that the hepatic lesions were new (Figure 1). Given the rapid development of these lesions and concern for potential liver abscesses the decision was made for a drainage procedure. An attempt at interventional radiology guided drainage was unsuccessful and subsequently the patient underwent a surgical exploration.
Intra-operative findings were notable for multiple palpable hepatic masses through out both lobes of the liver. By gross inspection the hepatic lesions measured 4-6 cm in size, with complete replacement of the left hepatic lobe and a large firm mass at the surface of the right hepatic lobe. A wedge biopsy of one of the liver masses was performed and frozen section showed morphologic features similar to the previously resected tumor consistent with metastatic large cell neuroendocrine carcinoma. An additional intra-operative finding was a small bowel enterocutaneous fistula 10 cm proximal to the previous anastomotic site, which was resected and reanastomized. Two weeks later, the patients condition deteriorated with development of a second enterocutaneous small bowel fistula. At that time the family withdrew care and the patient subsequently expired.
Discussion
Neuroendocrine tumors (NETs) are rare neoplasms with wide spectrum of clinical presentations that are classified according to differentiation, grade, and stage. Differentiation refers to the degree in which the neoplastic cells resemble their non-neoplastic equivalent (1). The term well-differentiated refers to neoplastic cells that closely resemble their non-neoplastic counter equivalent having organoid and nesting appearances; while poorly-differentiated is reserved for neoplasms that bear less resemblance to their cells of origin, and have diffuse architecture and irregular nuclei (1). Histologic grade refers to the aggressiveness of the neoplasm with high-grade having a more aggressive and less predictive course; poorly-differentiated NETs are traditionally considered high grade (1). Tumor stage refers to the extend of tumor spread.
Majority of NETs are carcinoid tumors, which are well-differentiated and have a better prognosis than the usual adenocarcinoma. Large-cell neuroendocrine carcinoma (LCNEC) is a rare subtype of NETs with an aggressive nature and a poor prognosis due to its tendency for early metastasis (2). While NETs can arise in different organs, colonic NETs are exceptionally rare (2),(3). A study by Bernick et al showed that 0.6% of patients with colorectal cancer had neuroendocrine carcinoma and only 0.2% of those were large cell neuroendocrine carcinomas (4),(5).
While the colonic LCNET are rare tumors, they share histological features with the more well described large cell neuroendocrine carcinomas of the lung. The histological classification of LCNETs of the lung was initially proposed by Travis et al (6) and subsequently adopted by the World Health Organization. These tumors are characterized by (i) neuroendocrine appearance under light microscopy (including an organoid, nesting, trabecular, rosette, and palisading pattern) (7), (ii) large cells with a polygonal shape, ample cytoplasm, coarse chromatin and frequent nucleoli, (iii) very high mitotic rate (greater than 10/10 high-power fields) along with frequent necrosis, and evidence of neuroendocrine features by immunohistochemistry or electron microscopy (4),(6).
The tumor in this case met the morphological criteria proposed by Travis et al (6), and had neuroendocrine immunohistochemical features including diffuse cytoplasmic staining for synaptophysin. Unlike adenocarcinomas, most poorly differentiated LCNETs, like the one in this case, are negative for CK-20 (a tumor marker traditionally confined to the intestinal epithelial, urothelial, and Merkel cells) (8). Nonetheless, several case reports of CK-20 positive LCNEC have been reported in the literature, suggesting a potentially common precursor for these tumors and the conventional colonic adenocarcinomas (2),(3),(9).
Ki-67 antige is a surrogate marker for cell proliferation and is detected in the nucleus of actively cycling cells. Since Ki-67 is strictly related to cell replication and not to DNA repair, it can serve as an excellent marker for tumor growth (10). In the case presented, 90% of the tumor cell nuclei stained positive for Ki-67, demonstrating its highly aggressive nature with rapid metastases to the liver. While tumor aggressiveness of the tumor has been associated to the extent of Ki-67 expression in some studies (11),(12), others have argued that the prognostic value of Ki-67 is dependent on the tissue type and that it may not be generalized to all tumors (13). Similarly, prognostic value of Ki-67 in LCNECs is unclear at this time.
Similar to the adenocarcinomas of the colon, liver is the most common site of metastasis for the NETs of the colon (14). Given the aggressive nature of colonic NET, patients most often present with metastatic disease at time of initial diagnosis (4). The aggressive nature of colonic large cell neuroendocrine tumors is evident in this case by the rapid progression of the tumor and near replacement of the liver by tumor in a period spanning approximately 10 days from the time of initial presentation to the time of second operative exploration.
In conclusion, colonic large-cell neuroendocrine carcinomas are rare and aggressive tumors. Most are located in the cecum or the rectum, are metastatic at presentation, and have a poor prognosis with median overall survival reported to be 10.4 months ( range of 0 to 263.7 months) (4). While surgical resection is the primary treatment modality, the benefit of chemo- or radiation therapy, as used for conventional colorectal adenocarcinomas, has not been established for colonic LCNET (3),(4),(15),(16). Interestingly a recent case report indicated clinical benefit to post-operative chemoradiation in a patient with LCNET (17). Thus further studies are needed to determine the molecular genetics of these rare tumors and define the optimal systemic and local therapies.
Footnotes
No potential conflict of interest.
References
- 1.Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 2.Park JS, Kim L, Kim CH, Bang BW, Lee DH, Jeong S, et al. Synchronous large-cell neuroendocrine carcinoma and adenocarcinoma of the colon. Gut Liver. 2010;4:122–5. doi: 10.5009/gnl.2010.4.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 4.Bernick PE, Klimstra DS, Shia J, Minsky B, Saltz L, Shi W, et al. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47:163–9. doi: 10.1007/s10350-003-0038-1. [DOI] [PubMed] [Google Scholar]
- 5.Gravante G, Markiewicz D, Madeddu F, Giordano P. Colonic large-cell neuroendocrine tumours. Can J Surg. 2009;52:E49–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB, Jr, Nieman L, et al. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–53. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton Stanley R., Aaltonen Lauri A. Pathology and Genetics of Tumours of the Digestive System. Lyon (France): IARCPress; 2000. World Health Organization Classification of Tumours. [Google Scholar]
- 8.Chan JK, Suster S, Wenig BM, Tsang WY, Chan JB, Lau AL. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol. 1997;21:226–34. doi: 10.1097/00000478-199702000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Kato T, Terashima T, Tomida S, Yamaguchi T, Kawamura H, Kimura N, et al. Cytokeratin 20-positive large cell neuroendocrine carcinoma of the colon. Pathol Int. 2005;55:524–9. doi: 10.1111/j.1440-1827.2005.01864.x. [DOI] [PubMed] [Google Scholar]
- 10.Vilar E, Salazar R, Pérez-García J, Cortes J, Oberg K, Tabernero J. Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer. 2007;14:221–32. doi: 10.1677/ERC-06-0074. [DOI] [PubMed] [Google Scholar]
- 11.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 12.Gabler D, Mandal C, Harrington C, Adamczyk M, Linthicum DS. Kinetic and energetic parameters of imipramine binding to monoclonal antibodies as measured by fluorescence spectroscopy. Hybridoma. 1992;11:301–10. doi: 10.1089/hyb.1992.11.301. [DOI] [PubMed] [Google Scholar]
- 13.Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 14.Vilallonga R, Espín Basany E, López Cano M, Landolfi S, Armengol Carrasco M. [Neuroendocrine carcinomas of the colon and rectum. A unit's experience over six years] Rev Esp Enferm Dig. 2008;100:11–6. doi: 10.4321/s1130-01082008000100003. [DOI] [PubMed] [Google Scholar]
- 15.Jansson D, Gould VE, Gooch GT, Rittenhouse HG, Shin SS, Manderino GL, et al. Immunohistochemical analysis of colon carcinomas applying exocrine and neuroendocrine markers. APMIS. 1988;96:1129–39. doi: 10.1111/j.1699-0463.1988.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 16.Vortmeyer AO, Lubensky IA, Merino MJ, Wang CY, Pham T, Furth EE, et al. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89:1448–53. doi: 10.1093/jnci/89.19.1448. [DOI] [PubMed] [Google Scholar]
- 17.Nojima H, Seike K, Kosugi C, Shida T, Koda K, Oda K, et al. Advanced moderately differentiated neuroendocrine carcinoma of the rectum with favorable prognosis by postoperative chemoradiation. World J Surg Oncol. 2010;8:29. doi: 10.1186/1477-7819-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]