Abstract
Described are the first antemortem diagnosis made via fecal examination using the Baermann technique and the first postmortem recovery of endemic Angiostrongylus vasorum in dogs from North America, specifically the Avalon peninsula of Newfoundland. In one dog, A. vasorum was recovered and identified at postmortem; gross and histologic lesions are described.
Angiostrongylus vasorum is a metastrongylid nematode that infects the pulmonary artery and right ventricle of wild and domestic canids. Red fox and other species of wild fox serve as natural definitive hosts (1). Infections are considered endemic in various parts of Europe (France, Denmark, Germany, Italy, Spain, Switzerland), England, Ireland, Africa (Uganda), Turkey, countries of the former USSR, South America (Brazil, Colombia), and Canada (Newfoundland) (2,3). In Newfoundland, A. vasorum was first reported in 2 red foxes in 1973 (3). Angiostrongylus vasorum is pathogenic in dogs; effects of infection range from subclinical to fatal cardiopulmonary disease. Previous reports of A. vasorum infection in dogs in North America involved animals that had been imported and had most likely acquired the infection in Europe (4,5).
In June 1996, a 5-year-old, male beagle with a history of a persistent cough (case 1) was presented for veterinary care. The owner of the dog lived in the eastern part of the Avalon peninsula of Newfoundland (St. John's) and the dog had no history of travel outside the province. On physical examination, the dog had a normal temperature, but the abdomen was distended. The lung fields appeared congested on radiographs. Over the course of the next several months, the dog was treated with various therapeutic regimens involving antibiotics, corticosteroids, diuretics, and bronchodilators. The dog was administered 60 mg tetracycline hydrochloride, 60 mg novobiocin, 1.5 mg prednisolone (Delta-Albaplex tablets; Janssen Animal Healthy, Toronto, Ontario), PO, q12h for 10 d but showed only slight improvement. The dog was then treated with furosemide (Lasix; Aventis Pharma, Laval, Quebec), 2 mg/kg body weight (BW), PO, q12h for 5 d, and theophylline (Theo-dur; Astra Pharma Inc., Mississauga, Ontario), 20 mg/kg BW, PO, q12h for 7 d, then q24h for 4 d, but it did not respond to these therapies. Cephalexin (Novolexin; Novopharm, Toronto, Ontario), 250 mg, PO, q12h for 5 d, and prednisone (Prednisone; Novopharm), 5 mg, PO, q12h for 5 d, then q24h for 5 d, and then q48h thereafter were also administered. The dog's condition became progressively worse, despite the therapy prescribed. The possibility of lungworm infection was considered, based on thoracic radiographs that showed a diffuse interstitial pattern. A fecal sample was submitted for examination. Numerous first-stage larva of A. vasorum were recovered from feces by the Baermann technique. Larva of A. vasorum measured 345.1 ± 16.1 μm in length (range, 322.8 to 368.6 μm) and the tail had a severe kink and a dorsal spine (Figure 1). A few larva of Crenosoma vulpis were also recovered from the fecal sample. The C. vulpis larva were about 300 μm in length; the tails had a slight bend but lacked a severe kink and a dorsal spine.
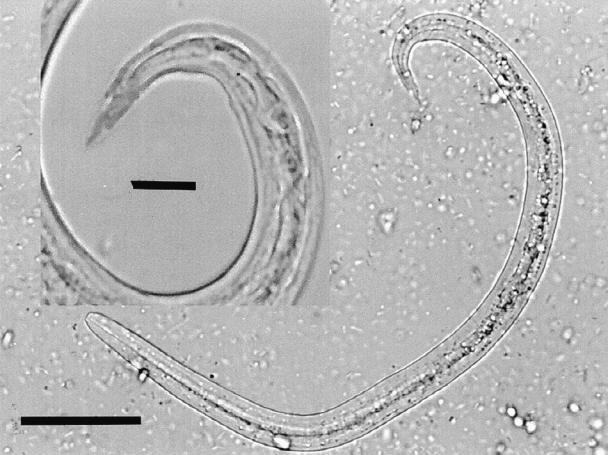
Figure 1. Composite micrograph of a first-stage larva of A. vasorum recovered from feces using the Baermann technique. The insert shows the larval tail at a higher magnification. Note the kinked tail and the distinctive dorsal spine. Bar = 50 μm; insert bar = 12.5 μm.
The dog was treated with ivermectin (Ivomec; Merial Canada, Baie D'urfé, Quebec) 0.2 mg/kg BW SC; 2 doses separated by 1 wk. On follow-up examination at 14 d posttreatment, the feces remained positive for A. vasorum. The course of ivermectin treatment (2 more treatments) was repeated. No posttreatment complications were observed and feces were negative on examination 2 wk posttreatment. Although the clinical signs had greatly improved, the dog continued to suffer from a chronic cough and was placed on prednisone. The dog was euthanized 14 mo after the initial date of diagnosis due to right-sided congestive heart failure. Permission for postmortem examination was not given by the owners.
Fecal samples from 2 other dogs in the household (6-year-old female Brittany spaniel and a 2-year-old male beagle) were examined by the Baermann technique. Both dogs were asymptomatic. The male beagle was positive for A. vasorum and the Brittany spaniel for C. vulpis. Both dogs were treated with ivermectin (0.2 mg/kg BW, SC; a single dose to treat C. vulpis; 2 doses, 1 wk apart, to treat A. vasorum) without any clinical complications. Posttreatment fecal examinations were not done on these dogs due to the lack of clinical signs and the disinterest of the owner.
A 7 month-old, female, mixed breed dog (case 2) was submitted for postmortem examination. The owner of the dog lived in the eastern part of the Avalon peninsula of Newfoundland (Ferryland) and the dog had no history of travel outside the province. The dog had appeared healthy in the morning but later that day was seen frothing at the mouth, exhibited nuchal rigidity, and died soon after. The dog had been free to roam the neighborhood. The owner reported that a number of dogs in the immediate area had died and poisoning was suspected. Samples of lung, liver, spleen, brain, stomach, kidney, and heart were fixed in phosphate-buffered 10% formalin and submitted for histologic examination. Stomach and intestinal contents were submitted for organochlorine and organophosphate screening. The entire lung and carcass were frozen and later submitted for further examination.
Grossly, the lungs contained many firm nodules that were particularly prominent near the apical borders of all lung lobes. Fourteen nematodes, approximately 1.5 to 2 cm in length, were collected from pulmonary arteries; their dark intestines intertwined with the white reproductive tissue, which gave them a “barber pole” appearance, similar to that of Haemonchus spp.
Microscopically, the lungs contained numerous, multifocal to coalescing pyogranulomas composed of epitheliod macrophages, multinucleated giant cells, and neutrophils. The center of these inflammatory nodules frequently contained the remnants of parasite eggs and, occasionally, larva. Pyogranulomas replaced large areas of pulmonary parenchyma. Lymphocytes and plasma cells were present at the periphery of the granulomas and fibroblastic proliferation was also apparent. Large adult nematodes were seen in medium-sized pulmonary arteries (Figure 2). Occasionally, organized and often recanalized thrombi were present within and partially occluded medium-sized arteries. Adjacent alveoli contained foamy macrophages and large numbers of eosinophils were occasionally present. Less affected areas of lung exhibited moderate congestion and edema.

Figure 2. Cross-section of a pulmonary artery from a 7-month-old, female, mixed breed dog. The vessel contains an adult nematode, later identified as Angiostrongylus vasorum. Bar = 100 μm, hematoxylin and eosin.
The meninges contained multifocal, small clusters of eosinophils, neutrophils, macrophages, lymphocytes, and plasma cells. Several small granulomas, which often contained nematode eggs, were scattered throughout the neural parenchyma of the cerebral cortex. Perivascular cuffing with mononuclear inflammatory cells was often present adjacent to cerebral granulomas.
Moderate numbers of variably sized granulomas were scattered throughout the renal cortex. Glomerular tufts were mildly thickened. The superficial mucosa and deep lamina propria of the stomach had several prominent clusters of mononuclear inflammatory cells admixed with moderate numbers of eosinophils. Occasionally, focal granulomas were observed within the myocardium. No identifiable parasite eggs or larva were seen in sections of kidney, stomach, or heart.
The stomach contents were analyzed and an organophosphate insecticide and pesticide called sulfatep was identified (quantification was not performed). The nematodes recovered from the pulmonary artery were identified as A. vasorum based on host, organ location, and morphology of the male bursa and spicules. The cause of death was attributed to acute toxicity due to organophosphate poisoning. The severe, multifocal to coalescing, interstitial pneumonia with intralesional nematode larva and multifocal, granulomatous meningoencephalitis with nematode eggs was attributed to parasitic infection. The pulmonary lesions described are typical of those found in dogs infected with A. vasorum (6). The multifocal granulomatous lesions found in the stomach, kidney, and myocardium may have been the result of aberrant nematode egg or larva deposition.
Canine angiostrongylosis has been reported extensively in endemic regions of Europe, Africa, and South America, but autochthonous infection has not been described previously in domestic dogs in North America. Angiostrongylus vasorum infection in Canada has been reported previously in a dog imported from Ireland, where transmission most likely occurred (5). These 2 cases represent the first reported endemic infections in dogs in Canada. Canids acquire A. vasorum infections by the ingestion of terrestrial gastropods (slugs and snails) or frog paratenic hosts (1,7). Larva are digested free of gastropod or frog tissue, penetrate the canine intestinal wall, and migrate to the heart and arteries by way of the lymphatics, portal circulation, liver, and caudal vena cava. Immature worms become established in pulmonary arteries as early as 10 d postinfection. The worms mature, mate, and produce undifferentiated eggs, which lodge in pulmonary capillaries. The eggs develop and release first-stage larva (L1), which break out into the airspace, are subsequently coughed up, swallowed, and passed with the feces about 45 to 57 d postinfection. Dogs, and presumably foxes, are infected and shed larva intermittently in the feces for the remainder of their lives. Dogs have been reported to shed as many as 280 000 larva/g feces (8). Antemortem diagnosis is by fecal examination to detect larva by using the Baermann technique or (less reliably) by standard fecal flotation. There are no anthelmintics approved for use in treating dogs with angiostrongylosis. Infected dogs have been treated successfully with fenbendazole (20 mg/kg BW, PO, q24h or q12h for 2 to 3 wk) or ivermectin (0.2 mg/kg BW, SC, repeated in 1 wk) (1). Potential posttreatment complications include severe dyspnea and ascites (cor pulmonale), which may need to be managed by administering diuretics, bronchodilators, and expectorants. Marked hypovolemic shock, resembling an anaphylactic reaction, attributed to the rapid killing effects of levamisole has also been reported as a potential posttreatment complication in a dog (9).
The disease syndrome observed in the antemortem case was typical of that reported for A. vasorum infection in dogs. Chronic cough, anorexia, weight-loss, exercise intolerance, dyspnea, and gagging are the most commonly observed clinical signs of angiostrongylosis (1,4,5,8). Bleeding tendencies (usually seen as subcutaneous hematomas), ascites, vomiting, syncope, signs of central nervous system disease, or sudden death have also been reported (4,5,8,10,11). Case 1 also illustrates the potential pathogenic nature of the parasite in that the dog was left with significant permanent cardiopulmonary damage after treatment that eventually resulted in euthanasia of the animal. The earliest possible diagnosis and treatment would likely improve the prognosis in dogs with angiostrongylosis. This dog was also infected with the lungworm C. vulpis. The role C. vulpis may have played in contributing to the clinical signs observed is unknown. Nonfatal chronic respiratory disease characterized primarily by a cough has been reported in dogs with lungworm infection (12). A recent survey of dogs in Atlantic Canada with a chronic cough indicated that C. vulpis was a causative agent in 24% of the cases (13).
The most common pathological changes reported in clinical cases of angiostrongylosis in dogs include interstitial pneumonia, pulmonary consolidation, and fibrosis; congestive heart failure; and coagulopathy. The presence of adults, larva, and eggs in many organ systems, including the central nervous system and the eye, have been reported (5,11,14). The presence of larva within blood vessels, in the lungs, and elsewhere appears to irritate the endothelium and stimulate intravascular coagulation, resulting in arterial thrombosis and inflammation. This vascular response has been hypothesized to lead to a consumptive coagulopathy, which, in a few reported cases, was thought to lead to the development of disseminated intravascular coagulation (10).
The presence of nematode eggs within the brain of the dog in the postmortem case suggests that eggs gained access to the systemic circulation via venous drainage of the lung to the left heart. Aberrant larval migration and subsequent egg production could not be definitively ruled out, but adult nematodes were not observed outside the lung. Although not a common manifestation of infection, disseminated angiostrongylosis has been described (5). Aberrant migration of parasite larva (larva that do not travel simply from the intestinal tract to the pulmonary artery and heart) via systemic circulation has been a commonly proposed pathogenesis for the presence of parasites in the brain, eye, liver, spleen, and even within femoral arteries (11).
The fox is reported to be less severely affected by infection than the dog, unless there is concurrent disease (sarcoptic mange) (15). In these cases, affected foxes tended to have larger worm burdens and more severe lesions in the lungs. A parasitologic survey of wild fox in a region of Denmark known to be an endemic focus for A. vasorum found a high prevalence of low-grade infections among wild foxes, suggesting a considerable degree of adaptation between the parasite and host (16). However, if animals were to be immunocompromised due to factors such as concurrent infection or starvation, host control of the parasite may be inadequate, resulting in parasite proliferation and significant disease. Red fox, as natural definitive hosts, serve as important reservoirs for the parasite and have epidemiological importance, particularly in areas where there is close coexistence of foxes and dogs.
When or how A. vasorum was introduced into Newfoundland and its distribution within the province remains unknown. Both cases occurred in the eastern region of the Avalon peninsula. Further studies are needed to determine the extent to which it occurs in the province. A great concern is the potential for the spread of A. vasorum from Newfoundland to the rest of Atlantic Canada. Any region containing the biological and climactic factors conducive to the transmission of C. vulpis would also be expected to favor that of A. vasorum, as they share similar intermediate and reservoir hosts. Given the volume and frequency of travel between Newfoundland and the rest of Atlantic Canada, the introduction of A. vasorum to the mainland appears likely to be inevitable.
In conclusion, dogs demonstrating clinical signs of lung, or heart disease, or both, or signs of coagulopathy with a history of traveling or living in Newfoundland should have angiostrongylosis included on the list of differential diagnoses. Many of the same conditions present in Newfoundland (climate, intermediate hosts, etc) are present elsewhere in eastern Canada. The possibility of parasite establishment on the mainland through the movement of domestic dogs exists. CVJ
Footnotes
Address all correspondence and reprint requests to Dr. Bourque, e-mail: abourque@upei.ca.
References
- 1.Bolt G, Monrad J, Koch J, Jensen AL. Canine angiostrongylosis: a review. Vet Rec 1994;135:447–452. [DOI] [PubMed]
- 2.Conboy G. Canine angiostrongylosis (French Heartworm). In: Bowman DD, ed. Companion and Exotic Animal Parasitology. International Veterinary Information System (www.ivis.org), May, 2000.
- 3.Smith FR, Threlfall W. Helminths of some mammals from Newfoundland. Am Midld Nat 1973;90:215–218.
- 4.Williams JF, Lindemann B, Padgett GA, Smith OL. Angiostrongylosis in a greyhound. J Am Vet Med Assoc 1985;186: 1101–1103. [PubMed]
- 5.Perry AW, Hertling R, Kennedy MJ. Angiostrongylosis with disseminated larval infection associated with signs of ocular and nervous disease in an imported dog. Can Vet J 1991;32:430–431. [PMC free article] [PubMed]
- 6.Prestwood AK, Greene CE, Mahaffey EA, Burgess DE. Experimental canine angiostrongylosis: I. Pathologic manifestations. J Am Anim Hosp Assoc 1981;17:491–497.
- 7.Bolt G, Monrad J, Frandsen F, Henriksen P, Dietz HH. The common frog (Rana temporaria) as a potential paratenic and intermediate host for Angiostrongylus vasorum. Parasitol Res 1993;79: 428–430. [DOI] [PubMed]
- 8.Martin MWS, Ashton G, Simpson VR, Neal C. Angiostrongylosis in Cornwall: Clinical presentations of eight cases. J Small Anim Pract 1993;34:20–25.
- 9.Scand J, Bolt G. Hypovolaemic shock after anthelmintic treatment of canine angiostrongylosis. J Small Anim Pract 1996;37:594–596. [DOI] [PubMed]
- 10.Ramsey IK, Littlewood JD, Dunn JK, Herrtage ME. Role of chronic disseminated intravascular coagulation in a case of angiostrongylosis. Vet Rec 1996;138:360–363. [DOI] [PubMed]
- 11.Cury MC, Lima WS. Rupture of femoral artery in a dog infected with Angiostrongylus vasorum. Vet Parasitol 1996;65:313–315. [DOI] [PubMed]
- 12.Bihr T, Conboy GA. Lungworm (Crenosoma vulpis) infection in dogs on Prince Edward Island. Can Vet J 1999;40:555–559. [PMC free article] [PubMed]
- 13.Conboy GA. Canine crenosomosis in Atlantic Canada (abstract). Proc 45th Ann Meet Am Assoc Vet Parasitol, Salt Lake City, Utah, 2000;45:121.
- 14.King MCA, Grose RMR, Startup G. Angiostrongylus vasorum in the anterior chamber of a dog's eye. J Small Anim Pract 1994;35: 326–328.
- 15.Simpson VR. Angiostrongylus vasorum infection in foxes (Vulpes vulpes) in Cornwall. Vet Rec 1996;139:443–445. [DOI] [PubMed]
- 16.Bolt G, Monrad J, Henriksen P, et al. The fox (Vulpes vulpes) as a reservoir for canine angiostrongylosis in Denmark. Acta Vet Scand 1992;33:357–362. [DOI] [PMC free article] [PubMed]


