Abstract
Background/Aims
Our study aimed to assess 1) the temporal trends in incidence and mortality of liver cancer and 2) age-period-cohort effects on the incidence in Canada.
Methods
We analyzed data obtained from the Canadian Cancer Registry Database and Canadian Vital Statistics Death Database. We first examined temporal trends by sex, age group, and birth cohort between 1972 and 2006. Three–year period rates and annual percentage change (APC) were calculated to compare the changes over the study period. We used age-period-cohort modelling to estimate underlying effects on the observed trends in incidence.
Results
The overall age-adjusted incidence rates increased from 2.6 and 1.5 per 100 000 in 1972–74 to 6.5 (APC: 2.9) and 2.2 (APC: 1.2) per 100 000 in 2004–06 among males and females, respectively. The age-adjusted mortality rates increased from 3.3 and 2.0 per 100 000 in 1972–74 to 6.0 (APC: 2.3) and 2.6 (APC: 1.2) per 100 000 in 2004–06 among males and females, respectively. The incidence increased most rapidly in men aged 45–54 years (APC: 4.1) and women aged 65–74 years (APC: 1.7) over the period of study.
Conclusions
The age-period-cohort analysis suggests that birth-cohort effect is underlying the increase in incidence. While the exact reason for the increased incidence of liver cancer remains unknown, reported increase in HBV and HCV infections, and immigration from high-risk regions of the world may be important factors.
Keywords: Incidence of liver cancer, immigration, HBV and HCV, age-period-cohort model
Introduction
Liver cancer is the sixth most common cancer worldwide and the third most common cause of cancer mortality (1). Although the incidence of liver cancer is low in North America and Europe, it is one of the few with an increasing incidence (1)-(3). The overall incidence to mortality ratio is near 1 (4). Porgnosis is very poor, with a 5-year relative survival rate of only 18% among Canadians diagnosed between 2004 and 2006 (5). As a result, the proportion of liver cancer death in Canada has increased from 2.1% (ranked 14th) of all cancer deaths in 2000 to 2.6% (ranked 9th) in 2007. Men are more vulnerable to developing liver cancer than women, with male to female ratios between 2:1 and 4:1 (1),(3),(6).
Liver cancer has several subtypes, including hepatocellular carcinoma (HCC), cholangiocarcinoma, hepatoblastoma, and angiosarcoma. HCC accounts for between 85% and 90% of all liver cancers, while most of the remaining liver cancers are cholangiocarcinoma (6),(7). Major risk factors for liver cancer include hepatitis B virus (HBV) and hepatitis C virus (HCV) infections and alcohol abuse, which together may be responsible for up to 90% of incident cases (6)-(8),(9).
In this study, we 1) analysed the temporal trends in incidence of and mortality due to liver cancer in Canada from 1972 to 2006; 2) examined the changes in incidence by age at diagnosis, time period and birth cohort; and used age-period-cohort modelling to assess the potential underlying effects on the incidence.
Materials and Methods
We obtained incidence data files for 1972–91 from the National Cancer Incidence Reporting System (NCIRS) and for 1992–2006 from the Canadian Cancer Registry (CCR), and mortality data for 1972–2006 from the Canadian Vital Statistics Death Database. The Health Statistics Division of Statistics Canada maintains the data used in this study, and the databases are considered to be very accurate and reliable. (A detailed description of the registry, including data sources, methodology and accuracy, is available on the Statistics Canada website (10) and elsewhere (11).) A very small percentage of incidence cases and deaths were excluded due to their unknown age. All the incidence records were converted to codes used in International Classification of Diseases, Ninth Revision (ICD–9) or International Classification of Diseases for Oncology, Third Edition (ICD–O–3) (12). To assess cause of mortality, we used codes from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD–10) (13) for deaths since 2000. The mortality data included liver unspecified cases because the coding for liver cancer changed slightly (14)-(16).
To examine the trends of liver cancer over the period of study, we used the codes ICD–9 155, ICD–O–3 C22 and ICD–10 C22 for liver cancer. First, we contrasted the average 3–year age-adjusted incidence and mortality rates for the period 1972–74 with that for 2004–06 for men and women separately. We then compared the incidence and mortality rates for five specific age groups that is 35–44, 45–54, 55–64, 65–74 and 75–84 years. We evaluated secular trends in the incidence and mortality of liver cancer through linear regression models using logarithms of the annual rates for all ages as well as for the five age groups. Correspondingly, the annual percent changes (APC) during the study period were derived from the regression coefficients of those models. All age-adjusted incidence and mortality rates were calculated using 1991 Canadian population serving as the standard.
Analyses integrating age at diagnosis, time period of diagnosis and birth cohort were conducted separately for men and women. We grouped age at diagnosis into 5-year intervals (35–39 years to 80–84 years) and categorized the period of diagnosis into 5-year intervals from 1972 through 2006 (1972–76 to 2002–06). Corresponding to these age intervals and time periods, 16 overlapping 10-year birth cohorts (1888–97 to 1963–72) were derived for the age-period-cohort analysis of the incidence. We thus computed and plotted the age-specific incidence rates for all the 16 birth cohorts. A Poisson regression model was used to estimate the age, period and cohort effects; the model assumes that the number of incident cases follows a Poisson distribution and that the incidence rates are a multiplicative function of the included model parameters, making the logarithm of the rates an additive function of the parameters (17)-(19). For example, the form of the age-period-cohort model was given by
| log(dij/pij) = µ + αi + βj + γk |
where log (dij/pij) is the rate of interest with dij denoting the number of the cases in the ith age group and jth period and pij is the population at risk in the ith age group and jth period; αi is the effect of the ith age group; βj is the effect of jth period category; and γk is the effect of the kth cohort category (k = I – i + j when I = 1, 2,…, I). Inherent in the three-factor age-period-cohort model is the well-known non-identifiability problem: parameters for age, period and cohort can not be uniquely estimated because of the exact linear dependence of the regression variables (cohort = period − age) (20),(21). Although there are several methods that can deal with the non-identifiability problem, there is no consensus in the literature as to which method is optimal. Hence, we selected two-factor models to calculate the relative risk as the log of regression coefficients by adjusting for the other factor. To test the effect of birth cohort and period of diagnosis individually after controlling for the effect of age, we compared respective two-factor models with the full model.
Parameters of the models were estimated by means of the maximum likelihood method with SAS procedure GENMOD (release SAS Enterprise Guide 4, SAS Institute Inc.). The goodness-of-fit of models was evaluated using the deviance, defined to be twice the difference between the maximum achievable log likelihood and the log likelihood at the maximum likelihood estimates of the regression parameters. Specific effects (e.g., period effects) were tested by comparing the difference in deviance between models with and without a term for the effect.
Results
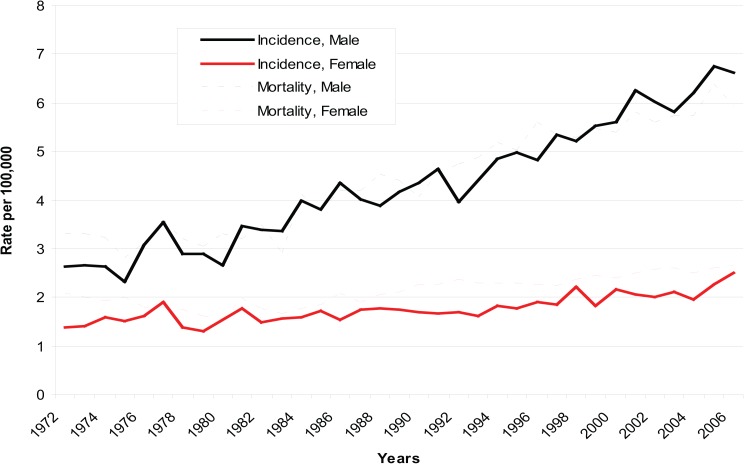
A total of 29 489 cases of liver cancer—19 859 (67.3%) in men and 9630 (32.7%) in women—were registered, and 31 568 deaths from liver cancer were reported in Canada between 1972 and 2006. The mortality rate exceeded the incidence rate among females and also in some years among males. The annual age–adjusted incidence rate increased by 145% for men (from 2.64 per 100 000 in 1972–74 to 6.46 per 100 000 in 2004–06) and by 52% for women (from 1.46 per 100 000 in 1972–74 to 2.22 per 100 000 in 2004–06). Mortality rates showed a similar increase, with the annual age–adjusted rate increasing by 84% (from 3.28 per 100 000 in 1972–74 to 6.02 per 100 000 in 2004–06) for men and 29% (from 2.01 per 100 000 in 1972–74 to 2.59 per 100 000 in 2004–06) for women. This trend appears more marked among men than women, especially since the early 1990s (Figure 1).
Figure 1. Age-adjusted Incidence and Mortality Rates of Liver Cancer by Gender, 1972-2006, Canada.
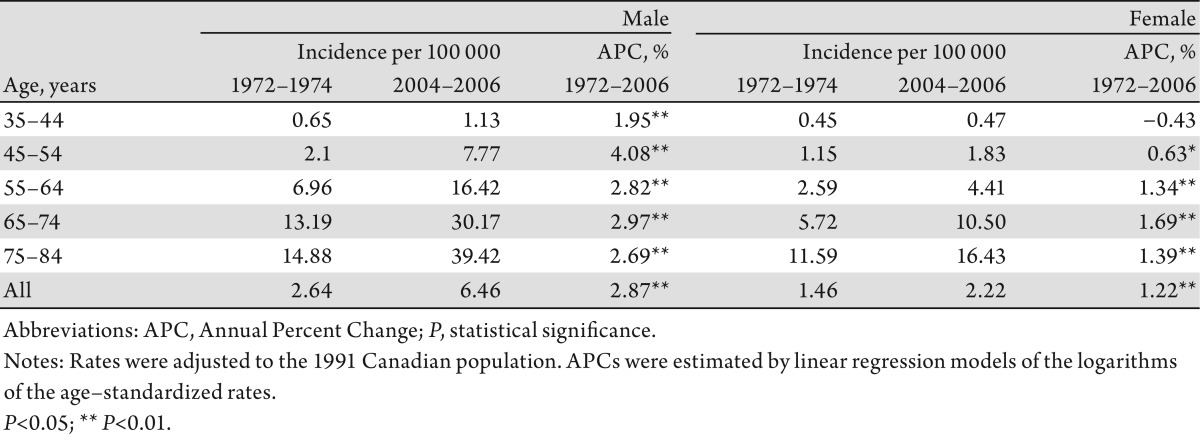
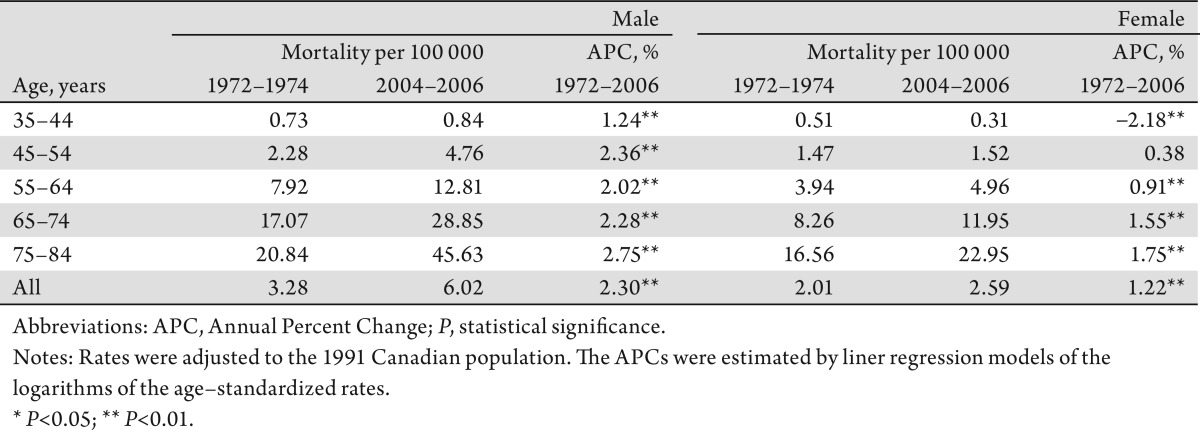
The increase in overall incidence rates of liver cancer among men was larger than that among women, with an APC of 2.9% and 1.2%, respectively. Among the respective age groups, men aged 45–54 years experienced the most rapid increase in incidence (APC: 4.1%), while women aged 65–74 years had the highest increase (APC: 1.7%) (Table1). The increase in mortality among men was higher than that among women (APC: 2.3% vs. 1.2%). Men aged 75–84 years had the most rapid increase (APC: 2.8%). Women aged 35–44 years of age had a statistically significant decrease (APC: −2.2%) over the study period, however (Table 2).
Table 1. Age–specific Incidence Rate of Liver Cancer (per 100 000 population) and Annual Percent Change, Canada, 1972–1974 to 2004–2006.
Table 2. Age–specific Mortality Rate of Liver Cancer (per 100 000 population) and Annual Percent Change, Canada, 1972–1974 to 2004–2006.
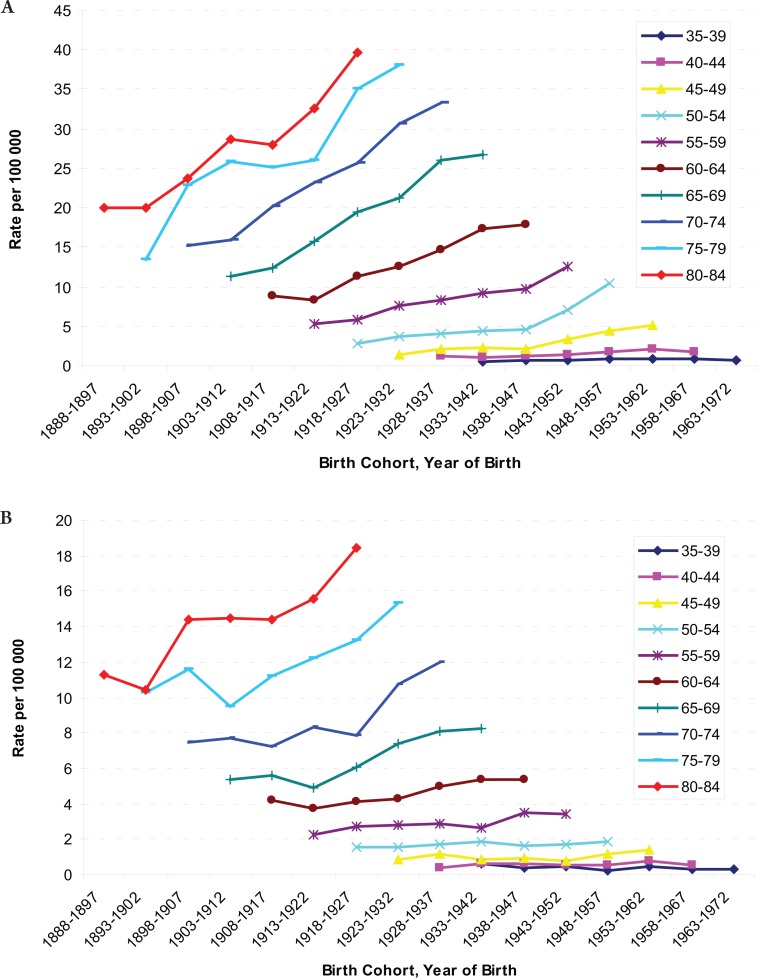
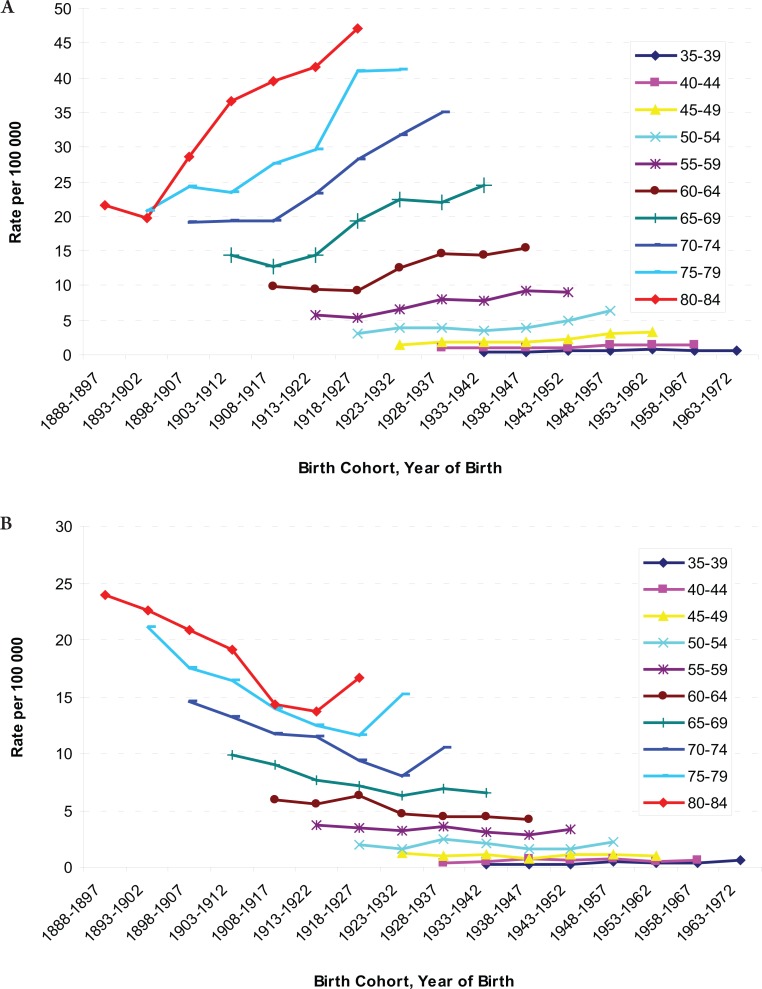
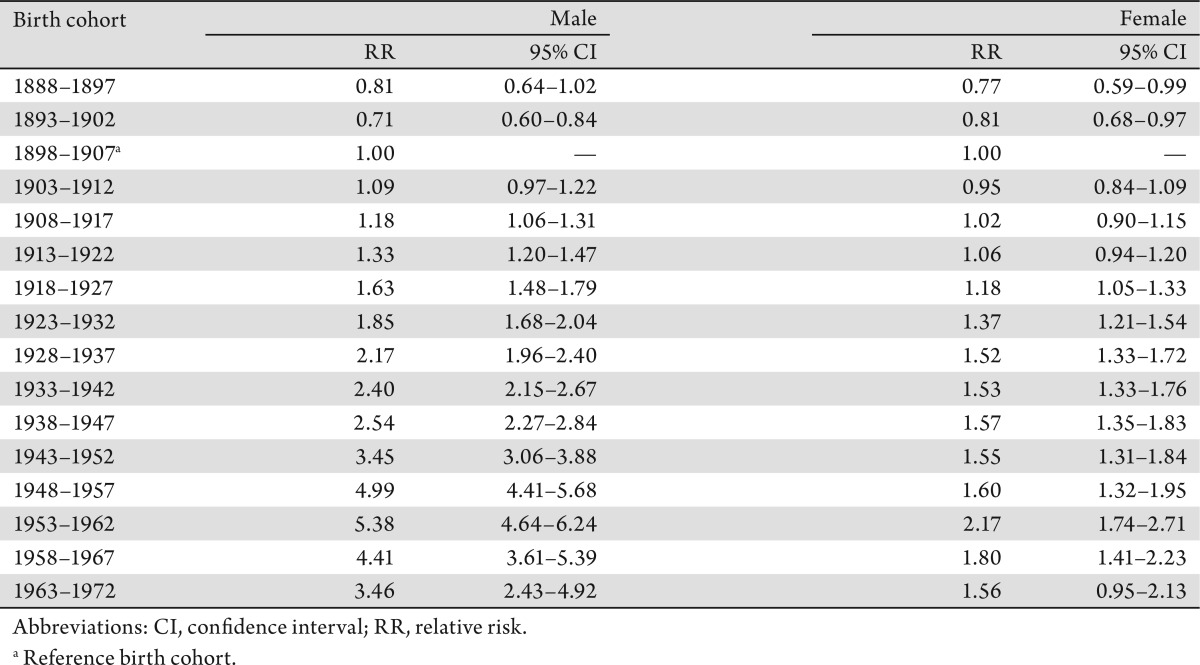
The age-specific incidence and mortality rates by birth cohort were plotted in Figure 2 and 3. The incidences increased as the birth cohort advanced, with more substantial increases in later birth cohorts for both men and women (Figure 2a and b). The highest mortality rates in aged 80–84 years among men, but a decreasing mortality rate in later birth cohorts in women were observed (Figure 3a and b). The results of fitting age-period-cohort models to the data are summarized (Table 3). The birth cohort effect was statistically significant among men and women; the period effect was statistically significant among women only. Further, comparison of the age-period model with the full age-period-cohort model showed an improvement, suggesting that the birth-cohort effect was stronger than the period effect among both men and women. Therefore, we selected the age-cohort model to estimate the relative risk of developing liver cancer. With the incidence rate in birth cohort 1898–1907 used as the reference, risk of diagnosis with liver cancer increased in subsequent birth cohorts. For example, the probability of the birth cohort of 1953–1962 being diagnosed with liver cancer was over five times as high for men and two times as high for women compared with the reference birth cohort of 1898–1907 (Table 4).
Figure 2. A: Age-specific Incidence Rates of Liver Cancer by Birth Cohort among Males, from 1888 through 1897 to 1963 through 1972; B: Age-specific Incidence Rate of Liver Cancer by Birth Cohort among Females, from 1888 through 1897 to 1963 through 1972.
Figure 3. A: Age-specific Mortality Rates of Liver Cancer by Birth Cohort among Males, from 1888 through 1897 to 1963 through 1972; B: Age-specific Mortality Rates of Liver Cancer by Birth Cohort among Females, from 1888 through 1897 to 1963 through 1972.
Table 3. Comparison and evaluation of age–period–cohort modelling of the incidence of liver cancer for age 35–84 years, 1972–2006.
Table 4. Relative Risks of Incidence of Liver Cancer in Canadians Aged 35–84 Years, 1972–2006, According to Age–Cohort Modeling.
Discussion
Our data showed that the overall age-adjusted incidence and mortality rates of liver cancer have increased substantially since the early 1970s for both men and women in Canada. The increases were 145% among men and 52% among women for incidence of liver cancer, and 84% among men and 29% among women for mortality from liver cancer between 1972–74 and 2004–06. A limitation of the disease coding is that mortality data includes liver, unspecified cases (14)-(16). Our age-period-birth cohort modelling of the data suggests that birth-cohort effect might have played an important role in the increase in liver cancer incidence, although time-period effect could also be involved. Our results are largely consistent with the reports from Britain, Italy and the United States (1),(2),(22)-(24). Thus this modeling indicates that increased exposures to risk factors over time might be responsible for the increasing incidence of liver cancer in Canada. The underlying causes could include: 1) change or increase in related conditions such as HBV and HCV infections and in other risk factors; 2) increase in immigrant population from high-risk areas such as Asia and Africa; 3) advances in diagnostic technology and completeness of registration of cases; and 4) increases in prevalence of obesity and diabetes mellitus among Canadians.
Epidemiological studies found that recent immigrants from HBV–endemic areas and their descendants were at high risk of chronic HBV infection and of HBV–related liver cancer (25)-(27). Immigration from high-risk areas of hepatitis B infection, drug abuse and needle sharing, blood transfusion of unscreened blood or blood products, and unsafe sexual practices in the 1960s and 1970s have been associated with an increase in the HBV- and HCV- related liver cancer (9),(23),(28),(29).
The total number of individuals born outside of Canada reached 6.2 million, 19.8% of the total population in 2006 (31). Canadian census data show an increase in immigration from Africa and Asia made up about 17% of the foreign-born population in 1981, increasing to 28% in 1996 and 42% in 2001. Concurrently, immigrants from Europe made up a decreasing proportion of the foreign-born population, beginning at 67% in 1981 and dropping to 55% in 1996 and then 42% in 2001 (28). Immigrants from high-risk areas for HBV infection showed elevated rates of several diseases including liver cancer (27),(29). The highest annual percentage change (APC) among 45–65 men liver cancer could be influenced by immigration from high-risk areas of hepatitis B infection. Previous epidemiological studies associated an increase in immigrants from high-risk areas with the rise in incidence and mortality of liver cancer in Canada (30),(32). Analysis by cultural background and region of birth revealed a high incidence of and mortality due to liver cancer for immigrants from certain specific regions. Chen et al. found that the risk of liver cancer was associated with a high proportion of immigration to the province of Ontario (28). Luo et al. examined the incidence of cancer among Chinese immigrants in Alberta and found that the overall cancer incidence was lower among immigrants, but the incidence rates of liver cancer were much higher (16.7/100 000) than that among Canadian-born residents (1.7/100 000) of Alberta (32). The increased incidence rates of liver cancer observed in those studies were likely to be associated with the high prevalence of HBV and HCV infections among high-risk groups. Immigrants might have acquired such infection before coming to Canada. One study found increased risk among immigrants from South–Eastern Asia infected with biliary liver flukes where consumption of raw fresh-water fish is a cultural practice. Biliary liver fluke has an infrequent cause of infection which the potential long-term consequences of chronic infection are highly associated with cholangiocarcinoma (33). Liver cancer is more prevalent in men than in women worldwide (1),(2). We observed a male to female ratio of around 2:1 for liver cancer incidence and mortality in Canada. We also observed that the increasing trends of incidence and mortality of liver cancer among men started at 45 years of age. The reasons for higher rates of liver cancer in males may be due to sex-specific differences in exposure to risk factors (27). Further, epidemiological studies have indicated that males are more sensitive to the effect of HBV infection than females. Wang et al. found that there was a greater risk difference between hepatitis B surface antigen carriers and noncarriers among males than among females, and that males had a significant synergistic effect for the interaction between sex and HBV infection on liver cancer mortality (34). A case-control study by Yu et al. found an inverse relation between exposure to estrogens and liver cancer, suggesting that estrogens may provide a protective effect against liver cancer (35). Naugler et al. found that estrogen-mediated inhibition of interleukin-6 production by Kupffer cells reduced the risk of liver cancer in females (36).
Heavy consumption of alcohol is a well-known risk factor for liver cancer. Donato et al. found a positive linear relation between the risk of hepatocellular carcinoma (HCC) and alcohol intake, although these was no substantial relative risk differences between men and women (37). Further, risk of liver cancer is thought to be affected by synergistic interactions between HBV or HCV infection and alcohol (38). A systematic review of epidemiological evidence concluded that HBV infection, HCV infection and alcohol intake are major causes of HCC worldwide, and the three main risk factors together account for approximately 85% of the total HCC cases (39). Boffetta et al. found that DNA damage occurred after heavy alcohol consumption, and alcohol-associated liver cirrhosis was the most important risk factor for HCC in populations with low prevalence of infection from HBV and HCV, such as in the United States and northern Europe (40). Heavy alcohol consumption may cause DNA damage by reducing intake of nutrition; this could also explain the synergistic effect of alcohol and HBV and HCV infections (41). In addition, other studies also reported a strong association between obesity and liver cancer, although the mechanisms for this association remain unknown (42),(43). The metabolic abnormalities related to excess weight include high plasma triglyceride, glucose and insulin levels, as well as insulin resistance.
Period effect identified by our age-period-cohort regression, though statistically significant only in women, is very likely due to an overall improvement in the quality of cancer registration data that took place in the 1970s and early 1980s in most Canadian provinces/territories, especially Quebec (21),(44). Changes to cancer diagnostic criteria and registration methodology over that period were already documented by the Canadian Cancer Registry; however, its impact on the cancer trends was too small to be quantified by earlier studies (11),(44). In addition, the slight decrease in relative risks in the two most recent birth cohorts (Table 4) may indirectly indicate a likelihood that risks for liver cancer attributable to exposure to the risk factors identified above have yet to appear in younger generations.
In summary, substantial increases in incidence and mortality from liver cancer have occurred over the last 3 decades, with increases in rates among men over twice that for women. The underlying birth-cohort pattern observed for liver cancer incidence in the Canadian population suggests an increasing proportion of immigrants from high-risk regions, an increased prevalence of persons infected with HBV and HCV, and increasing rates of obesity are responsible for the increasing risk of developing liver cancer. Direct epidemiological evidence, however, is needed, as other yet unrecognized etiologic factors may remain to be identified.
Acknowledgments
We are grateful to Statistics Canada for access to the data provided to Public Health Agency of Canada. The cooperation of the provincial and territorial cancer registries that supply the data to Statistics Canada is gratefully acknowledged. The authors particularly thank Robert Semenciw and Larry Ellison for their critical review of the manuscript.
Footnotes
No potential conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Tsao L, Hsing AW, Devesa SS, Fraumeni JF., Jr International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–6. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 3.Pocobelli G, Cook LS, Brant R, Lee SS. Hepatocellular carcinoma incidence trends in Canada: analysis by birth cohort and period of diagnosis. Liver Int. 2008;28:1272–9. doi: 10.1111/j.1478-3231.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Ellison LF, Wilkins K. An update on cancer survival. Health Rep. 2010;21:55–60. [PubMed] [Google Scholar]
- 6.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 7.McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3–23. doi: 10.1016/j.bpg.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.ElSaadany S, Giulivi A. Epidemiology of hepatocellular carcinoma in Canada, 1995: analysis of death certificates. Chronic Dis Can. 2006;27:125–9. [PubMed] [Google Scholar]
- 10.Statistics Canada Canadian Cancer Registry[Internet] Health Statistics Division. 1992[cited 2011 February]. Available from: http://www.statcan.gc.ca/cgi-bin/imdb/p2SV.pl?Function=getSurvey&SDDS=3207&land=en&db=imdb&adm=8&dis=2.
- 11.Gaudette L, Lee J, Silberberger C. Cancer: incidence and deaths in Canada, 1990. Health Rep. 1993;5:348–54. [PubMed] [Google Scholar]
- 12.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology. 3rd edition. Geneva: World Health Organization. :2005. [Google Scholar]
- 13.Slee V. Geneva: World Health Organization; 1992. International statistical classification of diseases and related health Problems, 10th Revision (ICD-10) [Google Scholar]
- 14.World Health Organization . Geneva: WHO; 1967. International Classification of Diseases, Eighth Revision (ICD-8) [Google Scholar]
- 15.World Health Organization . Geneva: WHO; 1977. International Classification of Diseases, Ninth Revision (ICD-9) [Google Scholar]
- 16.World Health Organization . Geneva: WHO; 1992. International Classification of Diseases, Tenth Revision (ICD-10) [Google Scholar]
- 17.Clayton D, Schifflers E. Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med. 1987;6:449–67. doi: 10.1002/sim.4780060405. [DOI] [PubMed] [Google Scholar]
- 18.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med. 1987;6:469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 19.Holford TR. Approaches to fitting age-period-cohort models with unequal intervals. Stat Med. 2006;25:977–93. doi: 10.1002/sim.2253. [DOI] [PubMed] [Google Scholar]
- 20.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–57. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Semenciw R, Waters C, Wen SW, Mery LS, Mao Y. Clues to the aetiological heterogeneity of testicular seminomas and non-seminomas: time trends and age-period-cohort effects. Int J Epidemiol. 2000;29:826–31. doi: 10.1093/ije/29.5.826. [DOI] [PubMed] [Google Scholar]
- 22.Dal Maso L, Lise M, Zambon P, Crocetti E, Serraino D, Ricceri F, et al. Incidence of primary liver cancer in Italy between 1988 and 2002: an age-period-cohort analysis. Eur J Cancer. 2008;44:285–92. doi: 10.1016/j.ejca.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 23.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 24.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751–8. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hislop TG, Teh C, Low A, Li L, Tu SP, Yasui Y, et al. Hepatitis B knowledge, testing and vaccination levels in Chinese immigrants to British Columbia, Canada. Can J Public Health. 2007;98:125–9. doi: 10.1007/BF03404323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor VM, Tu SP, Woodall E, Acorda E, Chen H, Choe J, et al. Hepatitis B knowledge and practices among Chinese immigrants to the United States. Asian Pac J Cancer Prev. 2006;7:313–7. [PubMed] [Google Scholar]
- 27.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Yi Q, Mao Y. Cluster of liver cancer and immigration: a geographic analysis of incidence data for Ontario 1998-2002. Int J Health Geogr. 2008;7:28. doi: 10.1186/1476-072X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.McDermott S, Desmeules M, Lewis R, Gold J, Payne J, Lafrance B, et al. Cancer incidence among Canadian immigrants, 1980-1998: results from a national cohort study. J Immigr Minor Health. 2011;13:15–26. doi: 10.1007/s10903-010-9347-3. [DOI] [PubMed] [Google Scholar]
- 31.Statistics Canada[Internet] Population by immigrant status and period of immigration, 2006 counts, for Canada, provinces and territories - 20% sample data[modified 2009 March 27; cited 2011 February] Available from: http://www12.statcan.ca/census-recensement/2006/dp-pd/hlt/97-557/T403-eng.cfm?Lang=E&T=403&GH=4&SC=1&S=99&O=A.
- 32.Luo W, Birkett NJ, Ugnat AM, Mao Y. Cancer incidence patterns among Chinese immigrant populations in Alberta. J Immigr Health. 2004;6:41–8. doi: 10.1023/B:JOIH.0000014641.68476.2d. [DOI] [PubMed] [Google Scholar]
- 33.Stauffer WM, Sellman JS, Walker PF. Biliary liver flukes (Opisthorchiasis and Clonorchiasis) in immigrants in the United States: often subtle and diagnosed years after arrival. J Travel Med. 2004;11:157–9. [PubMed] [Google Scholar]
- 34.Wang N, Zheng Y, Yu X, Lin W, Chen Y, Jiang Q. Sex-modified effect of hepatitis B virus infection on mortality from primary liver cancer. Am J Epidemiol. 2009;169:990–5. doi: 10.1093/aje/kwn418. [DOI] [PubMed] [Google Scholar]
- 35.Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, et al. Hormonal markers and hepatitis B virus-related hepatocellular carcinoma risk: a nested case-control study among men. J Natl Cancer Inst. 2001;93:1644–51. doi: 10.1093/jnci/93.21.1644. [DOI] [PubMed] [Google Scholar]
- 36.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 37.Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 38.Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Ikematsu H, Noguchi A, et al. An epidemiologic study of effects of alcohol in the liver in hepatitis B surface antigen carriers. Am J Epidemiol. 1988;128:277–84. doi: 10.1093/oxfordjournals.aje.a114968. [DOI] [PubMed] [Google Scholar]
- 39.Alcohol drinking. Biological data relevant to the evaluation of carcinogenic risk to humans. IARC Monogr Eval Carcinog Risks Hum. 1988;44:101–52. [PMC free article] [PubMed] [Google Scholar]
- 40.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–56. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 41.Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia C, et al. The impact of obesity and diabetes mellitus on the risk of hepatocellular carcinoma. Ann Oncol. 2009;20:353–7. doi: 10.1093/annonc/mdn565. [DOI] [PubMed] [Google Scholar]
- 42.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol. 2005;42:218–24. doi: 10.1016/j.jhep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y, Canadian Cancer Registries Epidemiology Research Group Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159:259–68. doi: 10.1093/aje/kwh041. [DOI] [PubMed] [Google Scholar]
- 44.Gaudette LA, Lee J. Ottawa: Statistics Canada Marketing Department, Minister of Industry; 1997. Cancer incidence in Canada, 1969-1993. [Google Scholar]