Abstract
Aims
There are limited data on isoniazid (INH) pharmacokinetics in infants and young children and, therefore, uncertainty on appropriate dosing.
Methods
Pharmacokinetic data were obtained from perinatally HIV-exposed South African infants ages 3–24 months receiving INH 10–20 mg/kg/day orally for Mycobacterium tuberculosis (TB) prophylaxis. INH pharmacokinetic parameters were characterized with a population pharmacokinetic approach. Dosing simulations were performed to evaluate weight-based INH doses in children based on N-acetyltransferase 2 enzyme (NAT2) genotype, age, maximum concentrations (Cmax) ≥ 3mg/L, and area under the curve (AUC0-24) ≥ 10.52 mg*hr/L.
Results
In 151 infants (53% female, 48% HIV positive) receiving a mean INH dose of 14.5 mg/kg/day, mean (±SD) Cmax at 3, 6, and 23 months of age were 10.0 (3.5), 8.6 (2.6), and 9.3 (3.8) mg/L, respectively, mean (±SD) AUC0-24 were 53.6 (26.8), 42 (19.9), and 44 (30.7) mg*hr/L, respectively, and mean (±SD) half-life were 2.1 (0.7), 1.9 (0.6), and 1.8 (0.9) hours, respectively. A trimodal apparent oral clearance of INH as a function of NAT2 genotype was apparent as early as 3 months. INH was well tolerated. At an average INH dose of 14.5 mg/kg/day, 99% of infants ages 3–24 months have an INH Cmax ≥ 3 mg/L and 98% have an INH AUC0-24 ≥ 10.52 mg*hr/L.
Conclusions
INH at an average dose of 14.5 mg/kg once daily was well tolerated in infants and achieved INH Cmax values ≥ 3 mg/L and AUC0-24 values ≥ 10.52 mg*hr/L.
Keywords: isoniazid, pharmacokinetics, dosing, infants, children
Introduction
Tuberculosis (TB) is a leading cause of death in children including those with HIV infection and the third most commonly identified pathogen in children dying from acute severe pneumonia.(1) In children exposed to Mycobacterium tuberculosis (MTB), progression to active TB is most likely to develop in the very young (less than 4 years) and those who are immunocompromised.(1) Thus, treatment and prevention of TB is of critical importance in the pediatric population.
Isonicotinyl hydrazine or isoniazid (INH) has been the mainstay of TB treatment and prevention in both adults and children. When given orally in the fasted state, INH is rapidly absorbed with the maximum concentration (Cmax) occurring 1–2 hours post-dose. Food delays and decreases the extent of absorption. Acetylation by the N-acetyltransferase 2 (NAT2) enzyme is a major pathway for the metabolism of INH.(2) NAT2 is polymorphic and persons are classified as slow, intermediate, or fast acetylators. Distribution of acetylator phenotype varies by race and geographic region.(3) INH half-life is approximately 70 minutes vs. 2–5 hours in fast and slow acetylators, respectively.(4) The primary side effects associated with INH are peripheral neuropathy and hepatotoxicity (acute liver cell necrosis). Slow acetylators have a greater risk for peripheral neuropathy and hepatotoxicity from INH(4) and fast acetylators have decreased early bactericidal INH activity relative to slow acetylators(5). INH pharmacokinetic-pharmacodynamic relationships are not well-identified and there are no data on INH pharmacokinetic-dynamic relationships in the setting of TB prevention.
IMPAACT P1041 was a prospective, multicenter, randomized, double-blind, placebo controlled trial of INH 10–20 mg/kg once daily for the prevention of TB disease and latent infection in South African infants born to women with HIV infection.(6) A pharmacokinetic substudy was performed as part of IMPAACT P1041. Our objectives were to describe the clinical pharmacokinetics of INH in these infants, explore pharmacokinetic-dynamic relationships, and use this information in dosing simulations to guide INH dosing in infants and children.
Materials and Methods
Subjects and Pharmacologic Evaluations
Infants were randomized at 91–120 days of life to INH 10–20 mg/kg orally once daily or placebo. For dosing, 100 mg INH tablets (or matching placebo; Be-Tabs Pharmaceuticals (Pty) Ltd., Republic of South Africa) were crushed, mixed in warm water, and administered with an oral syringe 30 minutes prior to or 2 hours following a feeding. A complete description of study subjects and enrollment criteria is provided in the parent study.(6) One hundred sixty eight HIV-infected and 168 HIV-exposed, uninfected infants from Cape Town and Durban were invited to participate in a substudy of INH pharmacokinetics. Infants’ primary caregivers provided informed consent. The study protocol was approved by the Medicines Control Council in South Africa and the Division of AIDS at the NIH. Infants were randomized to one of two sampling groups and one of two sampling arms: Group 1 was sampled at first dose (week 0) and week 84 and Group 2 was sampled at weeks 12 and 84. Infants were sampled at either 1 and 3 hours (Arm A) or 2 and 4 hours (Arm B) post-observed dose. INH quantification in plasma, and subject classification to fast, intermediate or slow INH acetylation were done as described.(7–9)
A population pharmacokinetic model was developed and validated (NONMEM vVI) using the available INH concentration data. The development of the population model has been described in a separate publication.(9) Modeling methods were developed to account for the influence of NAT2 genotype and enzyme maturation on INH apparent oral clearance (CL/F) in infants; four factors were found to be associated with INH CL/F using this model: NAT2 genotype, enzyme maturation, size (body weight), and relative bioavailability changes. Subsequently, we obtained estimates of each individual infant’s CL/F and volume of distribution (V/F) at each study week using this model. From these values, each individual infant’s elimination half-life, steady-state area under the concentration time curve from 0 to 24 hours (AUC0-24), Cmax, and time to Cmax (Tmax) were determined.
Pharmacokinetic-pharmacodynamic relationships
Predictors of clinical outcomes (TB and adverse events) were examined using generalized estimating equations in the (geepack package of R, version 2.13).(10, 11) Cases of definite, probable, and possible TB were combined for analyses. Definite TB was defined as isolation of Mycobacterium tuberculosis from any site. Probable TB was defined as a positive acid fast bacilli stain plus either two clinical algorithm criteria(6) or at least one abnormal chest x-ray feature suggestive of TB. Possible TB was defined as chest x-ray findings suggestive of pulmonary TB and either a score >=6 on a TB clinical screening algorithm or a reactive tuberculin skin test. The association between INH pharmacokinetic characteristics and the following two adverse events was determined: liver function tests greater than or equal to five times the upper limit of normal (i.e., grade 2 or higher) and symptoms of peripheral neuropathy of grade 2 or higher according to the 1994 Division of AIDS Toxicity Table for Grading Severity of Pediatric (>3 months of age) Adverse Experiences.(12) Given the exploratory nature of these analyses, no adjustments were made for multiple comparisons.
INH Dosing Simulations
Two types of dosing simulations were performed. The first simulation sought to determine the percentage of infants and children 3–24 months who would have an INH Cmax < 3 mg/L and an AUC0-24 < 10.52 mg*hr/L at a dose of 14.5 mg/kg/day if 1000 infants had been sampled at each age group in this study. The second simulation sought to determine the INH doses needed to achieve Cmax ≥ 3 mg/L and AUC0-24 ≥ 10.52 mg*hr/L in 95% of children ages 3–60 months. An INH Cmax of greater than 3 mg/L has been previously proposed as a response-linked pharmacokinetic parameter.(13–15) INH AUC has also been linked with TB killing. Donald et al. pooled data obtained from patients in the same geographic area where this study was performed to determine the INH AUC associated with 90% of the maximal killing of metabolically active bacilli present in the sputum during the first two days of TB treatment (EB90). EB90 was reached at an INH AUC0-∞ of 10.52 mg*hr/L.(16) Wilkins et al. recently used this AUC0-24 target of 10.52 mg*hr/L in dosing simulations using current guideline-recommended INH doses in adults.(17) Steady-state INH Cmax and AUC0-24 values were simulated for a population of infants and children 3–60 months receiving various INH doses (mg/kg). A range of possible body weights were simulated for each age period using the 50th percentiles for body weight on a pediatric growth chart.(18) Mean body weights used in dosing simulations at 3, 6, 9, 12, 15, 18, 24, 36, 48, and 60 months of age were 5.9, 7.5, 9.0, 9.8, 10.7, 11.4, 12.4, 14.0, 16.0, and 18.0 kg, respectively. The coefficient of variation for the mean body weight for each age group in the dosing simulations was 17%, which was consistent with the range at each age group in the IMPAACT P1041 pharmacokinetic substudy. Cmax and AUC0-24 were simulated for 1,000 children at each age group.
Results
Subjects
168 HIV-uninfected and 169 HIV-infected infants were enrolled in the pharmacokinetic substudy. The mean (±SD) INH dose was 14.5 (+/− 2.8) mg/kg/day. 368 INH concentrations from 151 infants receiving active INH (80 female, 73 HIV-infected) were used to develop the population pharmacokinetic model.(9) Some infants were lost to follow-up, while others did not undergo a second pharmacokinetic sampling due to premature closure of study. Forty-eight (32%), 67 (44%), and 36 (24%) infants were slow, intermediate, and fast NAT2 acetylators. Of the 73 infants who were HIV-1 seropositive, 13 and 28 were on antiretroviral therapy at the first and second pharmacokinetic sampling, respectively. The majority received stavudine, lamivudine plus lopinavir/ritonavir; other regimens included zidovudine, lamivudine plus lopinavir/ritonavir, zidovudine, lamivudine plus nevirapine, and stavudine, lamivudine, plus ritonavir.
INH Pharmacokinetics and Pharmacodynamics
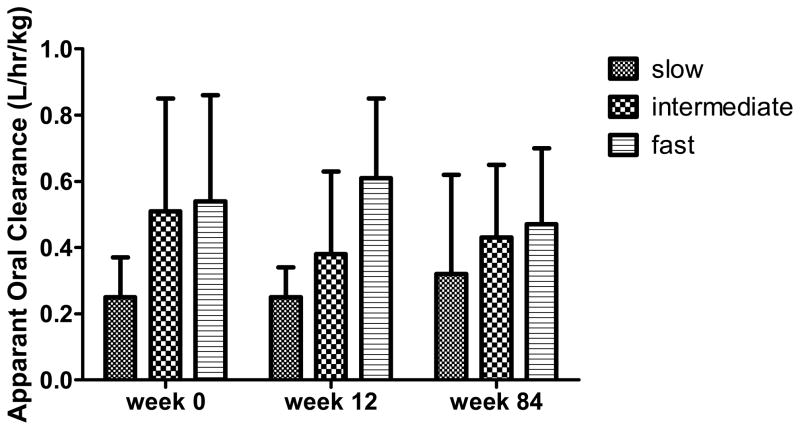
Concentration data used to develop the population pharmacokinetic model are shown in Figure 1. The INH pharmacokinetic parameters in these infants by study week and NAT2 genotype are shown in Table 1. There was a clear separation of INH CL/F by NAT2 genotype (Figure 2), apparent at all 3 study visits.
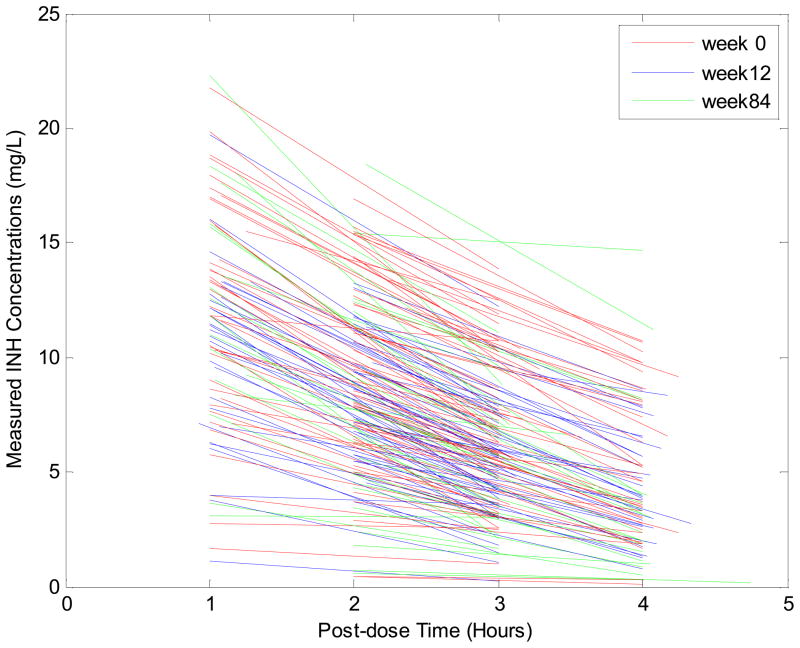
Figure 1.
INH Concentration data by time post-dose and visit week (red=week 0, blue=week 12, and green=week 84) in 151 South African Infants receiving isoniazid. 62 infants were sampled at first dose (week 0), 48 at week 12, 7 at week 84, 19 at both weeks 0 and 84, and 15 at both weeks 12 and 84. Three infants were sampled later than week 84 (1 at 96 weeks, 2 at 120 weeks). Mean (SD) concentrations at 1, 2, 3, and 4 hours post dose were 11.55 (4.55), 8.52 (3.95), 5.94 (2.98), and 4.58 (3.08) mg/L, respectively.
Table 1.
Isoniazid Pharmacokinetics in 151 South African Infants Receiving Isoniazid for M. Tuberculosis Prophylaxis
| Week 0 | Week 12 | Week 84 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (months) Median (Range) | 3.34 (3.03–4.04) | 6.23 (5.6–6.9) | 22.97 (21.6–33.47) | ||||||
| Weight (kg) Median (Range) | 5.8 (3.5–8.3) | 7.5 (4.5–10.4) | 11.6 (8.4–18.1) | ||||||
| NAT2 Genotype | Slow | Intermediate | Fast | Slow | Intermediate | Fast | Slow | Intermediate | Fast |
| CL/F (L/hr) Mean (SD) | 1.4 (0.55) | 2.9 (2.1) | 3.5 (2.05) | 1.85 (0.6) | 2.86 (1.87) | 4.3 (1.7) | 3.9 (3.6) | 4.7 (2.8) | 6.1 (3.1) |
| CL/F (L/hr/kg) Mean (SD) | 0.25 (0.12) | 0.51 (0.34) | 0.54 (0.32) | 0.25 (0.09) | 0.38 (0.25) | 0.61 (0.24) | 0.32 (0.3) | 0.43 (0.22) | 0.47 (0.23) |
| V/F (L) Mean (SD) | 5.4 (1.36) | 6.7 (2.9) | 6.74 (2.87) | 7.35 (1.8) | 6.9 (2.63) | 6.8 (1.8) | 13.6 (8.8) | 9.8 (4.0) | 9.6 (4.2) |
| AUC0-24 (mg*hr/L) Mean (SD) | 71.6 (25.8) | 42.5 (19.3) | 37.8 (20.5) | 61.4 (20.7) | 41.1 (15.4) | 25.3 (9.8) | 74.7 (49) | 36 (15.5) | 34 (16.3) |
| Cmax (mg/L) Mean (SD) | 11.2 (3.36) | 9.09 (3.29) | 9.16 (3.88) | 9.7 (2.54) | 8.78 (2.67) | 7.2 (2.2) | 10.2 (5.3) | 8.7 (3.1) | 9.7 (3.7) |
| Tmax (hr) Mean (SD) | 2.09 (0.09) | 2.09 (0.08) | 2.06 (0.28) | 1.79 (0.11) | 1.8 (0.09) | 1.7 (0.1) | 1.67 (0.11) | 1.54 (0.08) | 1.54 (0.1) |
| T1/2 (hr) Mean (SD) | 2.83 (0.41) | 1.77 (0.31) | 1.45 (0.28) | 2.8 (0.35) | 1.8 (0.25) | 1.15 (1.17) | 3.14 (0.99) | 1.5 (0.26) | 1.2 (0.2) |
Figure 2.
Mean (SD) weight-adjusted INH CL/F by NAT2 genotype (as shown in Table 1) at weeks 0, 12, and 84
There were three cases of definite TB, two of probable TB, 10 of possible TB, and two of latent TB among the infants in the pharmacokinetic substudy. Ten infants in the pharmacokinetic substudy died, three of who had possible TB. Ten infants had AST elevations (9, grade 2; 1, grade 3) and 16 infants had ALT elevations (9, grade 2; 5, grade 3; and 2, grade 4). Thirty nine infants had peripheral neuropathy (38, grade 2; 1, grade 4).
INH AUC0-24 and Cmax did not significantly predict the development of TB (p= 0.737 and p=0.294, respectively) or death (p=0.193 and p=0.508, respectively) in these infants. HIV infected (n=73; 48%) infants had 1.62 times the odds of having grade 2 or higher symptoms of peripheral neuropathy (p=0.0002) compared with HIV uninfected children (n=78; 52%). After adjustment for HIV status, INH AUC0-24 (p=0.453) and Cmax (p=0.328) did not significantly predict symptoms of peripheral neuropathy. INH Cmax significantly (p=0.05) predicted the development of liver enzyme elevations, whereas INH AUC0-24 did not (p=0.122). The median (IQR) INH Cmax was 11.8 mg/L (8.9–13.6) vs.8.7 (7.0–11.3) mg/L in those infants with vs. without ALT elevations greater than or equal to five times the upper limit of normal.
Simulations
For the first dosing simulation, the percentage of subjects who would have an INH Cmax < 3 mg/L and AUC0-24 < 10.52 mg*hr/L in a simulated population of 1000 infants at each age group receiving 14.5 mg/kg/d sampled at each of the three time periods (weeks 0, 12, and 84) are shown in Table 2. At a dose of 14.5 mg/kg/day, less than 1% of infants would have an INH Cmax < 3 mg/L and less than 2% would have an INH AUC0-24 < 10.52 mg*hr/L.
Table 2.
Percentages of Cmax < 3 mg/L and AUC0-24 < 10.52 mg*hr/L by NAT2 genotype for infants receiving 14.5 mg/kg/d. One thousand infants were simulated at each study visit.
| week 0 | week 12 | week 84 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| slow | intermediate | fast | slow | intermediate | fast | slow | intermediate | fast | |
| Cmax < 3 mg/L | 0 | 0.2% | 0.7% | 0 | 0 | 0.2% | 0 | 0 | 0 |
| AUC0-24 < 10.52 mg*hr/L | 0% | 0.2% | 1.5% | 0% | 0% | 1% | 0% | 0% | 1.5% |
For the second simulation, the INH dosing simulations found that the doses needed to attain Cmax ≥ 3 mg/L in 95% of infants and children aged 3 months to 5 years ranged from 5.7–10.8 mg/kg/day (Table 3) and the doses needed to attain an AUC0-24 ≥ 10.52 mg*hr/L in 95% ranged from 3.1–11.1 mg/kg/day (Table 4) depending on age and NAT2 genotype.
Table 3.
INH Doses (mg/kg) needed to achieve Cmax ≥ 3 mg/L in 95% of infants and young children based on age. One thousand infants/young children were simulated at each age group. Slow, intermediate, and fast represent acetylation phenotype based on NAT2 genotype.
| Age (month) | Mean Body Weight (kg) | INH daily dose (mg/kg) | ||
|---|---|---|---|---|
| Slow | Intermediate | Fast | ||
| 3 | 5.9 | 8.5 | 9.7 | 10.8 |
| 6 | 7.5 | 7.2 | 8.5 | 9.8 |
| 9 | 9.0 | 6.7 | 8.0 | 9.5 |
| 12 | 9.8 | 6.5 | 7.9 | 9.4 |
| 15 | 10.7 | 6.3 | 7.8 | 9.3 |
| 18 | 11.4 | 6.2 | 7.8 | 9.2 |
| 24 | 12.4 | 6.1 | 7.8 | 9.1 |
| 36 | 14.0 | 6.0 | 7.8 | 8.9 |
| 48 | 16.0 | 5.8 | 7.8 | 8.8 |
| 60 | 18.0 | 5.7 | 7.8 | 8.6 |
Table 4.
INH Doses (mg/kg) needed to achieve AUC0-24 ≥ 10.52 mg*hr/L in 95% of infants and young children based on age. One thousand infants/young children were simulated at each age group. Slow, intermediate, and fast represent acetylation phenotype based on NAT2 genotype.
| Age (month) | Mean Body Weight (kg) | INH daily dose (mg/kg) | ||
|---|---|---|---|---|
| Slow | Intermediate | Fast | ||
| 3 | 5.9 | 5.6 | 8.3 | 10.9 |
| 6 | 7.5 | 4.6 | 7.4 | 10.9 |
| 9 | 9.0 | 4.1 | 7.1 | 10.9 |
| 12 | 9.8 | 3.9 | 7.2 | 11.0 |
| 15 | 10.7 | 3.7 | 7.3 | 11.1 |
| 18 | 11.4 | 3.7 | 7.3 | 11.1 |
| 24 | 12.4 | 3.5 | 7.4 | 10.9 |
| 36 | 14.0 | 3.4 | 7.7 | 10.6 |
| 48 | 16.0 | 3.3 | 7.9 | 10.3 |
| 60 | 18.0 | 3.1 | 7.9 | 10.0 |
Discussion
The pharmacokinetics of INH were characterized in 151 South African infants ages 3–24 months receiving INH 10–20 mg/kg orally once daily for TB prevention. We found this dose of INH was well tolerated and that 99% of infants had an INH Cmax ≥ 3 mg/L and 98% had an INH AUC0-24 ≥ 10.52 mg*hr/L. This substudy of IMPAACT P1041 represents the largest dataset to date of INH pharmacokinetics in infants and young children and the first study of INH pharmacokinetics in a substantial number of infants.
At a dose of 10–20 mg/kg once daily, INH concentrations in these infants were similar to older children (median age 3.8 years) receiving 10 mg/kg once daily (8) and adults receiving 5–10 mg/kg once daily (19). Weight-adjusted CL/F values in adults range from 0.18–0.42 L/hr/kg.(20) In these infants, the mean weight-adjusted CL/F in fast acetylators exceeded adult values at weeks 0, 12 and 84 and intermediate acetylators exceeded adult values at weeks 0 and 84. The weight-adjusted CL/F observed in this study is similar to that reported by Rey et al. in 34 young children (27 of which were greater than 4 months old).(21)
IMPAACT P1041 was closed to accrual in June 2008 following an interim data safety monitoring board review that found the primary outcome rates (TB disease/death) did not differ by study treatment arm (INH or placebo) in either the 548 HIV-infected or 804 HIV-exposed uninfected infants.(6) If INH Cmax and a value ≥ 3 mg/L and/or AUC0-24 and a value ≥ 10.52 mg*hr/L is the clinically relevant pharmacokinetic parameter(s) for predicting efficacy of INH, then the pharmacokinetic findings in this study do not provide an explanation for the failure of INH to reduce the primary outcomes of TB disease/death. In fact, dosing simulations predict a slightly lower dose than used in this study would produce Cmax ≥ 3 mg/L and AUC0-24 ≥ 10.52 mg*hr/L in 95% of children ages 3 months to 5 years (Tables 3 and 4). These simulations indicate dosing requirements may actually decrease with age in the majority of children. The decrease in dosing requirements may be due to a decrease in the liver mass to body mass ratio as children grow.(23) However, the decrease in dosing requirements is less pronounced in the intermediate and fast acetylators indicating a contribution of fast allele maturation to the CL/F of INH in these children. The final pharmacokinetic model found that the relative bioavailability of INH increased during the course of the study from 0.72 at 3 months to 0.95 at 24 months. The increase in INH relative bioavailability may possibly be due to improved caregiver adherence with medication administration over time, dose retention with increasing age, and/or changes in enzyme activity and gastrointestinal tract environment during the first two years of life. Additionally, INH absorption and therefore bioavailability is reduced in the presence of food, and the increased relative bioavailability may be related to improved separation of dose administration from feeding of the infant. The increase in INH relative bioavailability will contribute to changing dose requirements over time.
A study of 263 South African children, including from one of the centers where our study was undertaken, reported that INH at 8–12 mg/kg/day was effective in preventing TB in children (median age 24.7 months)(22), which also argues against suboptimal concentrations as an explanation for the study findings.
Our Cmax dosing simulations are consistent with the clinical findings of McIlleron et al.(15) In a study of 56 South African children (median age 3.22 years) receiving treatment for TB, 21/30 (70%) of those on a dose of 4–6 mg/kg had an INH Cmax < 3 mg/L, whereas 17/17 (100%) on INH doses of 8–12 mg/kg had an INH Cmax > 3 mg/L. The data from the present study indicate that INH doses of 5.7 to 10.8 mg/kg based on NAT2 genotype and age would produce Cmax ≥ 3 mg/L in 95% of children ages 3 months to 5 years.
There are limitations to our study. We chose a sparse sampling population pharmacokinetic approach because conducting intensive pharmacokinetic studies in infants over time would be expensive and require frequent blood draws. Thus, individual AUC0-24 and Cmax values were estimated from our population pharmacokinetic model and may have some imprecision.(9) However, the estimated pharmacokinetic parameters of INH in these infants are consistent with older children and adults indicating little bias in our parameters with the use of this minimally invasive strategy. We were limited in our ability to investigate pharmacokinetic-dynamic relationships for INH since there were very few cases of TB and adverse events in these young children. Additional limitations relate to the dosing simulations and pharmacodynamic relationships. First, dosing requirements were simulated for children ages 3 months to 5 years (Tables 3 and 4) using our population pharmacokinetic model, but it should be appreciated the data used to develop these simulations were based on concentrations collected in infants and children through a median of 2 years of age. The AUC0-24 and Cmax targets used in our dosing simulations are potential concentration targets for INH in the setting of TB treatment; it is unknown if these are appropriate targets for TB prevention.
At the time of IMPAACT P1041 initiation, TB treatment and prevention guidelines differed in their INH dosing recommendations for infants and children. South African guidelines recommended a dose of 5 mg/kg daily.(24) These guidelines were updated in 2008 and the INH dose for TB prevention increased to 10–15 mg/kg daily.(25) The WHO increased the INH dose recommendation to between 10 and 15 mg/kg daily in September 2009, as recommended by the CDC.(26) In this pharmacokinetic study, we found an average INH dose of 14.5 mg/kg/day was well tolerated and achieved desired Cmax and AUC targets in 98% of infants. These data provide strong support that the INH dose of 5 mg/kg/day (as recommended by the South African guidelines and WHO at the time P1041 was initiated) is likely to be subtherapeutic.
Conclusion
The pharmacokinetic data for INH in these infants and children demonstrated a dose of 10–20 mg/kg/day orally was well tolerated and that more than 98% of infants achieved Cmax and AUC values above those considered therapeutic, based on the available knowledge of INH exposure-response relationships. Therefore, these pharmacokinetic data do not provide an explanation for the lack of prevention of TB disease and latent infection observed in IMPAACT P1041 and motivate investigations of other reasons for the lack of benefit. These pharmacokinetic data do indicate thatfuture studies of INH for TB prevention (or treatment) in infants and children should ensure the dosing regimen accounts for the effects of and changes in age, weight and maturation of NAT2 acetylation and perhaps individual NAT2 genotype on INH pharmacokinetics. The dosing regimen should be designed to achieve concentrations above therapeutically-linked target values, which need further elucidation, in essentially all children.
Acknowledgments
Funding:
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement 5U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and 1U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C). This work was supported in part by grant NIH/NIBIB P41-EB001978 (DZD) and grant NIH/NIAID U01-AI068632 (CVF).
Dr. Charles Mitchell, protocol co-chair; Drs. S. Nachman and A. Violari, protocol vice-chairs; S. Kim, protocol statistician; and the entire P1041 protocol team. We gratefully acknowledge all of the sites that conducted this study, particularly Tygerberg Children’s Hospital at the Stellenbosch University, Tygerberg, South Africa and the University of Kwazulu-Natal. We are indebted to all of the study subjects and their families who participated in this trial.
References
- 1.Swaminathan S, Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Suppl 3):S184–94. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 2.Weber WW, Hein DW. Clinical pharmacokinetics of isoniazid. Clinical pharmacokinetics. 1979;4(6):401–22. doi: 10.2165/00003088-197904060-00001. [DOI] [PubMed] [Google Scholar]
- 3.Walker K, Ginsberg G, Hattis D, et al. Genetic polymorphism in N-Acetyltransferase (NAT): Population distribution of NAT1 and NAT2 activity. Journal of toxicology and environmental health. 2009;12(5–6):440–72. doi: 10.1080/10937400903158383. [DOI] [PubMed] [Google Scholar]
- 4.Preziosi P. Isoniazid: metabolic aspects and toxicological correlates. Current drug metabolism. 2007;8(8):839–51. doi: 10.2174/138920007782798216. [DOI] [PubMed] [Google Scholar]
- 5.Donald PR, Sirgel FA, Venter A, et al. The influence of human N-acetyltransferase genotype on the early bactericidal activity of isoniazid. Clin Infect Dis. 2004;39(10):1425–30. doi: 10.1086/424999. [DOI] [PubMed] [Google Scholar]
- 6.Madhi SA, Nachman S, Violari A, et al. Primary isoniazid prophylaxis against tuberculosis in HIV-exposed children. The New England journal of medicine. 2011;365(1):21–31. doi: 10.1056/NEJMoa1011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seifart HI, Gent WL, Parkin DP, et al. High-performance liquid chromatographic determination of isoniazid, acetylisoniazid and hydrazine in biological fluids. Journal of chromatography. 1995;674(2):269–75. doi: 10.1016/0378-4347(96)82886-6. [DOI] [PubMed] [Google Scholar]
- 8.Schaaf HS, Parkin DP, Seifart HI, et al. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Archives of disease in childhood. 2005;90(6):614–8. doi: 10.1136/adc.2004.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu R, Kiser JJ, Mitchell C, et al. The Pharmacogenetics of NAT2 Enzyme Maturation in Perinatally HIV Exposed Infants Receiving Isoniazid. Journal of Clinical Pharmacology. 2012;52(4):511– 9. doi: 10.1177/0091270011402826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Fine J. Estimating equations for association structures. Statistics in medicine. 2004;23(6):859–74. doi: 10.1002/sim.1650. discussion 75–7,79–80. [DOI] [PubMed] [Google Scholar]
- 11.Hojsgaard S, Halekoh U, Yan J. The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software. 2005;15(2):1–11. [Google Scholar]
- 12.DIVISION OF AIDS (DAIDS) TABLE FOR GRADING SEVERITY OF PEDIATRIC (> 3 MONTHS OF AGE) ADVERSE EXPERIENCES. 1994 Apr; Available from: http://hsc.unm.edu/som/gcrc/ftp/ToxicityTables_Pediatric_Over3monthsAge.pdf.
- 13.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62(15):2169–83. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Chideya S, Winston CA, Peloquin CA, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48(12):1685–94. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIlleron H, Willemse M, Werely CJ, et al. Isoniazid plasma concentrations in a cohort of South African children with tuberculosis: implications for international pediatric dosing guidelines. Clin Infect Dis. 2009;48(11):1547–53. doi: 10.1086/598192. [DOI] [PubMed] [Google Scholar]
- 16.Donald PR, Parkin DP, Seifart HI, et al. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. European journal of clinical pharmacology. 2007;63(7):633–9. doi: 10.1007/s00228-007-0305-5. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins JJ, Langdon G, McIlleron H, et al. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br J Clin Pharmacol. 2011;72(1):51–62. doi: 10.1111/j.1365-2125.2011.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The National Center for Health Statistics and the National Center for Chronic Disease Prevention and Health Promotion. 2000 [cited 2010 June]; Available from: https://www.cdc.gov/growthcharts/
- 19.Parkin DP, Vandenplas S, Botha FJ, et al. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. American journal of respiratory and critical care medicine. 1997;155(5):1717–22. doi: 10.1164/ajrccm.155.5.9154882. [DOI] [PubMed] [Google Scholar]
- 20.Kergueris MF, Bourin M, Larousse C. Pharmacokinetics of isoniazid: influence of age. European journal of clinical pharmacology. 1986;30(3):335–40. doi: 10.1007/BF00541539. [DOI] [PubMed] [Google Scholar]
- 21.Rey E, Gendrel D, Treluyer JM, et al. Isoniazid pharmacokinetics in children according to acetylator phenotype. Fundamental & clinical pharmacology. 2001;15(5):355–9. doi: 10.1046/j.1472-8206.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 22.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ (Clinical research ed. 2007;334(7585):136. doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology--drug disposition, action, and therapy in infants and children. The New England journal of medicine. 2003;349(12):1157–67. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 24.National Tuberculosis Control Programme Practical Guidelines 2000. 2000 [cited 2010 December 11]; Available from: http://www.doh.gov.za/tb/index.html.
- 25.South African National Tuberculosis Guidelines 2008. 2008 [cited 2012 February 13]; Available from: http://hivfshealth.org/document/2011/03/14/national-tuberculosis-management-guidelines.
- 26.Treatment of Tuberculosis: American Thoracic Society, CDC, and Infectious Diseases Society of America. 2003 [cited 2010 December 11]; Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5211a1.htm.