Abstract
Williams-Beuren Syndrome (WBS) is caused by a submicroscopic deletion on chromosome 7q11.23 that encompasses the entire elastin (ELN) gene. Elastin, a key component of elastic fibers within the lung, is progressively destroyed in emphysema. Defects in the elastin gene have been associated with increased susceptibility towards developing chronic obstructive pulmonary disease (COPD) and emphysema in both humans and mice. We postulate that hemizygosity at the elastin gene locus may increase susceptibility towards the development of COPD and emphysema in subjects with WBS. We describe an adult subject with WBS who was a lifelong non-smoker and was found to have moderate emphysema. We also examined the pulmonary function of a separate cohort of adolescents and young adults with WBS. Although no significant spirometric abnormalities were identified, a significant proportion of subjects reported respiratory symptoms. Thus while significant obstructive disease does not appear to be common in relatively young adults with WBS, subclinical emphysema and lung disease may exist which possibly could worsen with advancing age. Further investigation may elucidate the pathogenesis of non-smoking related emphysema.
Keywords: Elastin, emphysema, pulmonary function tests, Williams Syndrome
Introduction
Williams-Beuren Syndrome (OMIM 194050) is a contiguous gene deletion disorder typically characterized by mild-to-moderate mental retardation, characteristic facial dysmorphology, and vascular abnormalities including supravalvular aortic stenosis (SVAS) [Ewart 1993]. The disorder is estimated to have a prevalence of 1 in 7500 [Stromme 2002]. The causative defect is a submicroscopic deletion at chromosome 7q11.23 approximately 1.5-1.8 Mb in size that includes the entire elastin (ELN) gene in 97-98% of cases [Del Campo 2006; Lowery 1995]. Though the deletion is usually the result of a de novo mutation [Urban 1996], transmission from parent to child in an autosomal dominant fashion has been documented [Morris 1993; Sadler 1993].
Elastin is a key component of elastic fibers within the lung. Emphysema, a subtype of chronic obstructive pulmonary disease (COPD), is characterized by progressive destruction and loss of elastic fibers [Shifren and Mecham 2006]. Imbalances between proteases and anti-proteases increase susceptibility towards developing COPD; the best described imbalance remains severe deficiency of α-1 antitrypsin, an antiprotease which acts against neutrophil elastase. Defects within the elastin gene may also contribute to COPD susceptibility - a functional mutation in the elastin gene has been found in two unrelated subjects with early-onset COPD [Cho 2009; Kelleher 2005]. Mutations in the elastin gene have also been described in cases of autosomal dominant cutis laxa (OMIM 123700) associated with severe, premature emphysema [Rodriguez-Revenga 2004; Urban 2005]. Mice deficient in elastin (Eln -/-) exhibit impaired terminal airway development which morphologically resembles emphysema [Wendel 2000].
Despite biological plausibility, respiratory impairment is not typically described in conjunction with Williams-Beuren Syndrome (WBS). To date, there has been no published report of pulmonary function testing in subjects with WBS; we hypothesized that WBS subjects would have increased risk for COPD due to hemizygosity at the ELN locus. In the following communication, we describe pulmonary function data from adolescents and young adults with WBS in conjunction with a separate adult patient who was a lifelong non-smoker and was found to have emphysema.
Methods
Study population at the Williams-Beuren Syndrome music camp
The protocol was approved by the Partners Healthcare institutional review board. The parents of teenagers and young adults attending a summer music camp for children with WBS at Belvoir Terrace in Lenox, MA in 2003 were sent a letter requesting their participation in the study. Sixteen camp participants enrolled in the study. Informed consent for participation and for review of the subjects’ medical records was obtained from the subjects and their parents or guardians. All camp participants reported a clinical diagnosis of WBS. Eleven subjects were personal patients of one of the authors (B.R.P.) and previously had ELN deletions confirmed by FISH analysis as part of their clinical care. We attempted to re-contact the five remaining subjects in whom the ELN gene deletions had not been confirmed; three additional subjects had ELN deletions confirmed by FISH analysis under the care of their private physicians.
Spirometry testing, questionnaire, and medical history
Spirometry testing was performed using a Collins Survey Tach spirometer. At least three maneuvers were attempted for each subject. Acceptability criteria for spirometry measurements included 1) back extrapolated volume <0.15L, 2) effort free from cough in the first second, 3) difference between two largest volumes for forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) achieved <200 mL [Miller 2005]. Spirometry results are expressed in terms of percent predicted values normalized for race, age, gender, and height [Hankinson 1999]. A questionnaire on basic medical history, smoking behavior, exposure to secondhand smoke, and respiratory symptoms was completed by the subject and his or her parent or guardian.
Reference population
Population based control subjects were drawn from a cohort from East Boston and Watertown, MA which has previously been described [Silverman 1998]. The analysis was restricted to subjects who had never smoked and were under the age of 40.
Case study
An adult patient with the diagnosis of WBS followed by two of the authors (B.R.P. and E.K.S.) was incidentally noted to have evidence of emphysema on chest computed tomography (CT). Non-contrast images were obtained using a Siemens Dual Source CT Scanner and were analyzed using 3D Slicer v2.8 (www.slicer.org). The diagnosis of WBS was confirmed by FISH analysis when the subject was in his 20’s. The patient and his mother/guardian provided consent to the review of his de-identified medical records and images for the purposes of this case study.
Results
Basic demographic information, respiratory symptoms, and spirometry results are summarized in Table 1. Of the 16 subjects who consented to the study, twelve were able to perform spirometry meeting reproducibility criteria for FEV1 measurements [Miller 2005]. While FEV1 measurements were of reasonable quality, most subjects were not able to sustain expiration ≥6 seconds or to reach a plateau on their volume-time curve – therefore analyses of FVC and FEV1/FVC ratio were not performed. All FEV1 measurements obtained were within the normal range. Despite a normal FEV1, a surprisingly high proportion of subjects were reported by their parents/guardians to have respiratory complaints such as wheezing and coughing. None of the participants had a previous diagnosis of COPD, emphysema, or chronic bronchitis by history (data not shown).
Table I.
Characteristics of adolescent and young adults with WBS
| Cases | Controls | |
|---|---|---|
| N | 16 | 22 |
| Age (range) | 20 * (15-27) | 27.6 (20-35) |
| Sex (% male) | 56.3 | 63.6 |
| Race (% white) | 93.8 | 100 |
| BMI (range) | 23.1 (16.2-41.6) | 25.9 (17.3-40.3) |
| Smoking (% ever) | 0 | 0 |
| Passive exposure to cigarette smoke | 12.5 | 13.6 |
| FEV1 % predicted | 101.3 (18.4) § | 92.2 (7.6) |
| Shortness of breath † | 50 * | 4.5 |
| Wheezing causing shortness of breath | 31.3 | 18.2 |
| Required medication for wheezing | 31.3 | 18.2 |
| Cough | 43.8 | 22.7 |
Data presented as mean (SD) or percent unless otherwise indicated
p-value <0.05 when compared to controls,
N=12 for FEV1 analysis
- Ever bothered by shortness of breath
- Shortness of breath walking up a hill
- Shortness of breath walking on the level
- Shortness of breath walking < 100 yards
- Shortness of breath with dressing or undressing
Case report
An adult patient with the diagnosis of WBS since childhood was incidentally noted to have moderate paraseptal emphysema on computed tomography during evaluation of unrelated symptoms (Figure 1). Quantitative analysis of the high resolution CT images revealed the 15th percentile value of the histogram to be -966.1 Hounsfield units (HU). Mean lung density was -892 HU and the lung volume by CT imaging was 5.01 liters. The percentage of emphysema in the upper, middle, and lower thirds of the lung was 25.8%, 26.4%, and 23% respectively at -950 HU – these values are substantially elevated when compared to a cohort of asymptomatic young adults (0.35%)[Irion 2009] and are suggestive of additional emphysema beyond the grossly evident paraseptal collections. The patient was 31 years old at time of diagnosis of emphysema, had been a lifelong non-smoker, and was subsequently found to have a normal alpha-1 antitrypsin level. Post-bronchodilator FEV1 was 3.15 liters (106% predicted), FVC was 4.01 liters (106% predicted), and FEV1/FVC ratio was 79 (92% predicted). Total lung capacity and DLCO were 117% and 100% of predicted, respectively, while inspiratory capacity was 2.94 liters (108% predicted) and residual volume was 1.43 liters (129% predicted). There was no significant spirometric response to inhaled bronchodilators. Clinically, he denied any respiratory complaints, was on no medications, worked full time as a clerk and denied any history of occupational exposures to respiratory irritants. Family history was negative for COPD or any other respiratory disease.
Figure I.
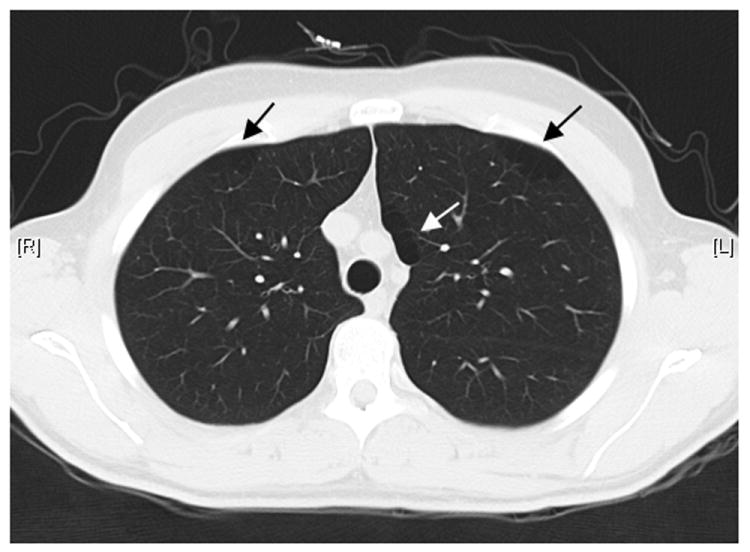
Paraseptal emphysema in a lifelong non-smoking patient with Williams-Beuren Syndrome.
Discussion
Respiratory disease in WBS has not been previously reported. Our study suggests that significant obstructive lung disease, as assessed by spirometry, is not typically seen in adolescents and young adults with WBS. However, this does not preclude the possibility of significant subclinical pulmonary disease, as illustrated by the finding of substantial emphysema in a non-smoking WBS patient.
Haploinsufficiency at the elastin locus, a deletion shared by nearly all patients with WBS, may predispose subjects towards premature development of emphysema. Possible mechanisms for this increased susceptibility may involve increased sensitivity to oxidative and mechanical stress due to differences in elastin gene dosage. This hypothesis is supported by murine models employing mice with a heterozygous deletion of the elastin gene (Eln +/-). While mice homozygous for the elastin gene deletion (Eln -/-) develop extensive changes in baseline lung architecture, heterozygous mice (Eln +/-) exhibit normal lung development and morphology despite having only approximately 45% of the lung elastin levels of wild type mice. However, when compared to wild type (Eln +/+) mice, heterozygous mice exhibit an augmented inflammatory response and are more susceptible to developing emphysema when exposed to cigarette smoke [Shifren 2007].
In the absence of a smoking history, other possible factors contributing to emphysema susceptibility may exist. In addition to increased sensitivity towards oxidative stress, reduced elastin levels in Eln +/- mouse lungs alter the mechanical properties of the lung [Shifren 2007]. Mechanical stress, in the absence of inflammation or oxidative stress, may contribute to the pathogenesis and propagation of emphysema [Brenner 1998; Suki 2003; West 1971]. Patients with WBS have also been purported to exhibit accelerated signs of aging such as premature cataracts, gray hair, and presbycusis [Cherniske 2004]. “Senile emphysema”, which is distinct from the normal aging lung [Verbeken 1992a; Verbeken 1992b], may be a part of the spectrum of premature aging in these individuals.
The lack of literature on pulmonary function and the development of emphysema in this population may reflect the general paucity of literature on adult WBS patients. Alternatively, because subjects with WBS often have other more salient systemic problems, pulmonary problems may not assume priority and may be underreported. Because many WBS patients have limited exercise capacity due to cognitive, musculoskeletal, or cardiovascular deficits [Giordano 2001], their pulmonary reserve may be adequate to avoid respiratory symptoms in the course of their usual activities. When exertional dyspnea is reported, it may be difficult to discern cardiovascular etiologies from intrinsic pulmonary disease. Increased dyspnea in our WBS cohort is not unexpected and may be due in part to the higher incidence of comorbid cardiovascular disease in this population [Pober 2008].
Besides dyspnea, a substantial proportion of our WBS patients reported respiratory complaints, such as wheezing and coughing, which have less salient overlap with possible cardiac etiologies. Although the differences between our WBS and reference populations did not reach statistical significance, this likely reflects low power due to our small sample sizes. Given the lack of obstructive pulmonary disease in our WBS patients, investigation into other lung impairments such as diffusion abnormalities or chronic bronchitis may represent an area that warrants further examination.
We acknowledge several additional limitations to this largely descriptive study. Our small cohort may be subject to self-selection bias with regards to participation. The diagnosis of WBS was previously confirmed by FISH analysis of the ELN gene deletion in the majority of our patients; the remaining minority may be subject to misclassification bias, though we feel this scenario is very unlikely in the setting of their typical WBS features. By using traditional spirometry to assess lung function, only subjects who were physically and mentally able to perform this test were included in the analysis; a possible alternative would be to utilize techniques which require limited subject participation similar to techniques employed for infants and young children. Longitudinal follow up and review of radiographic data in additional WBS subjects in the future would allow for improved detection of subclinical emphysema and lung disease. If emphysema is not a common finding among patients with WBS, more in-depth analysis of our case report subject may yield additional insights on the pathogenesis of non-smoking related emphysema.
References
- Brenner M, McKenna RJ, Jr, Gelb AF, Fischel RJ, Wilson AF. Rate of FEV1 change following lung volume reduction surgery. Chest. 1998;113(3):652–659. doi: 10.1378/chest.113.3.652. [DOI] [PubMed] [Google Scholar]
- Cherniske EM, Carpenter TO, Klaiman C, Young E, Bregman J, Insogna K, Schultz RT, Pober BR. Multisystem study of 20 older adults with Williams syndrome. Am J Med Genet A. 2004;131(3):255–264. doi: 10.1002/ajmg.a.30400. [DOI] [PubMed] [Google Scholar]
- Cho MH, Ciulla DM, Klanderman BJ, Hersh CP, Litonjua AA, Sparrow D, Raby BA, Silverman EK. Analysis of exonic elastin variants in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;40(6):751–755. doi: 10.1165/rcmb.2008-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo M, Antonell A, Magano LF, Munoz FJ, Flores R, Bayes M, Perez Jurado LA. Hemizygosity at the NCF1 gene in patients with Williams-Beuren syndrome decreases their risk of hypertension. Am J Hum Genet. 2006;78(4):533–542. doi: 10.1086/501073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, Stock AD, Leppert M, Keating MT. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet. 1993;5(1):11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- Giordano U, Turchetta A, Giannotti A, Digilio MC, Virgilii F, Calzolari A. Exercise testing and 24-hour ambulatory blood pressure monitoring in children with Williams syndrome. Pediatr Cardiol. 2001;22(6):509–511. doi: 10.1007/s002460010285. [DOI] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Irion KL, Marchiori E, Hochhegger B, Porto Nda S, Moreira Jda S, Anselmi CE, Holemans JA, Irion PO. CT quantification of emphysema in young subjects with no recognizable chest disease. AJR Am J Roentgenol. 2009;192(3):W90–96. doi: 10.2214/AJR.07.3502. [DOI] [PubMed] [Google Scholar]
- Kelleher CM, Silverman EK, Broekelmann T, Litonjua AA, Hernandez M, Sylvia JS, Stoler J, Reilly JJ, Chapman HA, Speizer FE, Weiss ST, Mecham RP, Raby BA. A functional mutation in the terminal exon of elastin in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2005;33(4):355–362. doi: 10.1165/rcmb.2005-0206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery MC, Morris CA, Ewart A, Brothman LJ, Zhu XL, Leonard CO, Carey JC, Keating M, Brothman AR. Strong correlation of elastin deletions, detected by FISH, with Williams syndrome: evaluation of 235 patients. Am J Hum Genet. 1995;57(1):49–53. [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Morris CA, Thomas IT, Greenberg F. Williams syndrome: autosomal dominant inheritance. Am J Med Genet. 1993;47(4):478–481. doi: 10.1002/ajmg.1320470409. [DOI] [PubMed] [Google Scholar]
- Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest. 2008;118(5):1606–1615. doi: 10.1172/JCI35309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Iranzo P, Badenas C, Puig S, Carrio A, Mila M. A novel elastin gene mutation resulting in an autosomal dominant form of cutis laxa. Arch Dermatol. 2004;140(9):1135–1139. doi: 10.1001/archderm.140.9.1135. [DOI] [PubMed] [Google Scholar]
- Sadler LS, Robinson LK, Verdaasdonk KR, Gingell R. The Williams syndrome: evidence for possible autosomal dominant inheritance. Am J Med Genet. 1993;47(4):468–470. doi: 10.1002/ajmg.1320470406. [DOI] [PubMed] [Google Scholar]
- Shifren A, Durmowicz AG, Knutsen RH, Hirano E, Mecham RP. Elastin protein levels are a vital modifier affecting normal lung development and susceptibility to emphysema. Am J Physiol Lung Cell Mol Physiol. 2007;292(3):L778–787. doi: 10.1152/ajplung.00352.2006. [DOI] [PubMed] [Google Scholar]
- Shifren A, Mecham RP. The stumbling block in lung repair of emphysema: elastic fiber assembly. Proc Am Thorac Soc. 2006;3(5):428–433. doi: 10.1513/pats.200601-009AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman EK, Chapman HA, Drazen JM, Weiss ST, Rosner B, Campbell EJ, O’Donnell WJ, Reilly JJ, Ginns L, Mentzer S, Wain J, Speizer FE. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1770–1778. doi: 10.1164/ajrccm.157.6.9706014. [DOI] [PubMed] [Google Scholar]
- Stromme P, Bjornstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002;17(4):269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema: roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med. 2003;168(5):516–521. doi: 10.1164/rccm.200208-908PP. [DOI] [PubMed] [Google Scholar]
- Urban Z, Gao J, Pope FM, Davis EC. Autosomal dominant cutis laxa with severe lung disease: synthesis and matrix deposition of mutant tropoelastin. J Invest Dermatol. 2005;124(6):1193–1199. doi: 10.1111/j.0022-202X.2005.23758.x. [DOI] [PubMed] [Google Scholar]
- Urban Z, Helms C, Fekete G, Csiszar K, Bonnet D, Munnich A, Donis-Keller H, Boyd CD. 7q11.23 deletions in Williams syndrome arise as a consequence of unequal meiotic crossover. Am J Hum Genet. 1996;59(4):958–962. [PMC free article] [PubMed] [Google Scholar]
- Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest. 1992a;101(3):793–799. doi: 10.1378/chest.101.3.793. [DOI] [PubMed] [Google Scholar]
- Verbeken EK, Cauberghs M, Mertens I, Clement J, Lauweryns JM, Van de Woestijne KP. The senile lung. Comparison with normal and emphysematous lungs. 2. Functional aspects. Chest. 1992b;101(3):800–809. doi: 10.1378/chest.101.3.800. [DOI] [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23(3):320–6. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- West JB. Distribution of mechanical stress in the lung, a possible factor in localisation of pulmonary disease. Lancet. 1971;1(7704):839–841. doi: 10.1016/s0140-6736(71)91501-7. [DOI] [PubMed] [Google Scholar]


