Summary
Age-related changes in immunity are well documented in humans and laboratory mammals. Using blood samples collected from wild Soay sheep, we show that pronounced differences in T-cell subsets and inflammatory markers amongst age classes are also evident under natural conditions. These shifts parallel those observed in mammals experiencing protected environments. We found progressive declines in the proportion of naïve CD4 T cells with age, a precipitous drop in γδ T cells after the second year of life and an increase in acute phase protein levels amongst geriatric sheep. Our findings suggest immune aging patterns observed in laboratory and domestic mammals may generalize to more complex, challenging environments and could have fitness costs under natural conditions.
Keywords: lymphocytes, immunosenescence, inflammation, naïve T cells, soay sheep, γδ T cells
Research in humans and laboratory mammals has demonstrated profound changes in immunity with age, including declines in the ratio of naïve to memory T lymphocytes and increases in inflammatory markers (Linton & Dorshkind, 2004; Singh & Newman, 2011). Longitudinal studies suggest these changes may be important in age-related pathology and mortality in elderly humans and laboratory mice (Larbi et al., 2008; Singh & Newman, 2011). However, the wider evolutionary significance of such age-related changes in mammals remains uncertain (Shanley et al., 2009). We currently do not know whether immune aging patterns observed in the benign conditions experienced by modern humans and laboratory populations have any parallels in mammals experiencing parasite-rich, food-limited natural environments representative of those under which they actually evolved. Here, we present the first test for age-related differences in lymphocyte subsets and inflammatory markers in a wild mammal and report considerable similarity to the patterns observed in humans and laboratory mammals.
The population of Soay sheep (Ovis aries) in the Village Bay area of Hirta, St Kilda, has been closely monitored since 1985. It is unmanaged and unpredated with individuals experiencing food limitation over winter and challenges from micro- and macro-parasites (Clutton-Brock & Pemberton 2004). In August 2010, we collected blood samples from female lambs, yearlings, adults (2–6 years) and geriatrics (7–10 years) to examine age-related variation in immune measures known to change with age in humans or laboratory model systems (see Appendix S1).
T-cell populations were defined as helper (CD4+), naive (CD45RA+), regulatory (FoxP3+) or cytotoxic (CD8+), based on analogy with equivalent human and murine subpopulations. All measured T-cell subsets varied significantly amongst age classes, but we observed particularly notable declines in the proportion of naïve T helper cells and γδ T cells with age (Fig. 1). The proportion of T helper cells (CD4+) increased from around 25% of the total circulating lymphocyte population in lambs to 35% in geriatric sheep (F3,43 = 9.63, P < 0.001; Fig. 1A). Within this subset, the proportion of naïve helper T cells (CD4+ CD45RA+) declined progressively amongst age classes from around 35% to < 10% (F3,42 = 57.97, P < 0.001; Fig. 1B). Such a pattern is expected owing to declining thymic output of naïve T cells alongside their continuous antigenic activation and is consistent with findings in laboratory models and humans (Linton & Dorshkind, 2004), but has not previously been documented in a wild mammal to our knowledge. The proportion of regulatory T helper cells (CD4+ FoxP3+;’Tregs’), particularly those with a naïve phenotype (CD4+ FoxP3+ CD45RA+), declined with age (F3,43 = 12.19 and 18.15, respectively, both P < 0.001; Fig. 1C,D). The change in Tregs is in the opposite direction of that generally observed in mice and humans (Dejaco et al., 2006), and it is not clear why this is the case. However, the decline in naïve Tregs is consistent with previous findings in humans (Booth et al., 2010). The proportion of cytotoxic T cells (CD8+) was higher in geriatrics than other age classes but did not vary significantly between lambs and adults (F3,43 = 7.83, P < 0.001; Fig. 1E). Finally, the proportion of γδ T cells, which are known to circulate at high levels in young domestic ruminants (relative to humans and laboratory rodents) and decline with age in cattle (Hein & Mackay, 1991), decreased precipitously from around 20% in lambs and yearlings to < 5% in geriatric females (F3,43 = 32.22, P < 0.001; Fig. 1F).
Fig. 1.
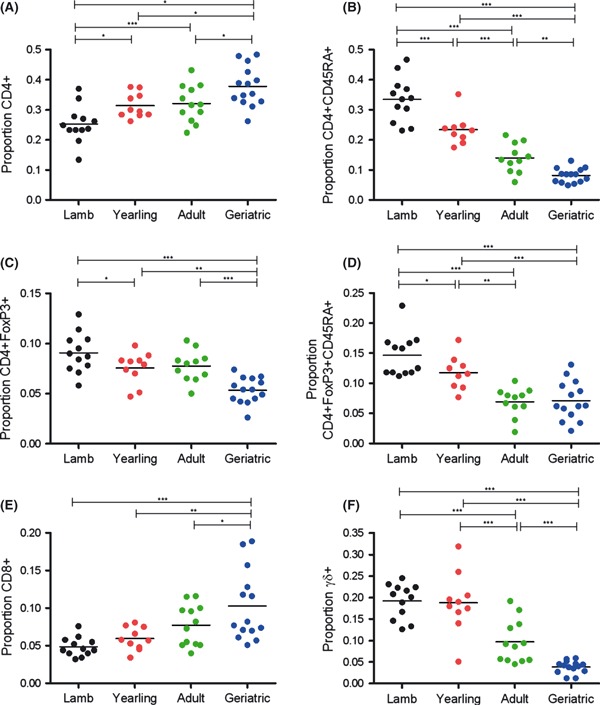
Age-related differences in T lymphocyte subsets in female sheep. Bars are mean values for each age class; lines above indicate significant post hoc tests comparing age groups (*P < 0.05; **P < 0.01; ***P < 0.001). Plots reflect the proportion of total lymphocyte population comprised of: (A) CD4+, (E) CD8+ and (F) γδ+ T cells; proportion of the CD4+ population comprised of (B) CD45RA+ naïve cells, (C) FoxP3+ regulatory cells; and (D) proportion of the CD4+ FoxP3+ cells that were CD45RA+ naïve cells.
Studies in humans frequently report increases in acute phase proteins and interleukin-6 (IL-6), a pro-inflammatory cytokine, in old age (Singh & Newman, 2011). We found that two acute phase proteins, haptoglobin and serum amyloid A, were higher on average in geriatric sheep than in yearlings and adults (F3,42 = 5.22 and F3,40 = 2.93, respectively, both P < 0.05; Fig. 2A,B). However, we did not find any age-related variation in IL-6 (F3,43 = 0.92, P = 0.44; Fig. 2C). IL-10, an anti-inflammatory cytokine, was significantly lower in lambs than in other age classes but did not vary from yearlings to geriatrics (F3,43 = 6.94, P < 0.001; Fig. 2D).
Fig. 2.
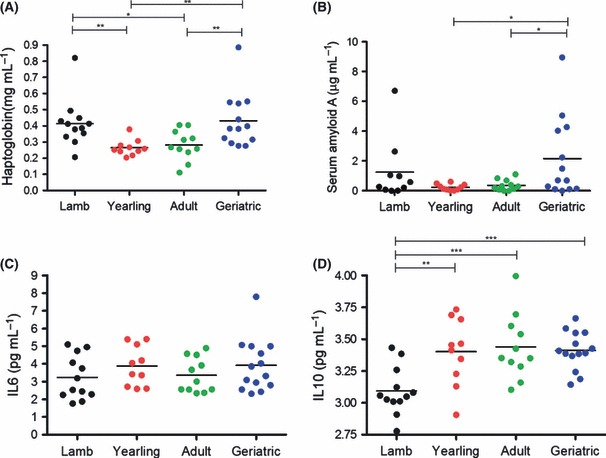
Age-related differences in (A) haptoglobin, (B) serum amyloid A, (C) interleukin-6 and (D) interleukin-10 in plasma from female sheep. Bars indicate mean values for each age class; lines above indicate significant post hoc tests comparing age groups (*P < 0.05; **P < 0.01; ***P < 0.001).
Our data complement accumulating evidence that declines in survival and reproduction with age are readily observable in the wild (Brunet-Rossinni & Austad, 2006) and that immune responses to antigenic challenge decrease with age in wild birds (Palacios et al., 2011). Studies testing evolutionary predictions in natural populations can provide important insights into the origins and maintenance of genetic variation underlying immunity (e.g. Graham et al., 2010; Räberg & Stjernman, 2003). Our relatively small, cross-sectional sample precluded us from detecting evolutionary trade-offs between growth and reproductive effort and our immune measures (see Appendix S2, Table S1). However, in providing the first evidence for age-related differences in T-cell subsets and acute phase proteins in a mammal experiencing ecologically realistic conditions, our data do suggest an important new degree of generality for patterns observed in the laboratory by immunologists. They also suggest that such age-dependent differences in immunity are targets for natural selection in wild mammals and highlight the potential for longitudinal research in wild animals to illuminate the evolutionary causes and consequences of variation in immunosenescence.
Acknowledgments
We thank all attendees of the Centre for Immunity, Infection and Evolution symposium on “Immunopathology & immunosenescence: an evolutionary perspective” for inspiring discussions. We are grateful to: Andrea Graham for inspiration and guidance throughout; Josephine Pemberton and the Soay sheep catch team 2010 for support with field work; National Trust for Scotland for permission to work on St Kilda; QinetiQ and Angus Campbell of Kilda Cruises for logistical support; Adam Hayward, Michael Evans, Emily Brown and Kathleen Morriss for help in the field/laboratory; Shonna Johnston and Fiona Rossi for assistance with flow cytometry analysis; and Rick Maizels, Colin Selman, Owen Jones, Ken Wilson, Tim Coulson and Simon Babayan for comments on early drafts. DHN was supported by a BBSRC David Phillips fellowship.
Author Contributions
DHN, TNM, KW and RZ planned the study and wrote the paper; TNM conducted the flow cytometry analysis; KW conducted the ELISA analysis; JGP and DHN coordinated sample collection.
Supporting information
Appendix S1 Experimental Procedures.
Appendix S2 Associations between immune measures and parasite burden, growth and reproduction.
Table S1 Associations between immune measures and parasite burden, growth and reproduction.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Booth NJ, McQuaid AJ, Sobande T, Kissane S, Agius E, Jackson SE, Salmon M, Falciani F, Yong K, Rustin MH, Akbar AN, Vukmanovic-Stejic M. Different Proliferative Potential and Migratory Characteristics of Human CD4(+) Regulatory T Cells That Express either CD45RA or CD45R0. J. Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- Brunet-Rossinni AK, Austad SN. Senescence in wild populations of mammals and birds. In: EJ Masoro, SN Austad., editors. Handbook of the Biology of Aging. Burlington, MA: Academic Press; 2006. pp. 243–266. [Google Scholar]
- Clutton-Brock TH, Pemberton JM. Soay Sheep: Dynamics and Selection in an Island Population. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- Dejaco C, Duftner C, Schirmer M. Are regulatory T-cells linked with aging? Exp. Gerontol. 2006;41:39–345. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–665. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]
- Hein WR, Mackay CR. Prominence of gamma-delta T-cells in the ruminant immune system. Immunol. Today. 1991;12:30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23:64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Palacios MG, Winkler DW, Klasing KC, Hasselquist D, Vleck CM. Consequences of immune system aging in nature: a study of immunosenescence costs in free-living tree swallows. Ecology. 2011;92:952–966. doi: 10.1890/10-0662.1. [DOI] [PubMed] [Google Scholar]
- Räberg L, Stjernman M. Natural selection on immune responsiveness in blue tits parus caeruleus. Evolution. 2003;57:1670–1678. doi: 10.1554/02-417. [DOI] [PubMed] [Google Scholar]
- Shanley DP, Aw D, Manley NR, Palmer DB. An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol. 2009;30:374–381. doi: 10.1016/j.it.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


