Abstract
Purpose
Erlotinib is clinically effective in patients with non–small-cell lung cancer (NSCLC) who have adenocarcinoma, are never or limited former smokers, or have EGFR mutant tumors. We investigated the efficacy of erlotinib alone or in combination with chemotherapy in patients with these characteristics.
Patients and Methods
Patients with advanced NSCLC (adenocarcinoma) who were epidermal growth factor receptor tyrosine kinase inhibitor and chemotherapy naive never or light former smokers (smokers of > 100 cigarettes and ≤ 10 pack years and quit ≥ 1 year ago) were randomly assigned to continuous erlotinib or in combination with carboplatin and paclitaxel (ECP) for six cycles followed by erlotinib alone. The primary end point was progression-free survival (PFS). Tissue collection was mandatory.
Results
PFS was similar (5.0 v 6.6 months; P = .1988) in patients randomly assigned to erlotinib alone (arm A; n = 81) or to ECP (arm B; n = 100). EGFR mutation analysis was possible in 91% (164 of 181) of patients, and EGFR mutations were detected in 40% (51 of 128) of never smokers and in 42% (15 of 36) of light former smokers. In arm A, response rate (70% v 9%), PFS (14.1 v 2.6 months), and overall survival (OS; 31.3 v 18.1 month) favored EGFR-mutant patients. In arm B, response rate (73% v 30%), PFS (17.2 v 4.8 months), and OS (38.1 v 14.4 months) favored EGFR-mutant patients. Incidence of grades 3 to 4 hematologic (2% v 49%; P < .001) and nonhematologic (24% v 52%; P < .001) toxicity was greater in patients treated with ECP.
Conclusion
Erlotinib and erlotinib plus chemotherapy have similar efficacy in clinically selected populations of patients with advanced NSCLC. EGFR mutations identify patients most likely to benefit.
INTRODUCTION
Lung cancer is the leading cause of cancer mortality in the United States and in the world, and more than 85% of patients with lung cancer have non–small-cell lung cancer (NSCLC).1 A majority of patients with lung cancer have stage IIIB or IV disease at the time of diagnosis, and palliative therapy with platinum-based double-agent chemotherapy is the standard therapy.2 The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) erlotinib is an effective treatment for patients with NSCLC for whom systemic chemotherapy has failed.3 The efficacy of EGFR TKIs, including erlotinib, is greatest in the subset of patients with NSCLC who are never or limited former cigarette smokers.3 This is likely because of the higher frequency of somatic mutations in the EGFR kinase domain in this phenotypic subset of patients with NSCLC.4,5 This observation has been confirmed in prospective clinical trials.6
A phase III trial evaluated the role of adding erlotinib to first-line carboplatin and paclitaxel chemotherapy in patients with advanced NSCLC.7 This strategy did not result in improvement in response rate (RR), time to progression, or overall survival (OS) in the intent-to-treat patient population.7 However, a subset analysis of patients who were never smokers revealed significant improvement in RR (30% v 11%; P = .02), time to progression (hazard ratio [HR], 0.50; 95% CI, 0.31 to 0.80; median, 6.0 and 4.3 months, respectively), and OS (HR, 0.49; 95% CI, 0.28 to 0.85; median, 22.5 and 10.1 months, respectively) for those treated in the erlotinib-containing arm compared with the chemotherapy and placebo arms.7 One potential reason for this clinical observation is a combined benefit of chemotherapy and erlotinib in the subset of patients likely to benefit from erlotinib therapy. Alternatively, the outcome differences may have been solely the result of increased efficacy of erlotinib in never smokers and/or in patients with EGFR-mutant advanced NSCLC.3 We thus developed a randomized phase II trial to investigate the efficacy of erlotinib alone and in combination with chemotherapy in patients selected based on clinical characteristics associated with known erlotinib benefit. At the time this trial was developed, routine EGFR mutation testing was not available. However, because of an interest in investigating the impact of EGFR mutations on the outcome of erlotinib-based therapy, tissue submission and specific tissue requirements were part of the trial eligibility.
PATIENTS AND METHODS
Eligibility Criteria
Patients were required to have histologic documentation of primary lung adenocarcinoma; patients with bronchioloaveolar or adenosquamous carcinoma were eligible. Patients were required to have at least a core biopsy and be a never smoker (defined as smoking ≤ 100 cigarettes in lifetime) or former light smoker (defined as smoking > 100 cigarettes and ≤ 10 pack years and quit ≥ 1 year ago). Other eligibility criteria were stage IIIB disease with malignant pleural or pericardial effusion or stage IV disease, age ≥ 18 years, Eastern Cooperative Oncology Group performance status of 0 or 1, and measureable disease as defined by RECIST (Response Evaluation Criteria for Solid Tumors).8 Laboratory requirements were an absolute neutrophil count ≥ 1,500/mL, platelet count ≥ 100,000/mL, hemoglobin ≥ 9.0 g/dL, renal function ≤ 1.5 mg/dL, total bilirubin less than upper limit of normal, and AST ≤ 2.5× upper limit of normal. No prior therapy with chemotherapy, erlotinib, or other agents targeting the EGFR pathway was allowed; radiation therapy and major surgery had to be completed ≥ 3 weeks before enrollment. Patients with brain metastases were required to be ≥ 3 weeks from completion of radiation therapy and asymptomatic and could not be receiving corticosteroid therapy. Patients with NSCLC not otherwise specified or those whose pathology consisted of only a fine needle aspirate were not eligible. This trial was approved by the institutional reviews boards of the participating institutions, and patients were required to provide informed consent before enrollment. This trial was registered with ClinicalTrials.gov.
Treatment
Patients were randomly assigned to erlotinib 150 mg daily alone (arm A) or erlotinib 150 mg daily continuous in combination with paclitaxel 200 mg/m2 every 21 days and carboplatin area under the curve of 6 using the Calvert formula every 21 days (arm B) for up to six cycles; patients assigned to arm B continued to receive erlotinib after completion of chemotherapy.9 Patients in both arms continued to receive erlotinib until disease progression or unacceptable toxicity. One cycle was defined as 21 days in both arms. Dose reductions for erlotinib were to 100 mg and 50 mg daily; one dose-level reduction was performed for grade 3 rash or diarrhea and grade ≥ 2 conjunctivitis. Erlotinib was discontinued for interstitial pneumonitis, grade 4 diarrhea or rash, and grade ≥ 2 keratitis. Patients in arm B were required to have an absolute neutrophil count ≥ 1,500/mL and platelets ≥ 100,000/mL on day 1 of each cycle; treatment could be delayed up to 2 weeks. Standard dose reductions were used for paclitaxel and carboplatin. Patients developing toxicity with paclitaxel and/or carboplatin had the option to continue one of the chemotherapy agents alone along with erlotinib or with erlotinib alone. Management of rash, diarrhea, supportive care, and antiemetics was at the discretion of the treating physician.
Trial Design and Statistical Considerations
The primary objective was to estimate progression-free survival (PFS) in each arm; secondary objectives included overall RR (ORR), OS, toxicity, and determination of PFS in patients with and without EGFR mutations in each arm. A total of 180 eligible patients (arm A, 80; arm B, 100) were to be accrued. Sample size was determined to have adequate power to address the primary objective. For arm A, it was prespecified that if median PFS were ≤ 2.9 months, it would not be of further interest; if median PFS were ≥ 4.3 months, it would be worthy of further investigation. Assuming constant hazards, it was equivalent to test H0: 18-week PFS ≤ 37% v H1: 18-week PFS ≥ 52% for arm A. For arm B, it was determined that if treatment were associated with median PFS ≤ 4.0 months, it would not be of further interest; if median PFS were ≥ 6.0 months, the regimen would be worthy of further investigation. Assuming constant hazards, it was equivalent to test H0: 18-week PFS ≤ 49% v H1: 18-week PFS ≥ 62% for arm B. The size of arms A and B allowed the testing of each hypothesis at a one-sided significance level of .10 with approximately 90% power. This trial was not designed to have adequate power to compare the efficacy of the two arms.
The expected frequency of EGFR mutations in this patient population was 15%. It was assumed that the RRs for EGFR–wild-type and -mutant patients were 10% and 60% in arm A, respectively. With 80 patients in arm A, there was 96% power to detect a 50% increase in RR between EGFR–wild-type and -mutant patients, at a significance level of .05 with a two-sided χ2 test. With 76 events, the study had 95% power, at a significance level of .05 using a two-sided log-rank test, to detect an HR of 0.31 (3 v 9.5 months) for PFS in favor of EGFR-mutant patients. In arm B, it was assumed that the RRs were 25% and 75% for EGFR–wild-type and -mutant patients, respectively. With 100 patients, there was 97% power to detect a 50% increase in RR between EGFR–wild-type and -mutant patients, at a significance level of .05 with a two-sided χ2 test. With 89 events, the study had 83% power, at a significance level of .05 using a two-sided log-rank test, to detect an HR of 0.42 (5 v 12 months) for PFS in favor of EGFR-mutant patients.
PFS was defined as the time between random assignment and disease progression or death (whichever occurred first); OS was defined as the time from random assignment until death resulting from any cause. Kaplan-Meier product limit estimator was used to estimate median PFS and OS as well as 95% CIs.10 The proportion of patients who experienced a response (partial or complete) and the exact 95% CI were estimated. Rates of treatment-related adverse events by type between arms were compared by Fisher's exact test. All P values are two sided.
EGFR Mutation Analysis
EGFR mutations were performed at the Dana-Farber Cancer Institute (Boston, MA) using a sensitive heteroduplex method coupled with enzymatic digestion as previously described.11 All positive findings were independently verified and subjected to sequencing. The mutation analyses were blinded to the patients' clinical outcome.
On-Study Assessment
Patients were required to undergo history and physical examination, tumor measurements, complete blood count, and serum chemistries at baseline. Patients underwent computed tomography of the chest and abdomen including the liver and adrenals, bone scan, and computed tomography or magnetic resonance imaging of the brain before registration. Patients underwent reimaging every two cycles (6 weeks) until disease progression or unacceptable toxicity. Patients were evaluated every cycle (3 weeks) via history and physical examination, complete blood count, and serum chemistries, and toxicity was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
RESULTS
Patient Characteristics and Treatment Administration
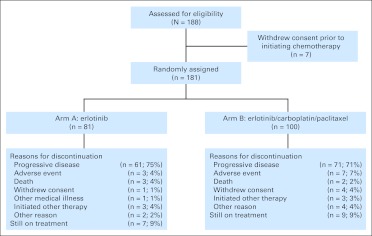
Between August 2005 and April 2009, 188 patients were enrolled; seven patients withdrew consent before initiating study therapy; 81 patients in arm A and 100 in arm B received study therapy (Fig 1). A majority of the patients were female (59%), were white (80%), had a performance score of 0 (54%), were never smokers (79%), and had adenocarcinoma histology (86%; Table 1). The median number of cycles of erlotinib in arm A was six (range, one to 70). The median number of cycles of therapy in arm B was eight (range, one to 70); the median number of cycles of the combination of erlotinib, carboplatin, and paclitaxel was three (range, one to six). Twenty-seven percent (48 of 181) of patients (arm A, 28%; arm B, 25%) received ≥ 18 cycles (1 year) of therapy.
Fig 1.
CONSORT diagram showing patient disposition.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Erlotinib |
Erlotinib Plus Carboplatin and Paclitaxel |
All Patients |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients | 81 | 100 | 181 | |||
| Age, years | ||||||
| Median | 58 | 60 | 59 | |||
| Range | 32-78 | 34-81 | 32-81 | |||
| Sex | ||||||
| Male | 32 | 40 | 42 | 42 | 74 | 41 |
| Female | 49 | 60 | 58 | 58 | 107 | 59 |
| Ethnicity | ||||||
| White | 61 | 75 | 84 | 84 | 145 | 80 |
| African American | 12 | 15 | 6 | 6 | 18 | 10 |
| Asian | 5 | 6 | 8 | 8 | 13 | 7 |
| Other | 2 | 3 | 1 | 1 | 3 | 2 |
| Unknown | 1 | 1 | 1 | 1 | 2 | 1 |
| ECOG performance status | ||||||
| 0 | 50 | 62 | 48 | 48 | 98 | 54 |
| 1 | 31 | 38 | 52 | 52 | 83 | 46 |
| Smoking history | ||||||
| Never smoker | 64 | 79 | 79 | 79 | 143 | 79 |
| Light former smoker | 17 | 21 | 21 | 21 | 38 | 21 |
| Histology | ||||||
| Adenocarcinoma | 71 | 88 | 84 | 84 | 155 | 86 |
| Bronhioloalveolar cancer | 2 | 2 | 2 | 2 | 4 | 2 |
| Adenocarcinoma with bronchioloalveolar features | 8 | 10 | 14 | 14 | 22 | 12 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Toxicity
In arm A, 23% of patients had a dose reduction in at least one cycle. In arm B, 27% of patients had a dose reduction in at least one cycle. The most common reason for treatment discontinuation in both arms was progressive disease: 61 patients (75%) in arm A and 71 patients (71%) in arm B. The rates of grades 3 to 4 hematologic toxicity were significantly higher (49% v 2%; P < .001) in arm B compared with arm A (Table 2). Similarly, the rates of nonhematologic toxicity (52% v 24%; P < .001) were greater in arm B compared with arm A. The rate of grade 3 acne/acneiform rash was similar in arms A and B (Table 2). Two patients in arm B experienced treatment-related death, one patient as a result of renal failure and one as a result of an adverse event not associated with a CTCAE term.
Table 2.
Common Adverse Events
| Adverse Event | Erlotinib |
Erlotinib Plus Carboplatin and Paclitaxel |
P* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 3 |
Grade 4 |
Grade 3 |
Grade 4 |
||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Hematologic | |||||||||
| Anemia | 1 | 1 | 0 | 0 | 7 | 7 | 0 | 0 | .0763 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 9 | 9 | 3 | 3 | < .001 |
| Neutropenia | 0 | 0 | 0 | 0 | 24 | 24 | 17 | 17 | < .001 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 1 | 1 | 4 | 4 | .0659 |
| Maximum | 2 | 2 | 0 | 0 | 29 | 29 | 20 | 20 | < .001 |
| Nonhematologic | |||||||||
| Allergic reaction (hypersensitivity) | 0 | 0 | 0 | 0 | 4 | 4 | 0 | 0 | .1291 |
| Diarrhea | 4 | 5 | 0 | 0 | 7 | 7 | 0 | 0 | .7567 |
| Fatigue | 1 | 1 | 0 | 0 | 16 | 16 | 1 | 1 | < .001 |
| Nausea | 1 | 1 | 0 | 0 | 7 | 7 | 0 | 0 | .0763 |
| Neuropathy (sensory) | 0 | 0 | 0 | 0 | 6 | 6 | 0 | 0 | .0338 |
| Rash (acne/acneiform) | 6 | 7 | 0 | 0 | 10 | 10 | 0 | 0 | .6068 |
| Vomiting | 1 | 1 | 0 | 0 | 7 | 7 | 0 | 0 | .0763 |
| Maximum | 18 | 22 | 2 | 2 | 39 | 39 | 13 | 13 | < .001 |
NOTE. Comparisons are for grades 3 to 4 toxicities between erlotinib and erlotinib plus chemotherapy.
Fisher's exact test.
Efficacy
Median follow-up for all patients was 38 months, and all 181 patients were evaluable for PFS and OS. In the treated patient population, the ORR in arm A was 35% (95% CI, 24 to 46; n = 28), and 28 patients (35%) experienced stable disease; median PFS and OS were 5.0 (95% CI, 2.9 to 7.0) and 24.6 months (95% CI, 18.4 to 33.8), respectively (Fig 2A). In arm B, the ORR was 46% (95% CI, 36 to 56; n = 46), and 38 patients (38%) experienced stable disease; median PFS and OS were 6.6 (95% CI, 5.4 to 8.2) and OS 19.8 months (95% CI, 14.4 to 27.8), respectively (Fig 2B). The primary end point was met in both arms of the study. The 18-week PFS rate for arm A was 52% (80% CI, 45 to 59); the lower limit of the 80% CI is above the prespecified limit of no interest in H0 (< 37%). Similarly, the 18-week PFS rate for arm B was 69% (80% CI, 62 to 74), and the lower limit of the 80% CI is above the prespecified limit of no interest in H0 (< 49%).
Fig 2.
(A) Progression-free survival and (B) overall survival in all patients. E; erlotinib, ECP; erlotinib plus carboplatin and paclitaxel.
Efficacy in EGFR-Mutant and Wild-Type Patients
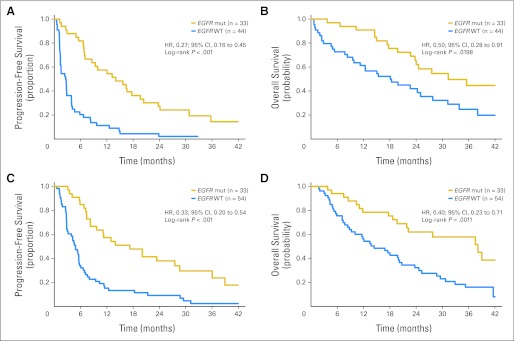
EGFR mutation analysis was successfully performed in 164 patients (91%); 17 patients had insufficient material or DNA for analysis (demographics listed in Appendix Table A1, online only). The mutational analysis was successfully performed in 77 (95%) of 81 patients in arm A and 87 (87%) of 100 patients in arm B. EGFR-activating drug-sensitive mutations, deletions of exon 19, and L858R point mutations were detected in 33 patients (43%) in arm A and 33 patients (38%) in arm B. In arm A, exons 19 and 21 mutations were detected in 23 and 10 tumor respectively; in Arm B exon 19 and 21 mutations were detected in 16 and 17 tumors, respectively. Six patients had EGFR exon 20 insertion mutations associated with erlotinib resistance (Appendix Table A2, online only).12 The outcome analyses were limited to patients with exon 19 deletions and L858R mutations to compare findings from the current study with those of prospective clinical trials limited to exons 19 and 21 EGFR-mutant patients with NSCLC.13–15 The frequency of EGFR mutations in never smokers (40%; 51 of 128) was similar (P = .84) to the frequency in former light smokers (42%; 15 of 36), consistent with prior observations.16 In both arms, the ORR was significantly greater (P < .001) for patients with EGFR-mutant tumors (Table 3). Similarly, median PFS and OS were significantly longer for patients with EGFR-mutant tumors compared with those with EGFR–wild-type tumors in both arms of the study (Table 3; Figs 3A to 3D). PFS and OS for EGFR-mutant patients were similar in both arms of the study (Table 3; Appendix Fig A1, online only).
Table 3.
Efficacy Analysis by EGFR Mutation Status
| Outcome | Erlotinib |
Erlotinib Plus Carboplatin and Paclitaxel |
||||
|---|---|---|---|---|---|---|
| EGFR Mutant | EGFR WT | P* | EGFR Mutant | EGFR WT | P* | |
| No. of patients | 33 | 44 | 33 | 54 | ||
| ORR, % | 70 | 9 | < .001† | 73 | 30 | < .001† |
| 95% CI | 51 to 84 | 3 to 22 | 18 to 44 | 18 to 44 | ||
| PFS, months | < .001‡ | < .001‡ | ||||
| Median | 14.1 | 2.6 | 17.2 | 4.8 | ||
| 95% CI | 7.0 to 19.6 | 1.4 to 3.9 | 8.2 to 28.7 | 2.8 to 5.6 | ||
| OS, months | .0198‡ | .0011‡ | ||||
| Median | 31.3 | 18.1 | 38.1 | 14.4 | ||
| 95% CI | 23.8 to NA | 9.5 to 27.8 | 19.6 to NA | 8.7 to 20.2 | ||
Abbreviations: NA, not available; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; WT, wild type.
Mutant v WT.
Fisher's exact test.
Two-sided log-rank test.
Fig 3.
(A) Progression-free (PFS) and (B) overall survival (OS) based on EGFR mutation (mut) in arm A (erlotinib alone). (C) PFS and (D) OS based on EGFR mut in arm B (erlotinib plus carboplatin and paclitaxel). HR, hazard ratio; WT, wild type.
We also analyzed the outcome of patients based on the specific EGFR mutation (exon 19 deletion v L858R). Patients with EGFR exon 19 deletion mutations had a significantly greater RR to erlotinib (83% v 40%; P = .0349) compared with those with L858R mutations (Appendix Table A3, online only). PFS was numerically longer for patients with EGFR exon 19 mutations treated in either arm A or B compared with those with L858R mutations (Appendix Table A3). OS was similar for patients with EGFR exon 19 deletions and L858R tumors (Appendix Table A3).
DISCUSSION
Patients with NSCLC whose tumors harbor EGFR mutations derive the greatest degree of benefit from first-line EGFR TKI therapy.6 These observations have been validated in retrospective and prospective clinical trials, and currently EGFR TKIs are commonly used as first-line therapy for advanced EGFR-mutant NSCLC.6,13,15,17 Our findings are consistent with prior observations. The outcome (RR and PFS) of patients with EGFR mutations treated with erlotinib alone (arm A) was similar to that in other prospective studies, in both white and Asian patients, of erlotinib in treatment-naive EGFR-mutant patients.15,18 The poor PFS in the EGFR–wild-type patients treated with erlotinib alone was also similar to that in prior studies of EGFR inhibitors in EGFR–wild-type patients or in those clinically unlikely to harbor an EGFR mutation.6,19 The current study further reinforces the importance of molecular rather than phenotypic selection of patients for first-line EGFR TKI therapy.6,18 This is particularly important for white patients, in whom, even in this highly clinically enriched patient population, the frequency of EGFR mutations was only 40% compared with 60% in Asian patients with similar phenotypes.6 Clinical trials have demonstrated that in unselected white patients who are unlikely to have EGFR-mutant tumors, first-line erlotinib therapy is associated with a survival detriment when compared with platinum-based chemotherapy.19,20
An important unresolved issue that in part led to the design of this trial was whether in the population of patients most sensitive to erlotinib (ie, EGFR-mutant patients) there would be any additional clinical benefit with the addition of systemic chemotherapy. Our findings would suggest that there is little, if any, such benefit (as measured by RR and PFS) in combining chemotherapy with erlotinib compared with erlotinib alone in EGFR-mutant patients (Table 3; Appendix Fig A1, online only). However, these findings should not be considered definitive, because the two-arm phase II trial was not designed to make formal comparison of these end points between the regimens. Our study, using continuous erlotinib therapy, also does not support the notion that EGFR TKIs and chemotherapy are antagonistic with one another.21 In fact, even among EGFR–wild-type patients, in both arms, OS was longer than OS observed in recent phase III trials.22,23 A randomized phase II trial conducted in Asia using intermittent erlotinib with chemotherapy demonstrated significantly longer PFS compared with chemotherapy alone.24 However, this study did not include an erlotinib-only arm or detailed molecular analyses on the majority of patients to determine whether a similar outcome would have been observed with single-agent erlotinib therapy.24 Intriguingly, the findings from EGFR-mutant NSCLC are in contrast with those in HER2-amplified breast cancer, in which, although trastuzumab has single-agent activity, the majority of the clinical benefit is derived from the combination of trastuzumab with chemotherapy.25,26
Improving PFS of patients with EGFR-mutant NSCLC treated with erlotinib remains a critical therapeutic challenge. Our study suggests that this is unlikely to be achieved by adding chemotherapy to erlotinib. Current studies are evaluating the benefit of the addition of bevacizumab to erlotinib in EGFR-mutant NSCLC based on a subset analysis of a prior clinical trial.27 Additional efforts aimed at understanding the biology of EGFR-mutant NSCLC and/or the development of more effective EGFR-targeted therapies are necessary to improve the outcome of patients with EGFR-mutant NSCLC.28,29
Acknowledgment
Supported in part by Grants No. CA32291 (P.A.J., M.C.), CA33601 (X.W., L.G.), CA47559 (M.A.S., T.E.S.), CA47577 (J.C.), CA31983 (M.J.E.), CA77658 (M.A.V.-C.), CA16450 (R.K.), CA41287 (E.E.V.), and CA77651 (V.A.M.) from the National Cancer Institute (NCI); by Grants No. CA31946 to Cancer and Leukemia Group B (CALGB) and CA33601 to the CALGB Statistical Center from the NCI; and by the NCI Cancer Therapy Evaluation Program Translational Research Initiative administered by SAIC-Frederick.
Appendix
The following institutions participated in this study: Board of Regents of the University of Oklahoma, Oklahoma City, OK, Shubham Pant, MD (supported by Grant No. CA37447 from the National Cancer Institute [NCI]); Cancer Centers of the Carolinas, Greenville, SC, Jeffrey K. Giguere, MD (CA29165); Christiana Care Health Services, Wilmington, DE, Stephen Grubbs, MD (CA45418); Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, MD, PhD (CA32291); Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD (CA47577); Georgetown University Medical Center, Washington, DC, Minetta C. Liu, MD (CA77597); Hematology-Oncology Associates of Central New York Community Clinical Oncology Program (CCOP), Syracuse, NY, Jeffrey Kirshner, MD (CA45389); Long Island Jewish Medical Center, Lake Success, NY, Kanti R. Rai, MD (CA35279); Massachusetts General Hospital, Boston, MA, Jeffrey W. Clark, MD (CA32291); Memorial Sloan-Kettering Cancer Center, New York, NY, Clifford A. Hudis, MD (CA77651); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN, Rafat Ansari, MD (CA86726); Rhode Island Hospital, Providence, RI, William Sikov, MD (CA08025); Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, MD (CA59518); Southeast Cancer Control Consortium CCOP, Goldsboro, NC, James N. Atkins, MD (CA45808); State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD (CA21060); The Ohio State University Medical Center, Columbus, OH, Clara D. Bloomfield, MD (CA77658); University of California at San Diego, San Diego, CA, Barbara A. Parker, MD (CA11789); University of Chicago, Chicago, IL, Hedy L. Kindler, MD (CA41287); University of Iowa, Iowa City, IA, Daniel A. Vaena, MD (CA47642); University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, MD (CA31983); University of Minnesota, Minneapolis, MN, Bruce A. Peterson, MD (CA16450); University of Nebraska Medical Center, Omaha, NE, Anne Kessinger, MD (CA77298); University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD (CA47559); and Washington University School of Medicine, St Louis, MO, Nancy Bartlett, MD (CA77440).
Fig A1.
(A) Progression-free survival and (B) overall survival in EGFR-mutant patients. E; erlotinib, ECP; erlotinib plus carboplatin and paclitaxel.
Table A1.
Patient Demographics Based on Presence or Absence of EGFR Mutation
| Characteristic | Wild Type |
Mutant |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| No. of patients | 98 | 66 | |||
| Age, years | .7926† | ||||
| Median | 59 | 58 | |||
| Range | 32-81 | 38-79 | |||
| Sex | .5186 | ||||
| Male | 43 | 44 | 25 | 38 | |
| Female | 55 | 56 | 41 | 62 | |
| Race | |||||
| White | 79 | 81 | 54 | 82 | |
| Black | 11 | 11 | 5 | 7 | .5926‡ |
| Asian | 4 | 4 | 6 | 9 | .3213§ |
| Others | 4 | 4 | 1 | 2 | |
| ECOG PS | .7490 | ||||
| 0 | 53 | 54 | 38 | 58 | |
| 1 | 45 | 46 | 28 | 42 | |
| Smoking history | .8497 | ||||
| Never smoker | 77 | 79 | 51 | 77 | |
| Light former smoker | 21 | 21 | 15 | 23 | |
| Histology | .8251 | ||||
| Adenocarcinoma | 83 | 85 | 57 | 86 | |
| Other | 15 | 15 | 9 | 14 | |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
P values are two sided using Fisher's exact test unless otherwise stated.
Wilcoxon.
White v black.
White v Asian.
Table A2.
Outcome of Patients With EGFR Exon 20 Insertion Mutations
| Patient No. | Treatment Assigned | Best Response | PFS (months) | OS (months) |
|---|---|---|---|---|
| 1 | E | PD | 0.7 | 1.0 |
| 2 | ECP | PD | 1.1 | 7.5* |
| 3 | ECP | PD | 1.5 | 2.0 |
| 4 | ECP | PR | 2.7 | 19.8 |
| 5 | ECP | PR | 5.5 | 23.7 |
| 6 | ECP | CR | 5.4 | 19.9 |
Abbreviations: CR, complete response; E, erlotinib; ECP, erlotinib plus carboplatin and paclitaxel; PD, progressive disease; PFS, progression-free survival; PR, partial response; OS, overall survival.
Patient lost to follow-up.
Table A3.
Efficacy Analysis Based on EGFR Mutation Subtype
| Outcome | Erlotinib |
Erlotinib Plus Carboplatin and Paclitaxel |
||||
|---|---|---|---|---|---|---|
| Exon 19 Deletion | L858R | P* | Exon 19 | L858R Deletion | P* | |
| No. of patients | 23 | 10 | 16 | 17 | ||
| ORR, % | 83 | 40 | .0349† | 75 | 71 | .9999† |
| 95% CI | 61 to 95 | 12 to 74 | 48 to 93 | 44 to 90 | ||
| PFS, months | .4547‡ | .1937‡ | ||||
| Median | 15.7 | 12.6 | 27.5 | 11.2 | ||
| 95% CI | 6.9 to 20.4 | 1.6 to 23.8 | 7.4 to NA | 6.8 to 23.4 | ||
| OS, months | .4953‡ | .9506‡ | ||||
| Median | 31.3 | 29.8 | 37.5 | 40.0 | ||
| 95% CI | 22.7 to NA | 3.7 to NA | 18.7 to NA | 10.4 to NA | ||
Abbreviations: NA, not available; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
Exon 19 deletion v L858R.
Fisher's exact test.
Two-sided log-rank test.
Footnotes
Written on behalf of Cancer and Leukemia Group B.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00126581.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Pasi A. Jänne, Boehringer Ingelheim (C), Pfizer (U), AstraZeneca (C), Roche (C), Genentech (C); Thomas E. Stinchcombe, Genentech (C), Eli Lilly (C); Martin J. Edelman, Genentech (C), OSI Pharmaceuticals (C); Vincent A. Miller, Astellas Pharma (C), Boehringer Ingelheim (C), Clovis Oncology (C), Genentech (C) Stock Ownership: None Honoraria: Mark A. Socinski, Genentech; Jeffrey Crawford, Genentech; Vincent A. Miller, Astellas Pharma, Boehringer Ingelheim, Clovis Oncology, Genentech Research Funding: Mark A. Socinski, Genentech; Thomas E. Stinchcombe, Genentech, Pfizer, GlaxoSmithKline, Synta Pharmaceuticals; Miguel A. Villalona-Calero, Genentech; Vincent A. Miller, Boehringer Ingelheim, Genentech, ImClone Systems, Novartis, Pfizer Expert Testimony: None Other Remuneration: Pasi A. Jänne, Genzyme
AUTHOR CONTRIBUTIONS
Conception and design: Pasi A. Jänne, Xiaofei Wang, Mark A. Socinski, Robert Kratzke, Everett E. Vokes, Vincent A. Miller
Administrative support: Thomas E. Stinchcombe, Everett E. Vokes
Provision of study materials or patients: Pasi A. Jänne, Xiaofei Wang, Mark A. Socinski, Jeffrey Crawford, Thomas E. Stinchcombe, Marzia Capelletti, Martin J. Edelman, Miguel A. Villalona-Calero, Robert Kratzke, Everett E. Vokes, Vincent A. Miller
Collection and assembly of data: Xiaofei Wang, Mark A. Socinski, Jeffrey Crawford, Thomas E. Stinchcombe, Miguel A. Villalona-Calero, Robert Kratzke, Everett E. Vokes
Data analysis and interpretation: Pasi A. Jänne, Xiaofei Wang, Mark A. Socinski, Thomas E. Stinchcombe, Lin Gu, Marzia Capelletti, Martin J. Edelman, Vincent A. Miller
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non–small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Calvert AH, Newell DR, Gumbrell LA, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 11.Janne PA, Borras AM, Kuang Y, et al. A rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screening. Clin Cancer Res. 2006;12:751–758. doi: 10.1158/1078-0432.CCR-05-2047. [DOI] [PubMed] [Google Scholar]
- 12.Wu JY, Wu SG, Yang CH, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877–4882. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 14.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 15.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 16.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 17.Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: Results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 19.Gridelli C, Ciardiello F, Feld R, et al. International multicenter randomized phase III study of first-line erlotinib (E) followed by second-line cisplatin plus gemcitabine (CG) versus first-line CG followed by second-line E in advanced non-small cell lung cancer (aNSCLC): The TORCH trial. J Clin Oncol. 2010;28:540s. doi: 10.3816/CLC.2008.n.037. (suppl; abstr 7508) [DOI] [PubMed] [Google Scholar]
- 20.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol. 2008;26:863–869. doi: 10.1200/JCO.2007.13.2720. [DOI] [PubMed] [Google Scholar]
- 21.Davies AM, Ho C, Lara PN, Jr, et al. Pharmacodynamic separation of epidermal growth factor receptor tyrosine kinase inhibitors and chemotherapy in non-small-cell lung cancer. Clin Lung Cancer. 2006;7:385–388. doi: 10.3816/CLC.2006.n.021. [DOI] [PubMed] [Google Scholar]
- 22.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 23.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non–small-cell lung cancer. J Clin Oncol. 2009;27:5080–5087. doi: 10.1200/JCO.2008.21.5541. [DOI] [PubMed] [Google Scholar]
- 25.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 26.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 27.Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377:1846–1854. doi: 10.1016/S0140-6736(11)60545-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bivona TG, Hieronymus H, Parker J, et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Ercan D, Chen L, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462:1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]