Abstract
Purpose
We describe the health-related quality of life (HRQoL) of a cohort of children with brain tumors treated with proton radiotherapy.
Patients and Methods
We recruited 142 pediatric patients with brain tumors (age 2 to 18 years) and parents of such patients treated with proton radiation at Massachusetts General Hospital from 2004 to 2010. HRQoL was assessed using the PedsQL core, brain tumor, and cancer modules (range, 0 to 100). Assessments took place during radiation and annually thereafter. We examined correlations of HRQoL with disease, treatment, and cognitive and behavioral data.
Results
Overall reports of HRQoL during treatment were 74.8 and 78.1 for child self-report (CSR) and 67.0 and 74.8 for parent proxy report (PPR) for the core and brain tumor modules, respectively. PPR demonstrated lower HRQoL scores than CSR, but the two were highly correlated. Higher HRQoL scores were significantly associated with Wechsler Full Scale Intelligence Quotient scores (administered via the age-appropriate version) and better scores on two behavioral measures. Disease type also correlated with PPR core total HRQoL score at the beginning of treatment: medulloblastoma or primitive neuroectodermal tumors, 57.8; germ cell tumors, 63.5; ependymoma or high-grade glioma, 69.8; low-grade glioma, 71.5; and other low-grade neoplasms, 78.0 (P = .001). Craniospinal irradiation and chemotherapy were negatively correlated with HRQoL.
Conclusion
This is the first study to our knowledge of HRQoL in a cohort of children with brain tumors treated with proton radiation. This prospective study demonstrates the effect of disease type and intensity of treatment on HRQoL. It further suggests that where CSR is not possible, PPR is appropriate in most circumstances.
INTRODUCTION
Radiation therapy is an integral part of therapy for many pediatric brain tumors. However, radiotherapy is also associated with significant long-term toxicities in survivors. Radiation therapy to the brain can cause cognitive deficits, abnormal endocrine function, and hearing loss. These effects are correlated with radiation dose and volume and patient age and may also be influenced by chemotherapy or surgery.1–4 The toxicities are often associated with overall decreases in children's quality of life.
Proton radiotherapy can significantly reduce the dose of radiation to normal tissues, with a significant reduction in integral dose with protons compared with external-beam photons. This has been shown in numerous dosimetric studies.5–7 This reduction in radiation delivered to normal tissue should result in less toxicity and improved overall health outcomes, which has been seen in the rhabdomyosarcoma and medulloblastoma populations.6,8,9 This article seeks to build on the limited data of health outcomes with both patient- and parent-reported quality of life outcomes in a pediatric brain tumor cohort at baseline and follow-up.
This study is the first to our knowledge to report on the health-related quality of life (HRQoL) outcomes in a proton radiotherapy–treated pediatric cohort. Herein we describe the HRQoL of children with brain tumors who were treated with proton therapy and examine associations of HRQoL with cognitive and behavioral status, treatment characteristics, and tumor type. We further examined the trajectory of HRQoL for the cohort with data at 3 years after completion of radiation therapy. We hypothesized that poorer HRQoL would be associated with more aggressive treatment and lower scores on cognitive and behavioral measures. We also anticipated that HRQoL would increase over time since treatment.
PATIENTS AND METHODS
Patient Population
Institutional review board approval was obtained before enrollment of patients. We approached all eligible (N = 285) English- or Spanish-speaking patients and parents of patients between the ages of 2 and 18 years treated with proton radiation at the Massachusetts General Hospital within 2 weeks of the start of irradiation. Of these, 242 (85%) agreed to participate; 142 had brain tumors and formed the study cohort presented here. Among these 142, data from both child self-report (CSR) and parent proxy report (PPR) were available for 101 children. Children age 2 to 5 years were not eligible to provide self-reported data. In addition, some children were too sick to provide self-reported data. At 3 years after treatment, 43 patients were available for trend analysis with time. This number is smaller than the original cohort for several reasons: patients were removed from study because of tumor progression or death (n = 12); patients had not been in the study long enough to reach the 3-year assessment (n = 57); patients did not respond to the 3-year assessment (n = 23); and patients were older than 18 years of age and thus did not receive the PedsQL for this assessment (n = 7).
The study population includes patients who were enrolled from March 2004 to March 2010, with follow-up data extending into March 2011. When two parents were available, the parent who spent the most time with the child was asked to complete the survey. Children age 5 years or older also completed self-report forms. Study staff approached families in the radiation waiting room and offered to meet with them in a private space. After obtaining consent, parents filled out the parent proxy forms, and children filled out the self-report forms. A research assistant was available to read the questions to children. Families were allowed to take the assessments home and return the completed surveys via mail. Assessments were conducted in English and Spanish.
Follow-Up
Children were assessed twice over their radiation treatment course: during the first and last 2 weeks of treatment. We subsequently assessed them annually thereafter for up to 5 years. We report the 3-year trend, because our cohort with available data for later years after treatment is too small for analysis. All of the data shown here are from assessments at the beginning of treatment except for the trend data shown in the figures. Post-irradiation assessments were administered in person or by mail within 3 months of the annual anniversary of the end of radiation therapy.
Assessment Tools
HRQoL was assessed using the PedsQL core, brain tumor, and cancer modules. We used the age-appropriate versions. The PedsQL is a widely used generic pediatric HRQoL tool with a generic core scale suitable for use with both healthy populations and populations with acute and chronic health conditions. Physical, emotional, social, and school functioning are measured and reported as subscores. Parents respond based on a five-point Likert scale. The PedsQL has good psychometric properties and has been used in a wide variety of populations.10–12 All PedsQL scores are scaled from 0 to 100, with higher scores indicating better HRQoL. Normative samples composed of healthy children have scored on the high end of this scale, with an average score of 82.3 for a general group of children and 73.1 for children with chronic medical conditions.12 Mean HRQoL scores for healthy child samples range from 78 to 88, depending on the child's age.13
The PedsQL brain tumor module is similar to the PedsQL, but it consists of the following six scales: pain and hurt, nausea, procedural anxiety, worry, cognitive problems, and movement and balance.14 We also administered three scales from the PedsQL cancer module that are not part of the brain tumor module. They included treatment anxiety, perceived physical appearance, and communication.10
At the time of proton treatment, a total of 106 patients with brain tumors had data available for both QoL assessment and baseline cognitive and behavioral testing. Not all patients complied with neurocognitive and behavioral testing, but it was the policy to offer it to all patients.
Direct assessment of intelligence was conducted with children using an age-appropriate measure (eg, Wechsler Intelligence Scale for Children, fourth edition; Wechsler Preschool and Primary Scale of Intelligence, third edition). Measures of intelligence yielded a single score (IQ) summarizing overall intelligence and were comparable between tools. The IQ score is a standard score with a mean of 100 and standard deviation of 15. Child behavioral problems were assessed using the Behavior Assessment System for Children–Parent Form (second edition; BASC-2), a standardized written parent-reported measure with age-matched norms for ages 2 to 21 years (mean T-score, 50; standard deviation [SD], 10).15 The BASC-2 produces a composite T-score that describes the degree of overall problem behaviors (ie, BSI). We present data for children with scores of ≥ 60 (defined as problem behavior) or < 60 (defined as normal behavior). Adaptive behavior was assessed using the Scales of Independent Behavior–Revised (SIB-R), a standardized written questionnaire completed by the parent to assess child adaptive behavior and functional independence. The SIB-R yields a measure of functional independence expressed as a standard score (mean, 100; SD, 15).16 For the SIB-R, we examined a cutoff of < 90 to identify children with below-average functional independence.
Clinical data used to correlate with HRQoL scores include tumor type, radiation volume, (craniospinal irradiation [CSI] v partial brain irradiation), total radiation dose (low: < 45 GyRBE [ie, relative biologic effectiveness (RBE) –weighted absorbed dose] or high: ≥ 45 GyRBE), and surgery type. We classified children into four treatment groups: radiation only; radiation and surgery; radiation and chemotherapy; and radiation, surgery, and chemotherapy. We also extracted sociodemographic data (race/ethnicity [white v other], age at radiation therapy, and sex) from the clinical data.
Statistical Analyses
Patient demographics and basic clinical data are reported in Table 1. Mean HRQoL scores for the core, brain tumor, and cancer modules were computed, and PPR and CSR reports were correlated. We examined bivariate associations of the HRQoL summary scores with demographic, cognitive and behavioral functioning, and clinical data using two-sided P values. Finally, HRQoL scores were reported longitudinally and by irradiation field. Patients who underwent CSI received radiation therapy to the whole brain and spine as well as a boost to the tumor bed. Those patients who were treated with involved-field radiation required radiation only to the remaining tumor and tumor bed. Some patients with CNS germ cell disease were treated with whole- ventricle irradiation followed by a tumor bed boost. No patient in the IF irradiation group received whole-brain radiotherapy. Germ cell tumor patients who received whole-ventricle radiotherapy were grouped in the involved-field patients.
Table 1.
Child Demographics and Clinical Characteristics
| Characteristic | No. | % |
|---|---|---|
| Total No. of children | 142 | 100 |
| Demographics | ||
| Sex | ||
| Female | 65 | 45.8 |
| Male | 77 | 54.2 |
| Race | ||
| White | 115 | 81.0 |
| Other | 27 | 19.0 |
| Cognitive and behavioral functioning | ||
| IQ at baseline | ||
| < 90 | 19 | 17.9 |
| ≥ 90 | 87 | 82.1 |
| BASC-2 BSI T-score | ||
| Problem behavior (≥ 60) | 13 | 12.3 |
| Normal (< 60) | 93 | 87.7 |
| SIB-R | ||
| < 90 | 21 | 19.8 |
| ≥ 90 | 85 | 80.2 |
| Clinical data | ||
| Diagnosis | ||
| Medulloblastoma/PNET | 50 | 35.2 |
| Ependymoma/malignant glioma | 31 | 21.8 |
| Low-grade glioma | 20 | 14.1 |
| Other low-grade neoplasm | 23 | 16.2 |
| Germ cell tumor/germinoma | 18 | 12.7 |
| Tumor location | ||
| Posterior fossa | 72 | 50.7 |
| Other | 70 | 49.3 |
| Irradiation type | ||
| Craniospinal and boost | 61 | 43.0 |
| Involved field | 81 | 57.0 |
| Radiation dose, GyRBE | ||
| Low (< 45) | 6 | 4.2 |
| High (≥ 45) | 136 | 95.8 |
| Surgery type | ||
| No surgery/biopsy only | 23 | 16.2 |
| Definitive surgery | 119 | 83.8 |
| Chemotherapy | ||
| Yes | 88 | 62.0 |
| No | 54 | 38.0 |
| Treatment type | ||
| Radiation only | 10 | 7.0 |
| Radiation and surgery | 44 | 31.0 |
| Radiation and chemotherapy | 13 | 9.2 |
| Radiation, surgery, and chemotherapy | 75 | 52.8 |
Abbreviations: BASC-2, Behavior Assessment System for Children, second edition; BSI, Behavioral Symptoms Index; IQ, Wechsler Full Scale Intelligence Quotient; PNET, primitive neuroectodermal tumor; RBE, relative biologic effectiveness; SIB-R, Scale of Independent Behavior–Revised.
The protocol objectives were originally described with the primary aim of monitoring the pattern of HRQoL outcomes over time. The study was not designed to detect a prespecified effect size between defined patient subgroups at a fixed overall level of significance. The comparisons in Tables 2 and 3 explore whether differences in HRQoL are associated with baseline demographics, cognitive and behavioral status, tumor diagnosis, and treatment type. Because the marginal error rates rather than experiment-wise rates are of primary interest, the data analyses in Tables 2 and 3 have not been adjusted for multiple comparisons.
Table 2.
PedsQL Scores: Child Self-Report Versus Parent Proxy Report During Treatment
| PedsQL Module | Total No. of Pairs | Child Self-Report |
Parent Proxy Report |
Pearson Correlation Coefficient | P* | T Value | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Core, score | ||||||||
| Total | 99 | 74.8 | 16.1 | 67.0 | 20.6 | 0.77508 | < .001 | 5.93 |
| Physical health | 99 | 74.2 | 23.1 | 65.4 | 29.2 | 0.79839 | < .001 | 4.99 |
| Psychosocial health | 99 | 75.3 | 15.1 | 68.3 | 17.5 | 0.64637 | < .001 | 5.03 |
| Emotional | 99 | 73.8 | 20.4 | 63.7 | 19.7 | 0.63988 | < .001 | 5.90 |
| Social | 99 | 83.3 | 18.1 | 78.7 | 20.0 | 0.37917 | .0361 | 2.12 |
| School | 78 | 70.7 | 19.8 | 62.9 | 25.7 | 0.53473 | .0030 | 3.06 |
| Brain tumor, score | ||||||||
| Total | 101 | 78.1 | 12.1 | 74.8 | 13.0 | 0.57790 | .0053 | 2.85 |
| Pain and hurt | 99 | 80.1 | 22.6 | 74.8 | 22.6 | 0.69397 | .0036 | 2.98 |
| Nausea | 100 | 81.7 | 19.3 | 76.7 | 22.9 | 0.52031 | .0198 | 2.37 |
| Procedure anxiety | 99 | 52.3 | 35.1 | 46.0 | 37.4 | 0.67398 | .0373 | 2.11 |
| Movement and balance | 54 | 81.8 | 23.4 | 77.5 | 26.2 | 0.70576 | .1042 | 1.65 |
| Worry | 97 | 73.1 | 26.2 | 72.6 | 26.4 | 0.47216 | .8512 | 0.19 |
| Cognitive problems | 98 | 78.9 | 18.2 | 76.3 | 23.2 | 0.48291 | .2460 | −1.17 |
| Cancer, score | ||||||||
| Treatment anxiety | 100 | 83.3 | 21.4 | 70.8 | 26.9 | 0.43013 | < .001 | 4.80 |
| Perceived physical appearance | 98 | 81.4 | 22.1 | 75.9 | 24.4 | 0.39244 | .0357 | 2.13 |
| Communication | 99 | 73.3 | 26.9 | 75.2 | 26.1 | 0.48807 | .4745 | −0.72 |
Abbreviations: PedsQL, Pediatric Quality of Life Inventory; SD, standard deviation.
Paired t test.
Table 3.
Association Between PedsQL Total Core and Tumor Scores at Start of Treatment and Child Characteristics
| Charateristic | Child Self-Report |
Parent Proxy Report |
||||||
|---|---|---|---|---|---|---|---|---|
| Core Score |
Tumor Score |
Core Score |
Tumor Score |
|||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Demographics | ||||||||
| Age at start of treatment, years | ||||||||
| No. | 96 | 98 | 130 | 131 | ||||
| 2-4* | — | — | — | — | 70.5 | 20.5 | 79.2 | 11.8 |
| 5-7 | 73.2 | 11.5 | 77.5 | 10.9 | 64.6 | 19.9 | 74.1 | 11.0 |
| 8-12 | 78.7 | 18.2 | 79.5 | 13.0 | 68.9 | 21.2 | 75.5 | 14.5 |
| 13-17 | 70.5 | 16.3 | 76.5 | 12.3 | 62.2 | 19.8 | 73.5 | 13.2 |
| P† | .0910 | .5538 | .4389 | .3596 | ||||
| F value | 2.46 | 0.59 | −0.84 | 1.08 | ||||
| Sex | ||||||||
| No. | 101 | 102 | 134 | 135 | ||||
| Female | 74.5 | 15.5 | 77.6 | 10.6 | 66.2 | 21.4 | 74.7 | 12.7 |
| Male | 74.9 | 16.5 | 78.5 | 13.4 | 67.1 | 19.8 | 76.0 | 13.0 |
| P‡ | .8929 | .6987 | .7999 | .5529 | ||||
| T value | −0.13 | −0.39 | −0.25 | 0.59 | ||||
| Race | ||||||||
| No. | 101 | 101 | 134 | 135 | ||||
| White | 74.5 | 15.9 | 77.6 | 12.1 | 66.2 | 21.1 | 75.3 | 12.7 |
| Other | 75.6 | 16.6 | 80.4 | 11.9 | 68.8 | 17.9 | 75.9 | 13.6 |
| P‡ | .7985 | .3784 | .5641 | .8461 | ||||
| T value | −0.26 | −0.88 | −0.58 | −0.19 | ||||
| Cognitive and behavioral functioning | ||||||||
| IQ at baseline | ||||||||
| No. | 75 | 75 | 100 | 101 | ||||
| < 90 | 64.8 | 17.7 | 71.7 | 13.8 | 52.2 | 18.4 | 68.2 | 11.3 |
| ≥ 90 | 76.5 | 15.2 | 78.3 | 11.5 | 68.6 | 20.6 | 76.8 | 12.3 |
| P‡ | .0091 | .0496 | .0024 | .0076 | ||||
| T value | −2.68 | −2.00 | −3.11 | −2.73 | ||||
| BASC-2 BSI T-score | ||||||||
| No. | 75 | 75 | 100 | 101 | ||||
| Problem behavior (≥ 60) | 58.1 | 14.0 | 66.3 | 10.3 | 46.6 | 19.6 | 61.6 | 14.8 |
| Normal (< 60) | 76.1 | 15.6 | 78.5 | 11.8 | 68.9 | 20.0 | 77.0 | 11.1 |
| P‡ | .0010 | .0029 | < .001 | < .001 | ||||
| T value | −3.43 | −3.08 | −3.67 | −4.34 | ||||
| SIB-R | ||||||||
| No. | 74 | 74 | 100 | 101 | ||||
| < 90 | 60.4 | 14.2 | 69.6 | 12.5 | 52.1 | 18.3 | 65.1 | 11.2 |
| ≥ 90 | 76.8 | 15.6 | 78.3 | 11.7 | 69.8 | 20.4 | 77.5 | 11.8 |
| P‡ | < .001 | .0159 | < .001 | < .001 | ||||
| T value | −3.60 | −2.47 | −3.54 | −4.25 | ||||
| Clinical data | ||||||||
| Diagnosis | ||||||||
| No. | 101 | 102 | 134 | 135 | ||||
| Medulloblastoma/PNET | 67.1 | 13.9 | 75.5 | 12.2 | 57.8 | 22.5 | 74.0 | 12.3 |
| Ependymoma/high-grade glioma | 78.8 | 13.5 | 80.0 | 11.3 | 69.8 | 19.5 | 75.8 | 13.7 |
| Low-grade glioma | 78.3 | 17.7 | 79.5 | 11.2 | 71.5 | 16.2 | 77.1 | 11.5 |
| Other low-grade neoplasm | 80.8 | 11.2 | 81.0 | 10.7 | 78.0 | 11.6 | 80.2 | 10.4 |
| Germ cell tumor/germinoma | 74.5 | 20.2 | 75.8 | 14.9 | 63.5 | 21.9 | 69.8 | 15.8 |
| P† | .0186 | .4500 | .0010 | .1273 | ||||
| F value | 3.12 | 0.93 | 4.91 | 1.83 | ||||
| Tumor location | ||||||||
| No. | 101 | 102 | 134 | 135 | ||||
| Posterior fossa | 70.9 | 15.6 | 77.0 | 12.2 | 60.2 | 20.9 | 74.7 | 12.0 |
| Other | 77.6 | 15.7 | 78.9 | 12.1 | 72.9 | 18.1 | 76.1 | 13.6 |
| P‡ | .0373 | .4438 | < .001 | .5251 | ||||
| T value | −2.11 | −0.77 | −3.76 | −0.64 | ||||
| Irradiation type | ||||||||
| No. | 101 | 102 | 134 | 135 | ||||
| Craniospinal | 68.4 | 16.7 | 74.7 | 13.2 | 57.4 | 22.3 | 72.3 | 13.0 |
| Involved field | 79.1 | 14.0 | 80.3 | 10.8 | 73.2 | 16.3 | 77.6 | 12.3 |
| P‡ | < .001 | .0217 | < .001 | .0163 | ||||
| T value | −3.50 | −2.33 | −4.74 | −2.43 | ||||
| Radiation dose, GyRBE | ||||||||
| No. | 100 | 101 | 133 | 134 | ||||
| Low (< 45) | 82.8 | 14.0 | 83.3 | 12.2 | 66.2 | 12.0 | 72.3 | 13.5 |
| High (≥ 45) | 74.3 | 16.0 | 77.8 | 12.1 | 66.7 | 20.8 | 75.6 | 12.8 |
| P‡ | .2459 | .3203 | .9542 | .5394 | ||||
| T value | 1.17 | 1.00 | −0.06 | 0.38 | ||||
| Surgery type | ||||||||
| No. | 101 | 102 | 134 | 135 | ||||
| No surgery/biopsy only | 76.9 | 13.8 | 77.3 | 11.9 | 69.3 | 15.2 | 74.0 | 10.8 |
| Definitive surgery | 74.3 | 16.4 | 78.2 | 12.2 | 66.2 | 21.3 | 75.7 | 13.2 |
| P‡ | .5248 | .7782 | .5302 | .5814 | ||||
| T value | 0.64 | −0.28 | 0.63 | −0.55 | ||||
| Chemotherapy | ||||||||
| No. | 101 | 102 | 132 | 135 | ||||
| Yes | 71.6 | 16.7 | 76.5 | 12.7 | 61.6 | 21.5 | 73.6 | 12.8 |
| No | 79.5 | 13.6 | 80.4 | 10.9 | 74.5 | 16.1 | 78.3 | 12.4 |
| P‡ | .0146 | .1143 | < .001 | .0374 | ||||
| T value | −2.46 | −1.59 | −3.74 | −2.10 | ||||
| Treatment type | ||||||||
| No. | 100 | 101 | 133 | 134 | ||||
| Radiation only | 71.1 | 15.4 | 73.0 | 12.1 | 66.7 | 14.1 | 73.0 | 10.8 |
| Radiation and surgery | 81.2 | 12.8 | 82.0 | 10.1 | 76.1 | 16.2 | 79.3 | 12.5 |
| Radiation and chemotherapy | 80.6 | 11.9 | 80.1 | 11.5 | 71.2 | 16.2 | 74.7 | 11.2 |
| Radiation, surgery, and chemotherapy | 69.6 | 17.0 | 75.8 | 12.9 | 59.9 | 21.9 | 73.4 | 13.2 |
| P† | .0047 | .0739 | < .001 | .0996 | ||||
| F value | 4.61 | 2.38 | 6.55 | 2.13 | ||||
Abbreviations: BASC-2, Behavior Assessment System for Children, second edition; BSI, Behavioral Symptoms Index; IQ, Wechsler Full Scale Intelligence Quotient; PedsQL, Pediatric Quality of Life Inventory; PNET, primitive neuroectodermal tumor; RBE, relative biologic effectiveness; SD, standard deviation; SIB-R, Scale of Independent Behavior–Revised.
Children 2 to 4 years of age did not receive self-report forms.
Analysis of variance.
t test.
RESULTS
Table 1 lists the characteristics of the population. Mean age at the initiation of treatment was 8.5 years, with 46% of patients being female and 81% white. Approximately half of the population received IF radiotherapy, which targets the tumor or tumor bed and the surrounding area at risk and only irradiates part of the brain, and approximately half received CSI, which incorporates whole- brain and spine irradiation for a portion of the treatment followed by an additional dose to the tumor bed as well. A vast majority received at least 45 GyRBE (96%), and a majority of patients (62%) were treated with chemotherapy before, during, and/or after radiotherapy. The most common combination of treatments was the trimodality of radiation, surgery, and chemotherapy (53%), and the second-largest subset comprised those patients treated with irradiation and surgery (31%).
For those patients who received baseline cognitive and behavioral testing (n = 106), a majority had average to above-average overall intelligence (82%) as well as age-appropriate or advanced adaptive abilities and functional independence (80%). Only 12% had significant overall problem behavior (BASC-2 BSI T-score ≥ 60) at the time of proton therapy.
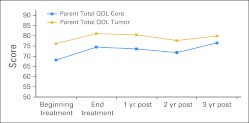
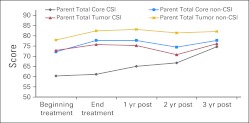
Figure 1 shows the trend in the reports of HRQoL over time for the two PPR measures for the 43 children for whom we had annual data up to 3 years after treatment. The data show an increase in HRQoL between the start and end of radiation treatment. Overall HRQoL scores rose from 68.1 at the beginning of treatment to 76.5 at year 3 after treatment, a statistically significant rise (P = .0057). For the tumor total HRQoL score, scores rose from 76.2 at the beginning of treatment to 79.9 at year 3, approaching statistical significance (P = .0599). Figure 2 shows similar findings by irradiation field. The overall trend toward increasing HRQoL occurred in both groups, reaching statistical significance in the total core PedsQL score in the CSI group (P = .0202).
Fig 1.
PedsQL total core and tumor scores for the cohort with at least 3 years post-treatment follow-up (n = 43). QOL, quality of life.
Fig 2.
PedsQL parent proxy total core and tumor scores for the cohort with at least 3 years post-treatment follow-up by radiation type. CSI, craniospinal irradiation.
Table 2 compares mean HRQoL scores at the beginning of treatment. For all core measures, parents reported worse HRQoL for their children than their children self-reported. PedsQL total summary scores were 74.8 based on CSR and 67.0 based on PPR (P < .001). Parent and child reports were significantly correlated, with Pearson correlation coefficients ranging from 0.38 (social score) to 0.80 (physical health score). Parents reported worse HRQoL than their children did for most of the tumor module scores.
In Table 2, among the core subscales, the school score was lowest, followed by the physical health and emotional scores. For the brain tumor and cancer modules, total mean score was 74.8 for PPR. Among the brain tumor and cancer module subscales based on PPR, HRQoL was lowest for procedural anxiety, followed by treatment anxiety, worry, and pain and hurt. CSR data mirrored PPR data.
Table 3 presents associations of HRQoL at the beginning of treatment with clinical variables. There were no statistically significant differences by age, sex, or race. The diagnoses of medulloblastoma or primitive neuroectodermal tumors (PNETs) and germ cell tumors had worse HRQoL based on the core total score by CSR and PPR at the time of treatment. Patients who underwent CSI reported worse core HRQoL scores, but reported tumor HRQoL scores similar to those of other patients. Extent of surgery type was not related to reported HRQoL. Chemotherapy adversely correlated with HRQoL on all measures except the CSR tumor module (where the differences were not significantly different). For each of the summary HRQoL measures, children in the lower IQ group reported worse HRQoL. Patients who showed evidence of problem behavior and who scored low on measures of functional independence tended to report lower levels of HRQoL on the summary scores.
DISCUSSION
The overall PPR HRQoL score at the beginning of radiation treatment (67.0) was considerably lower for the entire cohort of patients with brain tumors treated with proton radiation than for the normative populations of children overall (82.3) or by age group as well as for a general population of children with chronic conditions (73.1).12 However, overall HRQoL rose to 76.5 at the 3-year follow-up, closing the gap between healthy children and surpassing children with noncancer chronic health conditions. Among the domains on the core module, both parents and children reported physical health scores slightly lower than psychosocial health scores. Within the psychosocial score, school functioning was the worst, followed by the emotional and social scores. That the school score would be the worst among the psychosocial scores was to be expected for children receiving treatment, because the treatment disrupts the ability to attend school, and in most circumstances, patients traveled from out of state to receive radiation treatment and did not have the option of attending school at all.
Among the brain tumor– and cancer-specific subscores, procedural anxiety stood out as the domain of HRQoL ranked lowest by both parents and children. In another study of children with brain tumors, procedural anxiety also ranked as the lowest reported score based on both parent and child reports (PPR, 61.8; CSR, 68.2).17 However, treatment anxiety was not ranked nearly as low as procedural anxiety.
For CSR, communication and worry ranked relatively low compared with other domains. Communication questions ask about difficulty telling physicians how one feels, difficulty asking questions, and difficulty explaining the illness to others. Worry questions ask about worries related to treatment, adverse effects, and tumor recurrence. If providers give children and families more opportunities to express their concerns and address them, some of this worry may be alleviated. However, a certain amount of worry is appropriate given the seriousness of the condition, its treatment, and the uncertainty of ultimate outcome. For PPR, worry and pain and hurt followed procedural and treatment anxieties as the subscales with the lowest mean HRQoL reports. More work is needed to better understand what and how best to communicate with families given the complexities of the disease and treatment and the uncertainties of outcomes.
For all measures, parents reported worse HRQoL compared with children self-reports, which is consistent with other studies,18–21 including a similar prospective study of children with brain tumors, which showed consistently higher HRQoL for CSR,22 and a study of home- and hospital-stay patients with cancer.23 Perhaps parents are considering the possible long-term implications of their child having a brain tumor. PPR and CSR are highly correlated on almost all domains. An Asian study of children with brain tumors also showed high correlations between parent and child reports, and the parents again reported measures lower than the children did.24
HRQoL for this brain tumor proton cohort observed for 3 years showed an improvement in HRQoL after treatment, from a score of 68.1 (PPR) at the start of radiotherapy to 76.5 3 years after radiotherapy. This improvement was statistically significant overall and in the CSI group. This overall trend is likely because acute and subacute adverse effects from the tumor and treatments (radiotherapy with or without surgery and chemotherapy) remit with time. Furthermore, proton radiotherapy irradiates fewer normal tissues compared with photon treatments, which may partially mitigate the late or chronic adverse effects of therapy.25,26
This study did not show significant differences in HRQoL by sociodemographic characteristics. Data were not collected on socioeconomic status (SES), but this is the subject of an ongoing retrospective collection, and analysis to determine the effects of SES status on HRQoL in this population will be the subject of a future report. A majority of patients had to relocate to Boston for the duration of treatment, implying that many families had the means to do so, but there are also numerous low- or no- cost housing options for families in need. Importantly, other HRQoL reports have not found a relationship between SES and HRQoL,17,27 but it is certainly plausible that SES may have an effect.
The relatively consistent relationships between HRQoL and clinical variables suggest that HRQoL is influenced by disease, comorbidity, and treatment. All of the 12 associations of HRQoL with cognitive and behavioral measures indicated that worse cognitive and behavioral functioning was associated with worse HRQoL reports during treatment. Children with medulloblastoma or PNETs reported worse HRQoL than children with other tumors. Treatment including extent of irradiation or chemotherapy used also tracked with worse HRQoL during treatment. These outcomes are to be expected, because the greater the extent of irradiation, the more adverse effects, and the use of chemotherapy is also associated with adverse effects. Patients with medulloblastoma or PNETs received both CSI and chemotherapy, which are also associated with greater acute symptoms than partial brain irradiation alone. Furthermore, chemotherapy added to radiotherapy often enhances the adverse effects of radiation and adds known adverse effects to the treatment, which decreases HRQoL. To summarize, our findings demonstrate that more aggressive treatments such as combined modality therapies and poorer cognitive and behavioral functioning negatively influence HRQoL.
This prospective study of children with brain tumors treated with proton radiation demonstrates the effect on HRQoL of disease type and intensity of treatment. It further shows that although parents report lower HRQoL scores than their children, the reports of parents and children are highly correlated, suggesting that when CSR is not possible, PPR is appropriate in most circumstances. Findings showing relatively poorer HRQoL scores in the anxiety, communication, and worry domains suggest that providers could improve communication with patients to try to partially alleviate some concerns. Additionally, enlisting the help of mental health providers such as psychiatrists, psychologists, and social workers may also improve HRQoL outcomes.
Footnotes
See accompanying editorial on page 2028
Supported in part by the Susan McDaniel Brain Tumor Fund, Massachusetts General Hospital Marathon Fund for Pediatric Cancer, and Award No. P01CA021239 from the National Cancer Institute.
Presented at the International Society for Quality of Life Research, October 28-31, 2009, New Orleans, LA.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Nancy J. Tarbell, Procure (C) Stock Ownership: Nancy J. Tarbell, Procure Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Karen A. Kuhlthau, Margaret B. Pulsifer, Beow Y. Yeap, Nancy J. Tarbell, Torunn I. Yock
Financial support: Torunn I. Yock
Administrative support: Karen A. Kuhlthau, Jennifer Delahaye, Torunn I. Yock
Provision of study materials or patients: Jennifer Delahaye, Shannon M. MacDonald, Nancy J. Tarbell, Torunn I. Yock
Collection and assembly of data: Karen A. Kuhlthau, Margaret B. Pulsifer, Dianali Rivera Morales, Jennifer Delahaye, David Ebb, Shannon M. MacDonald, Torunn I. Yock
Data analysis and interpretation: Karen A. Kuhlthau, Beow Y. Yeap, Dianali Rivera Morales, Jennifer Delahaye, Kristen S. Hill, Annah N. Abrams, Nancy J. Tarbell, Torunn I. Yock
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Merchant TE, Kiehna EN, Li C, et al. Radiation dosimetry predicts IQ after conformal radiation therapy in pediatric patients with localized ependymoma. Int J Radiat Oncol Biol Phys. 2005;63:1546–1554. doi: 10.1016/j.ijrobp.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Merchant TE, Kiehna EN, Li C, et al. Modeling radiation dosimetry to predict cognitive outcomes in pediatric patients with CNS embryonal tumors including medulloblastoma. Int J Radiat Oncol Biol Phys. 2006;65:210–221. doi: 10.1016/j.ijrobp.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE, Kiehna EN, Miles MA, et al. Acute effects of irradiation on cognition: Changes in attention on a computerized continuous performance test during radiotherapy in pediatric patients with localized primary brain tumors. Int J Radiat Oncol Biol Phys. 2002;53:1271–1278. doi: 10.1016/s0360-3016(02)02828-6. [DOI] [PubMed] [Google Scholar]
- 4.Hua C, Bass JK, Khan R, et al. Hearing loss after radiotherapy for pediatric brain tumors: Effect of cochlear dose. Int J Radiat Oncol Biol Phys. 2008;72:892–899. doi: 10.1016/j.ijrobp.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald SM, Safai S, Trofimov A, et al. Proton radiotherapy for childhood ependymoma: Initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71:979–986. doi: 10.1016/j.ijrobp.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 6.Yock T, Schneider R, Friedmann A, et al. Proton radiotherapy for orbital rhabdomyosarcoma: Clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005;63:1161–1168. doi: 10.1016/j.ijrobp.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 7.St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727–734. doi: 10.1016/S0360-3016(03)01574-8. [DOI] [PubMed] [Google Scholar]
- 8.Yock TI, Yeap BY, Ebb D, et al. A phase II trial of proton radiotherapy for medulloblastoma: Preliminary results. J Clin Oncol. 2010;28(suppl):9507. abstr CRA9507. [Google Scholar]
- 9.Childs SK, Kozak KR, Friedmann AM, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: Clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2011;82:635–642. doi: 10.1016/j.ijrobp.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 11.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Varni JW, Burwinkle TM, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–341. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Varni J, Limbers C, Burwinkle T. Parent proxy-report of their children's health-related quality of life: An analysis of 13,878 parents' reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;5:2. doi: 10.1186/1477-7525-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer SN, Meeske KA, Katz ER, et al. The PedsQL Brain Tumor Module: Initial reliability and validity. Pediatr Blood Cancer. 2007;49:287–293. doi: 10.1002/pbc.21026. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds C, Kamphaus R. Behavior Assessment System for Children (ed 2) Circle Pine, MN: American Guidance Service; 2004. [Google Scholar]
- 16.Bruininks R, Woodcock R, Weatherman R, et al. SIB-R: Scales of Independent Behavior–Revised. Itasca, IL: Riverside Publishing Company; 1996. [Google Scholar]
- 17.Meeske K, Katz E, Palmer S, et al. Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer. 2004;101:2116–2125. doi: 10.1002/cncr.20609. [DOI] [PubMed] [Google Scholar]
- 18.Levi R, Drotar D. Health-related quality of life in childhood cancer: Discrepancy in parent-child reports. Int J Cancer Suppl. 1999;12:58–64. doi: 10.1002/(sici)1097-0215(1999)83:12+<58::aid-ijc11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Parsons S, Barlow S, Levy S, et al. Health-related quality of life in pediatric bone marrow transplant survivors: According to whom? Int J Cancer Suppl. 1999;12:46–51. doi: 10.1002/(sici)1097-0215(1999)83:12+<46::aid-ijc9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Britto M, Kotagal U, Chenier T, et al. Differences between adolescents' and parents' reports of health-related quality of life in cystic fibrosis. Pediatr Pulmonol. 2004;37:165–171. doi: 10.1002/ppul.10436. [DOI] [PubMed] [Google Scholar]
- 21.Sheffler LC, Hanley C, Bagley A, et al. Comparison of self-reports and parent proxy-reports of function and quality of life of children with below-the-elbow deficiency. J Bone Joint Surg Am. 2009;91:2852–2859. doi: 10.2106/JBJS.H.01108. [DOI] [PubMed] [Google Scholar]
- 22.Penn A, Lowis SP, Stevens MC, et al. Family, demographic and illness-related determinants of HRQL in children with brain tumours in the first year after diagnosis. Pediatr Blood Cancer. 2009;53:1092–1099. doi: 10.1002/pbc.22157. [DOI] [PubMed] [Google Scholar]
- 23.Speyer E, Herbinet A, Vuillemin A, et al. Agreement between children with cancer and their parents in reporting the child's health-related quality of life during a stay at the hospital and at home. Child Care Health Dev. 2009;35:489–495. doi: 10.1111/j.1365-2214.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 24.Yoo H, Ra Y, Park H, et al. Agreement between pediatric brain tumor patients and parent proxy reports regarding the Pediatric Functional Assessment of Cancer Therapy-Childhood Brain Tumor Survivors questionnaire, version 2. Cancer. 2010;116:3674–3682. doi: 10.1002/cncr.25200. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman K, Yock T. Radiation therapy for pediatric central nervous system tumors. J Child Neurol. 2009;24:1387–1396. doi: 10.1177/0883073809342275. [DOI] [PubMed] [Google Scholar]
- 26.McBride SM, Yock TI. Proton radiotherapy in pediatric malignancies. Radiat Med Rounds. 2010;1:491–510. [Google Scholar]
- 27.Bhat SR, Goodwin TL, Burwinkle TM, et al. Profile of daily life in children with brain tumors: An assessment of health-related quality of life. J Clin Oncol. 2005;23:5493–5500. doi: 10.1200/JCO.2005.10.190. [DOI] [PubMed] [Google Scholar]