Abstract
Purpose
The prognostic value of sex for esophageal cancer survival is currently unclear, and growing data suggest that hormonal influences may account for incidence disparities between men and women. Therefore, moving from the hypothesis that hormones could affect the prognosis of patients with esophageal cancer, we investigated the primary hypothesis that sex is associated with survival and the secondary hypotheses that the relationship between sex and survival depends, at least in part, on age, histology, and race/ethnicity.
Patients and Methods
By using the SEER databases from 1973 to 2007, we identified 13,603 patients (34%) with metastatic esophageal cancer (MEC) and 26,848 patients (66%) with locoregional esophageal cancer (LEC). Cox proportional hazards model for competing risks were used for analyses.
Results
In the multivariate analysis, women had longer esophageal cancer-specific survival (ECSS) than men in both MEC (hazard ratio [HR], 0.949; 95% CI, 0.905 to 0.995; P = .029) and LEC (HR, 0.920; 95% CI, 0.886 to 0.955; P < .001) cohorts. When age and histology were accounted for, there was no difference for ECSS between men and women with adenocarcinoma. In contrast, women younger than age 55 years (HR, 0.896; 95% CI, 0.792 to 1.014; P = .081) and those age 55 years or older (HR, 0.905; 95% CI, 0.862 to 0.950; P < .001) with squamous cell LEC had longer ECSS than men. In the squamous cell MEC cohort, only women younger than age 55 years had longer ECSS (HR, 0.823; 95% CI, 0.708 to 0.957; P = .011) than men.
Conclusion
Sex is an independent prognostic factor for patients with LEC or MEC. As secondary hypotheses, in comparison with men, women age 55 years or older with squamous cell LEC and women younger than age 55 years with squamous cell MEC have a significantly better outcome. These last two findings need further validation.
INTRODUCTION
Esophageal cancer is the eighth most common cancer worldwide, with 482,000 new cases (representing 3.8% of all new cancers) estimated in 2008, and the sixth most common cause of death from cancer with 407,000 deaths (representing 5.4% of all new cancers). Its incidence rates vary internationally more than 15-fold in men and almost 20-fold in women.1 In the United States, it was estimated that 16,640 new cases of esophageal cancer were diagnosed in 2010 and 14,500 deaths occurred. Esophageal cancer is highly lethal with 11,650 (88.7%) estimated deaths among men and 2,850 (81.2%) among women.2 Taken together with previous population studies,3–9 the latter suggests a survival benefit for women when compared with men.
The prevalence of the two main histologic subtypes—adenocarcinoma and squamous cell carcinoma—differs depending on geographic location. Squamous cell carcinoma of the esophagus (SCCE) predominates in the Middle East, Africa, Asia, and parts of Europe. In contrast, adenocarcinoma of the esophagus (ACE) is prevalent in Western countries.10 In the United States, the incidence of SCCE has steadily decreased in all ethnicities in the past three decades, with a concurrent increase in the incidence of ACE. In the white population, SCCE represents 27% of esophageal cancers. In contrast, SCCE remains a frequent malignancy in Hispanic, African American, and Asian populations (41%, 81%, and 70% of esophageal cancers, respectively).11
In the United States, both ACE and SCCE are more frequent in men than in women, mirroring parts of the world where SCCE largely predominates.1 Although this may represent various tumor-specific environmental exposures between sexes (eg, alcohol, tobacco), growing data suggest hormonal influences.12–14 Sex differences affect esophageal cancer incidence, yet the significance of sex as an independent prognostic marker is unclear. A major limitation of previous studies that examined the prognostic value of sex is the lack of adequate adjustment for other relevant clinical prognostic factors. Therefore, we used the SEER database to assess the influence of sex on the esophageal cancer–specific survival (ECSS) in locoregional esophageal cancer (LEC) and metastatic esophageal cancer (MEC). We evaluated metastatic diseases separately from locoregional diseases, because clinicopathologic prognostic factors and treatments may not have the same influence throughout the evolution of the malignancy. On the basis of our previous data,15 we hypothesized that hormonal status would influence survival in patients with esophageal cancer and that this influence might vary by histology and tumor stage.
PATIENTS AND METHODS
Study Design
The SEER public use database 1973 to 2007 (Version April 2010) was used for this analysis. The SEER Program, sponsored by the National Cancer Institute, collects information on cancer incidence and survival from 17 population-based cancer registries covering approximately 28% of the United States population.16
Study Population
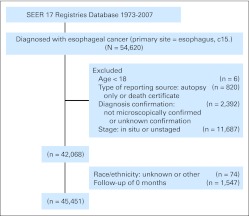
The criteria defined for inclusion in this study were primary histologically confirmed esophageal cancer and age at diagnosis of 18 years or older. We excluded a total of 14,169 patients (26%) from those diagnosed with esophageal cancer in the SEER database (n = 54,620) mainly because of unstaged or in situ tumors (n = 11,687), diagnosis not microscopically confirmed or unknown confirmation (n = 2,392), or no follow-up records (n = 1,547). A total of 40,451 patients with esophageal cancer matching the specified criteria were included in the final sample for this analysis (Appendix Figure A1, online only).
Statistical Analysis
The primary end point in this study was ECSS, defined as the period from diagnosis to death from esophageal or gastric cancer. ECSS was censored at the last follow-up, December 31, 2007, or 5 years after diagnosis, whichever came first. Of 40,451 patients, 27,414 died from esophageal or gastric cancer, and 5,776 died from other causes within the first 5 years after diagnosis. The median follow-up time for patients (n = 7,261) who were censored was 39 months, and 39% of them had been observed for at least 5 years. Proportional hazards regression model for competing risks, according to the method of Fine and Gray,17 was used to adjust for comorbidities that competed with death from esophageal cancer. The variables included in the model were sex, age, ethnicity, marital status, histology, tumor primary site, tumor grade, tumor stage, use of esophagectomy, use of radiation therapy, SEER registries, and year of diagnosis (Appendix Table A1, online only). Because detailed information on TNM staging in the early SEER database was not provided, stage was defined as locoregional (localized or regional in the SEER record description), or metastatic (distant). Because of differences in clinicopathologic factors and in treatments and the potentially different underlying hazard of (ECSS) failure, the analyses were conducted separately for patients with LEC or MEC. Pairwise interactions were examined by using stratified models and were tested by comparing corresponding likelihood ratio statistics between the baseline and nested Cox proportional hazards models, which included the multiplicative product terms. Departures of the proportional hazards assumption for the model were examined graphically by using smoothed plots of weighted Schoenfeld residuals. The primary hypothesis of this study was that sex was an independent prognostic factor for ECSS in both the LEC and MEC cohorts (ie, regardless of extent of disease). The secondary hypothesis tested was that the effect of sex on ECSS varied by age, histology, and/or race/ethnicity. All analyses were performed by using SAS software, version 9.2 (SAS Institute, Cary, NC) and library(cmprsk) in S-PLUS, version 3.3 (Statistical Sciences, Seattle, WA; S-PLUS 7.0 Enterprise Developer for Windows; S-PLUS, 1988, 2005, Insightful Corp.). Results were considered significant if a two-sided P < .05 was obtained. P values were not adjusted for multiple testing of secondary hypotheses.18
RESULTS
LEC
Patient characteristics.
This study included 26,848 patients with LEC diagnosed from 1973 to 2007. The proportion of patients diagnosed in 1973 to 1982, 1983 to 1995, and 1996 to 2007 was 12.2%, 25.4%, and 62.4%, respectively (Appendix Table A2, online only). ACE and SCCE were more commonly diagnosed in men than in women (ratio of men to women was 6.2:1 and 1.9:1, respectively).
Sex and LEC.
The median age for men was 67 years (range, 19 to 103 years), and the median age for women was 70 years (range, 19 to 107 years). When comparing by histology, women were older than men in both ACE and SCCE cohorts (ACE: women, age 72 years [range, 19 to 103 years]; men, age 67 years [range, 19 to 99 years]; SCCE: women, age 69 years [range, 24 to 107 years]; men, age 66 years [range, 20 to 103 years]). The demographic and clinicopathologic characteristics of men and women with LEC are provided in Table 1.
Table 1.
Demographic and Clinicopathologic Characteristics by Sex in Patients With LEC, SEER Data 1973-2007
| Characteristic | LEC (N = 26,848) |
||||
|---|---|---|---|---|---|
| Males (n = 19,957) |
Females (n = 6,891) |
P | |||
| No | % | No | % | ||
| Age, years | < .001 | ||||
| 18-44 | 614 | 3.1 | 139 | 2 | |
| 45-54 | 2,573 | 12.9 | 626 | 9.1 | |
| 55-64 | 5,422 | 27.2 | 1,563 | 22.7 | |
| 65-74 | 6,430 | 32.2 | 2,129 | 30.9 | |
| ≥ 75 | 4,918 | 24.6 | 2,434 | 35.3 | |
| Race | < .001 | ||||
| White | 15,026 | 75.3 | 5,196 | 75.4 | |
| African American | 2,811 | 14.1 | 1,094 | 15.9 | |
| Asian | 1,002 | 5 | 270 | 3.9 | |
| Hispanic | 1,040 | 5.2 | 301 | 4.4 | |
| Native American | 78 | 0.4 | 30 | 0.4 | |
| Histology | < .001 | ||||
| Squamous | 9,066 | 45.4 | 4,869 | 70.7 | |
| Adenocarcinoma | 8,736 | 43.8 | 1,410 | 20.5 | |
| Other | 2,155 | 10.8 | 612 | 8.9 | |
Abbreviation: LEC, locoregional esophageal cancer.
Sex, age, and survival for LEC.
In the multivariate model (Table 2 and Appendix Table A3, online only), women had significantly longer ECSS than men (4% absolute difference for 5-year ECSS rate). On the basis of our previous data in metastatic colorectal cancer, we examined the relationships between sex and ECSS by age, with patients being dichotomized below or above age 55 years for both men and women.15 The ECSS was longer for women than for men and did not differ significantly between age groups (Pinteraction = 0.32; 4% absolute difference for 5-year ECSS rate in both age groups; Appendix Table A4, online only).
Table 2.
Multivariate Analysis for ECSS in Patients With LEC, SEER Data 1973-2007
| Characteristic | LEC (N = 26,848) |
|||||
|---|---|---|---|---|---|---|
| No. of Patients | EC Death | 5-Year ECSS ± SE (%) | HR | 95% CI* | P* | |
| Sex | < .001 | |||||
| Male | 19,957 | 12,081 | 24 ± 0.3 | 1 (reference) | ||
| Female | 6,891 | 4,275 | 28 ± 0.5 | 0.920 | 0.886 to 0.955 | |
| Age, years | < .001 | |||||
| 18-44 | 753 | 438 | 31 ± 1.5 | 0.774 | 0.701 to 0.855 | |
| 45-54 | 3,199 | 1,889 | 29 ± 0.8 | 0.807 | 0.764 to 0.852 | |
| 55-64 | 6,985 | 4,124 | 29 ± 0.5 | 0.794 | 0.760 to 0.831 | |
| 65-74 | 8,559 | 5,169 | 26 ± 0.5 | 0.848 | 0.813 to 0.883 | |
| ≥ 75 | 7,352 | 4,736 | 19 ± 0.5 | 1 (reference) | ||
| Race | .031 | |||||
| White | 20,222 | 12,043 | 26 ± 0.3 | 1 (reference) | ||
| African American | 3,905 | 2,624 | 22 ± 0.6 | 1.068 | 1.016 to 1.123 | |
| Asian | 1,272 | 836 | 23 ± 1.0 | 1.089 | 0.999 to 1.186 | |
| Hispanic | 1,341 | 780 | 26 ± 1.1 | 0.989 | 0.916 to 1.067 | |
| Native American | 108 | 73 | 22 ± 3.3 | 1.133 | 0.838 to 1.533 | |
| Histology | .003 | |||||
| Squamous | 13,935 | 9,185 | 24 ± 0.4 | 1 (reference) | ||
| Adenocarcinoma | 10,146 | 5,506 | 27 ± 0.5 | 0.927 | 0.889 to 0.968 | |
| Other | 2,767 | 1,665 | 24 ± 0.7 | 0.965 | 0.911 to 1.022 | |
Abbreviations: EC, esophageal cancer; ECSS, esophageal cancer–specific survival; LEC, locoregional esophageal cancer; HR, hazard ratio.
Based on ECSS in competing risks regression model, including all variables in the table and marital status, radiation sequence (no radiation/surgery, neoadjuvant radiation, adjuvant radiation, intraoperative radiation or unknown), stage (localized v regional), SEER registries, and year of diagnosis (1973-1982, 1983-1995, 1996-2007).
Sex, age, histology, ethnicity, and survival for LEC.
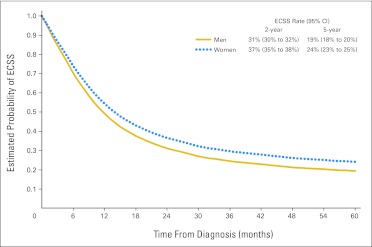
We found a significant difference in ECSS between sexes in adenocarcinoma and squamous cell carcinoma (pinteraction = 0.036, Appendix Table A4). ECSS was significantly longer for women when compared with men with squamous cell tumors (5% absolute difference for 5-year ECSS rate; Fig 1). In adenocarcinoma, this sex difference was not significant (Appendix Table A4).
Fig 1.
Adjusted survival curves of esophageal cancer–specific survival (ECSS) in patients with locoregional squamous cell carcinoma of the esophagus.
Finally, the relationship between sex, histology, and age was analyzed. In patients with adenocarcinoma, there was no significant difference in ECSS between men and women in patients younger than age 55 years or age 55 years or older. In contrast, women (n = 4,300) with squamous cell LEC age ≥ 55 years old had significantly longer ECSS than men (n = 7,660) of similar age (HR, 0.905; 95% CI, 0.862 to 0.950; P < .001; 5% absolute difference for 5-year ECSS rate). There was a trend in the association between sex (1,406 men and 569 women) and ECSS in squamous cell LEC for those younger than age 55 years (HR, 0.896; 95% CI, 0.792 to 1.014; P = .081; 4% absolute difference for 5-year ECSS rate). The effect of sex on ECSS was consistent across all ethnicities (Pinteraction > .05; Appendix Table A4).
MEC
Patient characteristics.
This study included 13,603 patients with MEC diagnosed from 1973 to 2007. The proportion of patients with MEC diagnosed in 1973 to 1982, 1983 to 1995, and 1996 to 2007 was 12.1%, 23.1%, and 64.8%, respectively (Appendix Table A2). ACE and SCCE were more commonly diagnosed in men than in women (ratio of men to women was 6.1:1 and 3.6:1, respectively).
Sex and MEC.
The median age for men was 64 years (range, 22 to 97 years), and the median age for women was 67 years (range, 21 to 95 years). When analyzing by histology, women were older in both ACE and SCCE cohorts (ACE: women, age 69 years [range, 21 to 95 years]; men, age 64 years [range, 22 to 97 years]; SCCE: women, age 66 years [range, 26 to 95 years]; men, age 64 years [range, 23 to 95 years]). The demographic and clinicopathologic characteristics of men and women with MEC are provided in Table 3.
Table 3.
Demographic and Clinicopathologic Characteristics by Sex in Patients With MEC, SEER Data 1973-2007
| Characteristic | MEC (N= 13,603) |
P | |||
|---|---|---|---|---|---|
| Males (n = 10,752) |
Females (n = 2,851) |
||||
| No. | % | No. | % | ||
| Age, years | < .001 | ||||
| 18-44 | 444 | 4.1 | 104 | 3.6 | |
| 45-54 | 1,776 | 16.5 | 377 | 13.2 | |
| 55-64 | 3,258 | 30.3 | 737 | 25.9 | |
| 65-74 | 3,202 | 29.8 | 889 | 31.2 | |
| ≥ 75 | 2,072 | 19.3 | 744 | 26.1 | |
| Race | < .001 | ||||
| White | 7,996 | 74.4 | 2,012 | 70.6 | |
| African American | 1,521 | 14.1 | 550 | 19.3 | |
| Asian | 555 | 5.2 | 128 | 4.5 | |
| Hispanic | 628 | 5.8 | 140 | 4.9 | |
| Native American | 52 | 0.5 | 21 | 0.7 | |
| Histology | < .001 | ||||
| Squamous | 4,162 | 38.7 | 1,660 | 58.2 | |
| Adenocarcinoma | 5,171 | 48.1 | 841 | 29.5 | |
| Other | 1,419 | 13.2 | 350 | 12.3 | |
Abbreviation: MEC, metastatic esophageal cancer.
Sex, age, and survival for MEC.
In the multivariate model (Table 4 and Appendix Table A3), women had significantly longer ECSS than men (2% absolute difference for 2-year ECSS rate). The difference in ECSS between sexes varied significantly by age in patients with metastatic disease (Pinteraction = .048; Appendix Table A4). Women with MEC younger than age 55 years had an ECSS better than men of similar age (5% absolute difference for 2-year ECSS rate). In contrast, this sex difference did not persist in patients ≥ 55 years old (2% absolute difference for 2-year ECSS rate).
Table 4.
Multivariate Analysis for ECSS in Patients With MEC, SEER Data 1973-2007
| Characteristic | MEC (N = 13,603) |
|||||
|---|---|---|---|---|---|---|
| No. | EC Death | 2-Year ECSS ± SE (%) | HR | 95% CI* | P* | |
| Sex | .029 | |||||
| Male | 10,752 | 8,769 | 9 ± 0.3 | 1 (reference) | ||
| Female | 2,851 | 2,289 | 11 ± 0.5 | 0.949 | 0.905 to 0.995 | |
| Age, years | < .001 | |||||
| 18-44 | 548 | 439 | 14 ± 1.3 | 0.816 | 0.742 to 0.898 | |
| 45-54 | 2,153 | 1,771 | 11 ± 0.6 | 0.910 | 0.856 to 0.968 | |
| 55-64 | 3,995 | 3,274 | 11 ± 0.4 | 0.921 | 0.872 to 0.973 | |
| 65-74 | 4,091 | 3,297 | 10 ± 0.4 | 0.907 | 0.858 to 0.958 | |
| ≥ 75 | 2,816 | 2,277 | 7 ± 0.4 | 1 (reference) | ||
| Race | .20 | |||||
| White | 10,008 | 8,166 | 10 ± 0.3 | 1 (reference) | ||
| African American | 2,071 | 1,683 | 9 ± 0.5 | 1.035 | 0.974 to 1.101 | |
| Asian | 683 | 551 | 11 ± 1.0 | 0.942 | 0.854 to 1.039 | |
| Hispanic | 768 | 597 | 11 ± 0.9 | 0.929 | 0.854 to 1.010 | |
| Native American | 73 | 61 | 7 ± 2.0 | 1.087 | 0.788 to 1.499 | |
| Histology | .027 | |||||
| Squamous | 5,822 | 4,695 | 10 ± 0.4 | 1 (reference) | ||
| Adenocarcinoma | 6,012 | 4,929 | 10 ± 0.4 | 1.071 | 1.019 to 1.125 | |
| Other | 1,769 | 1,434 | 9 ± 0.5 | 1.051 | 0.985 to 1.121 | |
Abbreviations: EC, esophageal cancer; ECSS, esophageal cancer–specific survival; HR, hazard ratio; MEC, metastatic esophageal cancer.
Based on ECSS in competing risks regression model, including all variables in the table and marital status, radiation sequence (no radiation/surgery, neoadjuvant radiation, adjuvant radiation, intraoperative radiation or unknown), SEER registries, and year of diagnosis (1973-1982, 1983-1995, 1996-2007).
Sex, age, histology, ethnicity, and survival for MEC.
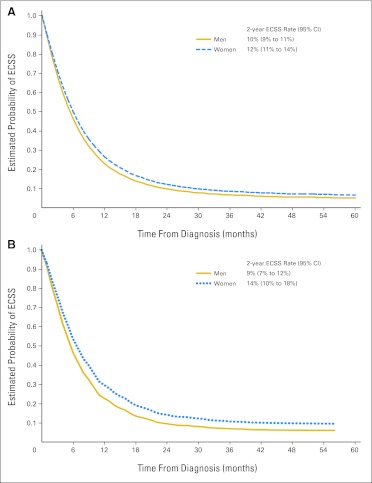
There was no significant difference for ECSS between women and men in both ACE and SCCE (Fig 2A and Appendix Table A4). However, when the relationship between sex, histology, and age was analyzed, women younger than age 55 years (n = 284) had significantly longer ECSS than men of the same age (n = 791; HR, 0.823; 95% CI, 0.708 to 0.957; P = .011; Fig 2B; 5% absolute difference for 2-year ECSS rate). In contrast, women with SCCE age ≥ 55 years (n = 1,376) had no significantly different ECSS than men of same age (n = 3,371; HR, 0.967; 95% CI, 0.902 to 1.037; P = .35). In patients with ACE, there was no significant difference in ECSS between men and women in age groups younger than age 55 years or age 55 years or older. The effect of sex on ECSS was consistent across all ethnicities (Pinteraction > .05; Appendix Table A4).
Fig 2.
Adjusted survival curves of esophageal cancer-specific survival (ECSS) in (A) all patients with metastatic squamous cell carcinoma of the esophagus and (B) in patients with metastatic squamous cell carcinoma of the esophagus younger than age 55 years.
DISCUSSION
The important burden of esophageal cancer in the United Stated is linked with its poor outcome (eighth position for cancer deaths), with both squamous cell and adenocarcinoma histology having poor prognosis.2 In the last 25 years, there has been a shift in esophageal histology with a decrease of SCCE and an increase in ACE. This shift has been attributed to the change in commonly reported risk factors.19–21 Although women experienced a shift in esophageal cancer histology similar to that of men, their rates of SCCE decrease and ACE increase were lower.22 Importantly, despite this gradual shift, SCCE remains a frequent malignancy representing 35% of all carcinomas of the esophagus.11
We demonstrate in one of the largest cohorts of patients with esophageal cancer that sex is an independent prognostic marker for patients with either LEC or MEC. Furthermore, our data suggest that sex may be prognostic only in squamous cell esophageal tumors. Previous studies have reported a prognostic value for sex in Asian patients with stage I to III SCCE.7,23,24 We hypothesized that hormonal influences, and thus menopause, could explain this prognostic difference. Age is commonly considered as a surrogate for menopause. On the basis of our previous study,15 we selected age 55 years as a surrogate for menopause. This predetermined cutoff was chosen because menopause occurs in only 5% of women after age 55 years.25 We found that in metastatic SCCE, only women younger than age 55 years had a lower risk of dying of esophageal cancer when compared with men of similar age. In contrast, women younger than or older than age 55 years with locoregional SCCE had a better prognosis, with the latter being statistically significant.
Estrogen receptors (ERs) are expressed in SCCE, and early preclinical studies26,27 have demonstrated that estrogens could inhibit squamous cell tumor growth. The influence of sex hormones was further demonstrated in in vivo studies.26,28 These antiproliferative functions are likely to occur through ER beta (ERβ), which is the predominant ER expressed in SCCE.29 In contrast to ER alpha (ERα), ERβ has antiproliferative functions.30 Unfortunately, the link between ER and esophageal cancer is currently poorly understood, mostly because of the confounding role of circulating estrogen and the concomitant expression of tumor androgen receptors (ARs). Current literature supports a biologic role of estrogens in early SCCE.12,31 These data contrast with our study, showing no survival difference between women younger than age 55 years and those age 55 years or older with localized SCCE. This apparent contradiction may be reconciled by taking into account the prosurvival signals induced by androgens. ARs are expressed in SCCE and have been shown to enhance tumor growth.26,27,32 Therefore, it is not surprising in our study that men with localized SCCE have an overall worse outcome. Our data show that the impact of age is different in localized and metastatic SCCE, suggesting that ERβ pathway signaling may be dynamic. Metastatic squamous cell tumors may lose their inhibitory ERβ capacity, which would reduce the survival benefit for women, particularly in postmenopausal women with low levels of ERβ ligand. Further studies are needed to clarify the mechanism and interaction of sex steroids in esophageal squamous cell tumors.
Although hormonal influences are strong candidates for explaining this sex difference in outcome, other prognostic factors should be considered. One candidate is the human papilloma virus (HPV). Oncogenic types of HPV have been reported to play a significant role in SCCE carcinogenesis in high-risk geographic areas (eg, East Asia). In contrast, its role in the United States is less clear, with low detection rates being reported.33,34 Moreover, in contrast to head and neck cancer in which HPV infection predominates in males and is correlated with better prognosis,35 no sex imbalance or prognostic value has been shown in SCCE.36,37 Other potential prognostic factors are alcohol or smoking status. Because the SEER database provides no information on smoking status or alcohol consumption, we could not investigate their potential prognostic influence.
In agreement with a recent SEER-based study,38 women with LEC or MEC and adenocarcinoma histology did not have a different outcome from men, irrespective of their menopausal status. ERβ and AR protein expression have been documented in adenocarcinoma of the esophagus.29,39,40 However to date, convincing preclinical data showing a direct influence of sex hormones on adenocarcinoma cells or xenografts are lacking. Altogether, although hormonal influences may play a role in the carcinogenesis of esophageal adenocarcinoma,14,41,42 ER or AR pathways may drive established adenocarcinoma of the esophagus only marginally, if at all.
Our study has several limitations related to the lack of details provided by the SEER database on the TNM tumor stage (only recently reported), on treatment information for the sequence of radiation therapy, on systemic treatment (no information), and on socioeconomic status. Despite these limitations, our results remained significant in the LEC cohort after adjusting for localized or regional stage. Our analyses also remained significant after adjusting for the year of diagnosis as a surrogate for type of therapy as follows: the study population was divided into three subsets based on year of diagnosis (1973 to 1982, 1983 to 1995, and 1996 to 2007). These cutoffs were selected on the basis of relevant publications likely to have driven a change of practice.43–45 Adjustment for the year of diagnosis also allowed controlling for the quality of the elder SEER database. Although details on socioeconomic status are not provided, our results remain significant after adjusting for marital status, previously shown to be prognostic in colorectal cancer.46
Because of the lack of details on comorbidities in the SEER database, we restricted our analyses to ECSS, which is influenced to a lesser degree than overall survival. However, we cannot exclude that our data reflect an imbalance between comorbidities between sexes. It is possible that misclassification of cardia adenocarcinoma might have obscured the prognostic influence of sex steroids in ACE.47 Finally, our findings derived from our secondary hypotheses have not been adjusted for multiple testing and therefore should be confirmed in futures studies. Despite these limitations, studies performed in population-based registries provide unique opportunities to evaluate a large number of patients. This approach is particularly useful in diseases such as esophageal cancer for which few large prospective studies are available.
Despite aggressive multimodality treatment strategies for localized SCCE, the expected 2-year survival rate remains between 20% and 50%.48 To set a benchmark, it has been estimated that adding concomitant chemotherapy to radiotherapy in localized SCCE provides a 7% reduction to 2-year mortality.49 Therefore, although the magnitude of the effect of sex in localized SCCE can be seen as small (5% absolute difference for 5-year ECSS), this may translate to the development of clinically meaningful treatment strategies in a disease in which there are a limited number of active agents. Similarly, in the metastatic setting in which no phase III trials have ever been reported, our data showing a 5% absolute difference for 2-year ECSS rate between men and women younger than age 55 years is intriguing. Nevertheless, when considering the relatively low prevalence of ECSS, the latter may have a modest impact in the United States. These data may be of critical importance for other parts of the world with a higher prevalence of SCCE.
In summary, this study shows that sex is an independent prognostic factor regardless of the extent of the disease. Several findings derived from our secondary hypotheses should be confirmed in another independent cohort: the better outcome seen in women with squamous cell MEC may be restricted to women younger than age 55 years; women younger than or older than age 55 years with squamous cell LEC have a better prognosis, with the latter being statistically significant; sex is not a significant prognostic factor in established esophageal adenocarcinoma. We suggest that these associations are possibly related to both estrogen and androgen exposition. Taken together, these data are important because SCCE remains an important burden despite the shift in histology occurring in the Western world. Moreover, in areas of the world where the SCCE incidence is high, this shift has yet not been observed with relatively stable SCCE incidences.50 If our secondary hypotheses are validated in other databases, future correlative studies in SCCE should focus on the pathways of sex steroids to elucidate the molecular mechanisms linked with poor outcome. With better understanding, the activation of these pathways may be modified, creating new approaches in SCCE treatment.
Appendix
Table A1.
Definitions of Variables and Cut Points
| Variable | Definitions and Cut Points |
|---|---|
| Sex | Male and female |
| Age | 18-44, 45-54, 55-64, 65-74, and ≥ 75 years |
| Race/ethnicity | White, African American, Asian/Pacific Islander, Hispanic (identified as having Spanish/Hispanic surname or of Spanish origin regardless of the category of race/ethnicity), and Native American |
| Histology | Adenocarcinoma, squamous cell carcinoma, and other (other histology or not specified) |
| Tumor primary site | Lower third (C152, C155), middle third (C151, C154), upper third (C150, C153), and overlapping or not specified (C158, C159) |
| Tumor grade/differentiation | Well differentiated (grade 1), moderately differentiated (grade 2), poorly differentiated or undifferentiated (grade 3 or 4), and not determined |
| Treatment | Esophagectomy (partial or total esophagectomy) and radiation therapy (beam radiation or radiation; method or source not specified). Detailed information on surgery of the primary site was not available prior to 1983. Records on chemotherapy were not available in the SEER database. |
| Radiation sequence | No radiation/surgery, neoadjuvant radiation, adjuvant radiation, and intraoperative radiation or unknown |
| Marital status at diagnosis | Not married (including never married, separated, divorced, widowed, and unknown) and married (including common law) |
| Stage | Metastatic (SEER historic stage A code 4) and locoregional (localized or regional corresponding to SEER historic stage A code 1 or 2). |
| SEER registry | 17 SEER registry sites (http://seer.cancer.gov/registries/list.html) |
| Year of diagnosis | Three intervals: 1973-1982, 1983-1995, and 1996-2007 |
Table A2.
No. of Patients by Three Intervals of Year of Diagnosis and Inclusion and Exclusion Criteria
| Variable | Year of Diagnosis |
|||||
|---|---|---|---|---|---|---|
| 1973-1982 |
1983-1995 |
1996-2007 |
||||
| No. | % | No. | % | No. | % | |
| Total No. of patients diagnosed with esophageal cancer in the SEER database | 7,400 | 14,112 | 33,108 | |||
| Total No. of patients with in situ or unstaged esophageal cancer | 2,008 | 27 | 3,667 | 26 | 6,012 | 18 |
| Total No. excluded from the analysis | 2,466 | 33 | 4,159 | 29 | 7,544 | 23 |
| Total No. included in the analysis | 4,934 | 67 | 9,953 | 71 | 25,564 | 77 |
| Stage in patients included in the analysis | ||||||
| Locoregional | 3,284 | 67 | 6,808 | 68 | 16,756 | 66 |
| Metastatic | 1,650 | 33 | 3,145 | 32 | 8,808 | 34 |
Table A3.
Multivariate Analysis for ECSS in Patients With EC by Stage, SEER Data 1973-2007
| Characteristic | LEC (n = 26,848)* |
MEC (n = 13,603)* |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | HR† | 95% CI | P† | No. of Patients | HR† | 95% CI | P† | |
| Site | ||||||||
| Lower third | 13,597 | 1 (reference) | 7,598 | 1 (reference) | ||||
| Middle third | 7,462 | 1.074 | 1.031 to 1.119 | 2,908 | 1.021 | 0.969 to 1.075 | ||
| Upper third | 2,868 | 0.920 | 0.868 to 0.975 | 964 | 0.958 | 0.884 to 1.039 | ||
| Overlapping or NOS | 2,921 | 1.094 | 1.037 to 1.154 | < .001 | 2,133 | 0.986 | 0.932 to 1.043 | .44 |
| Tumor grade | ||||||||
| 1 | 1,695 | 0.695 | 0.647 to 0.747 | 475 | 0.829 | 0.751 to 0.915 | ||
| 2 | 9,108 | 0.847 | 0.818 to 0.878 | 3,679 | 0.867 | 0.831 to 0.905 | ||
| 3 or 4 | 10,944 | 1 (reference) | 6,707 | 1 (reference) | ||||
| Not determined | 5,101 | 0.785 | 0.751 to 0.820 | < .001 | 2,742 | 0.868 | 0.825 to 0.912 | < .001 |
| Radiation | ||||||||
| No or unknown | 10,283 | 1 (reference) | 6,226 | 1 (reference) | ||||
| Yes | 16,565 | 0.993 | 0.952 to 1.035 | .73 | 7,377 | 0.806 | 0.775 to 0.838 | < .001 |
| Esophagectomy | ||||||||
| No or unknown | 18,793 | 1 (reference) | 12,746 | 1 (reference) | ||||
| Yes | 8,055 | 0.593 | 0.566 to 0.622 | < .001 | 857 | 0.651 | 0.600 to 0.707 | < .001 |
| Radiation sequence | ||||||||
| No radiation/surgery | 21,807 | 1 (reference) | 12,577 | 1 (reference) | ||||
| Neoadjuvant radiation | 2,886 | 0.873 | 0.818 to 0.932 | 346 | 0.700 | 0.619 to 0.793 | ||
| Adjuvant radiation | 1,967 | 0.930 | 0.875 to 0.989 | 641 | 0.936 | 0.866 to 1.012 | ||
| Intraoperative radiation or unknown | 188 | 0.914 | 0.777 to 1.074 | < .001 | 39 | 0.966 | 0.735 to 1.269 | < .001 |
| Marital status at diagnosis | ||||||||
| Not married | 11,383 | 1 (reference) | 5,698 | 1 (reference) | ||||
| Married | 15,465 | 0.893 | 0.864 to 0.923 | < .001 | 7,905 | 0.934 | 0.899 to 0.971 | < .001 |
Abbreviations: EC, esophageal cancer; ECSS, esophageal cancer–specific survival; HR, hazard ratio; LEC, locoregional esophageal cancer; MEC, metastatic esophageal cancer; NOS, not otherwise specified.
In all, 16,356 ECSS deaths and 4,154 non-ECSS deaths occurred among the LEC cohort, and 11,058 ECSS deaths and 1,622 non-ECSS deaths occurred among the MEC cohort.
Based on disease-specific survival in competing risks regression model, including all variables in this table plus sex, age, race, and histology, SEER registries, and year of diagnosis (1973-1982, 1983-1995, 1996-2007). Stage (localized v regional) was adjusted in patients with LEC.
Table A4.
Summarizing the Tests for Interaction: Testing for the Consistency for HR Comparing ECSS Between Women and Men, According to Age, Histology, and Race
| Characteristic | LEC |
MEC |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P for Interaction* | HR | 95% CI | P for Interaction* | |
| Age, years | .32 | .048 | ||||
| < 55 | 0.893 | 0.798 to 0.998 | 0.812 | 0.727 to 0.906 | ||
| ≥ 55 | 0.922 | 0.886 to 0.960 | 0.975 | 0.926 to 1.028 | ||
| Histology | .036 | .18 | ||||
| Squamous | 0.907 | 0.867 to 0.948 | 0.945 | 0.887 to 1.006 | ||
| Adenocarcinoma | 1.002 | 0.926 to 1.084 | 1.018 | 0.937 to 1.106 | ||
| Race | .80 | .70 | ||||
| White | 0.917 | 0.877 to 0.959 | 0.963 | 0.910 to 1.019 | ||
| African American | 0.901 | 0.825 to 0.984 | 0.896 | 0.807 to 0.996 | ||
| Asian | 0.909 | 0.764 to 1.080 | 0.889 | 0.718 to 1.100 | ||
| Hispanic | 1.035 | 0.864 to 1.239 | 1.058 | 0.859 to 1.304 | ||
Abbreviations: ECSS, esophageal cancer–specific survival; HR, hazard ratio; LEC, locoregional esophageal cancer; MEC, metastatic esophageal cancer.
Patients with other histology were excluded for testing interaction between sex and histology. Native American patients were excluded for testing interaction between sex and race because of their small number.
Fig A1.
A flowchart of patient selection from the SEER database.
Footnotes
Supported by the San Pedro Foundation, by Yvonne Bogdanovich, by Grant No. 5P30CA014089-27S1 from the National Institutes of Health P30CA, by Grant No. BIL KLS 02591-02-2010 from “Ligue Suisse contre le Cancer” (P.B.), and by grants from the Austrian Society of Hematology and Oncology, the Bank Austria Visiting Scientists Program, and the “Verein fuer Krebskranke” of the Medical University Graz (A.G.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Pierre Bohanes, Dongyun Yang, Ruchika S. Chhibar, Melissa J. Labonte, Thomas Winder, Yan Ning, Wu Zhang, Heinz-Josef Lenz
Financial support: Heinz-Josef Lenz
Administrative support: Rita El-Khoueiry
Collection and assembly of data: Dongyun Yang, Ruchika S. Chhibar, David Paez, Rita El-Khoueiry
Data analysis and interpretation: Pierre Bohanes, Dongyun Yang, Ruchika S. Chhibar, Melissa J. Labonte, Armin Gerger, Léonor Benhaim, Takeru Wakatsuki, Fotios Loupakis, Wu Zhang, Heinz-Josef Lenz
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber D, Rineer J, Vongtama D, et al. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol. 2010;5:244–250. doi: 10.1097/JTO.0b013e3181c5e34f. [DOI] [PubMed] [Google Scholar]
- 4.Salek R, Bezenjani SE, Saedi HS, et al. A geographic area with better outcome of esophageal carcinoma: Is there an effect of ethnicity and etiologic factors? Oncology. 2009;77:172–177. doi: 10.1159/000231887. [DOI] [PubMed] [Google Scholar]
- 5.Schwer AL, Ballonoff A, McCammon R, et al. Survival effect of neoadjuvant radiotherapy before esophagectomy for patients with esophageal cancer: A Surveillance, Epidemiology, and End Results study. Int J Radiat Oncol Biol Phys. 2009;73:449–455. doi: 10.1016/j.ijrobp.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Paulson EC, Ra J, Armstrong K, et al. Underuse of esophagectomy as treatment for resectable esophageal cancer. Arch Surg. 2008;143:1198–1203. doi: 10.1001/archsurg.143.12.1198. [DOI] [PubMed] [Google Scholar]
- 7.Morita M, Yoshida R, Ikeda K, et al. Advances in esophageal cancer surgery in Japan: An analysis of 1000 consecutive patients treated at a single institute. Surgery. 2008;143:499–508. doi: 10.1016/j.surg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Badwe RA, Patil PK, Bhansali MS, et al. Impact of age and sex on survival after curative resection for carcinoma of the esophagus. Cancer. 1994;74:2425–2429. doi: 10.1002/1097-0142(19941101)74:9<2425::aid-cncr2820740906>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Sugimachi K, Matsuoka H, Matsufuji H, et al. Survival rates of women with carcinoma of the esophagus exceed those of men. Surg Gynecol Obstet. 1987;164:541–544. [PubMed] [Google Scholar]
- 10.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 11.Howlader N, Noone AM, Krapcho M, et al., editors. Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975-2008. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 12.Wang QM, Qi YJ, Jiang Q, et al. Relevance of serum estradiol and estrogen receptor beta expression from a high-incidence area for esophageal squamous cell carcinoma in China. Med Oncol. 2011;28:188–193. doi: 10.1007/s12032-010-9457-8. [DOI] [PubMed] [Google Scholar]
- 13.Derakhshan MH, Liptrot S, Paul J, et al. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 14.Marmo R, Rotondano G, Piscopo R, et al. Combination of age and sex improves the ability to predict upper gastrointestinal malignancy in patients with uncomplicated dyspepsia: A prospective multicentre database study. Am J Gastroenterol. 2005;100:784–791. doi: 10.1111/j.1572-0241.2005.40085.x. [DOI] [PubMed] [Google Scholar]
- 15.Hendifar A, Yang D, Lenz F, et al. Gender disparities in metastatic colorectal cancer survival. Clin Cancer Res. 2009;15:6391–6397. doi: 10.1158/1078-0432.CCR-09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Surveillance, Epidemiology, and End Results, Overview of the SEER program. http://seer.cancer.gov/about/overview.html.
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 19.Engel LS, Chow WH, Vaughan TL, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 20.Corley DA, Kubo A, Levin TR, et al. Helicobacter pylori infection and the risk of Barrett's oesophagus: A community-based study. Gut. 2008;57:727–733. doi: 10.1136/gut.2007.132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 22.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang BY, Goan YG, Hsu PK, et al. Tumor length as a prognostic factor in esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91:887–893. doi: 10.1016/j.athoracsur.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Tong DK, Law S, Kwong DL, et al. Histological regression of squamous esophageal carcinoma assessed by percentage of residual viable cells after neoadjuvant chemoradiation is an important prognostic factor. Ann Surg Oncol. 2010;17:2184–2192. doi: 10.1245/s10434-010-0995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14:103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 26.Ueo H, Matsuoka H, Sugimachi K, et al. Inhibitory effects of estrogen on the growth of a human esophageal carcinoma cell line. Cancer Res. 1990;50:7212–7215. [PubMed] [Google Scholar]
- 27.Matsuoka H, Sugimachi K, Ueo H, et al. Sex hormone response of a newly established squamous cell line derived from clinical esophageal carcinoma. Cancer Res. 1987;47:4134–4140. [PubMed] [Google Scholar]
- 28.Utsumi Y, Nakamura T, Nagasue N, et al. Role of estrogen receptors in the growth of human esophageal carcinoma. Cancer. 1989;64:88–93. doi: 10.1002/1097-0142(19890701)64:1<88::aid-cncr2820640116>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Kalayarasan R, Ananthakrishnan N, Kate V, et al. Estrogen and progesterone receptors in esophageal carcinoma. Dis Esophagus. 2008;21:298–303. doi: 10.1111/j.1442-2050.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 30.Hartman J, Ström A, Gustafsson JA. Estrogen receptor beta in breast cancer: Diagnostic and therapeutic implications. Steroids. 2009;74:635–641. doi: 10.1016/j.steroids.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Nozoe T, Oyama T, Takenoyama M, et al. Significance of immunohistochemical expression of estrogen receptors alpha and beta in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2007;13:4046–4050. doi: 10.1158/1078-0432.CCR-07-0449. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita Y, Hirai T, Mukaida H, et al. Detection of androgen receptors in human esophageal cancer. Jpn J Surg. 1989;19:195–202. doi: 10.1007/BF02471585. [DOI] [PubMed] [Google Scholar]
- 33.Malik SM, Nevin DT, Cohen S, et al. Assessment of immunohistochemistry for p16INK4 and high-risk HPV DNA by in situ hybridization in esophageal squamous cell carcinoma. Int J Surg Pathol. 2011;19:31–34. doi: 10.1177/1066896910382005. [DOI] [PubMed] [Google Scholar]
- 34.Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721–728. doi: 10.1136/jcp.55.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tribius S, Ihloff AS, Rieckmann T, et al. Impact of HPV status on treatment of squamous cell cancer of the oropharynx: What we know and what we need to know. Cancer Lett. 2011;304:71–79. doi: 10.1016/j.canlet.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Antonsson A, Nancarrow DJ, Brown IS, et al. High-risk human papillomavirus in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2080–2087. doi: 10.1158/1055-9965.EPI-10-0033. [DOI] [PubMed] [Google Scholar]
- 37.Dreilich M, Bergqvist M, Moberg M, et al. High-risk human papilloma virus (HPV) and survival in patients with esophageal carcinoma: A pilot study. BMC Cancer. 2006;6:94. doi: 10.1186/1471-2407-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon N, Zhuge Y, Cheung M, et al. The roles of neoadjuvant radiotherapy and lymphadenectomy in the treatment of esophageal adenocarcinoma. Ann Surg Oncol. 2010;17:791–803. doi: 10.1245/s10434-009-0819-4. [DOI] [PubMed] [Google Scholar]
- 39.Akgun H, Lechago J, Younes M. Estrogen receptor-beta is expressed in Barrett's metaplasia and associated adenocarcinoma of the esophagus. Anticancer Res. 2002;22:1459–1461. [PubMed] [Google Scholar]
- 40.Tihan T, Harmon JW, Wan X, et al. Evidence of androgen receptor expression in squamous and adenocarcinoma of the esophagus. Anticancer Res. 2001;21:3107–3114. [PubMed] [Google Scholar]
- 41.van Soest EM, Siersema PD, Dieleman JP, et al. Age and sex distribution of the incidence of Barrett's esophagus found in a Dutch primary care population. Am J Gastroenterol. 2005;100:2599–2600. doi: 10.1111/j.1572-0241.2005.00305_6.x. [DOI] [PubMed] [Google Scholar]
- 42.van Blankenstein M, Looman CW, Johnston BJ, et al. Age and sex distribution of the prevalence of Barrett's esophagus found in a primary referral endoscopy center. Am J Gastroenterol. 2005;100:568–576. doi: 10.1111/j.1572-0241.2005.40187.x. [DOI] [PubMed] [Google Scholar]
- 43.Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 44.Advani SH, Saikia TK, Swaroop S, et al. Anterior chemotherapy in esophageal cancer. Cancer. 1985;56:1502–1506. doi: 10.1002/1097-0142(19851001)56:7<1502::aid-cncr2820560704>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 45.Ezdinli EZ, Gelber R, Desai DV, et al. Chemotherapy of advanced esophageal carcinoma: Eastern Cooperative Oncology Group experience. Cancer. 1980;46:2149–2153. doi: 10.1002/1097-0142(19801115)46:10<2149::aid-cncr2820461006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Wilson SE, Stewart DB, et al. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: Does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011;35:417–422. doi: 10.1016/j.canep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Souza RF, Spechler SJ. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA Cancer J Clin. 2005;55:334–351. doi: 10.3322/canjclin.55.6.334. [DOI] [PubMed] [Google Scholar]
- 48.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 49.Rebecca WO, Richard MA. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2003;1:CD002092. doi: 10.1002/14651858.CD002092. [DOI] [PubMed] [Google Scholar]
- 50.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident—Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–735. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]