Abstract
Extracellular matrix (ECM) hydrogels with patterned lumens have been used as a framework to generate more physiologically relevant models of tissues, such as vessels and mammary ducts, for biological investigations. However, these models have not found widespread use in research labs or in high-throughput screening applications in large part because the basic methods for generating the lumen structures are generally cumbersome and slow. Here we present viscous finger patterning, a technique to generate lumens through ECM hydrogels in microchannels that can be accomplished using manual or automated pipetting. Passive pumping is used to flow culture media through an unpolymerized hydrogel, creating a lumen through the hydrogel that is subsequently polymerized. Viscous finger patterning takes advantage of viscous fingering, the fluid dynamics phenomenon where a less viscous fluid will flow through and displace a more viscous fluid. We have characterized the technique and used it to create a variety of channel geometries and ECM hydrogel compositions, as well as for the generation of lumens surrounded by multiple hydrogel layers. Because viscous finger patterning can be performed with automated liquid handling systems, high-throughput generation of ECM hydrogels with patterned lumen is enabled. The ability to rapidly and cost-effectively create large numbers of lumens in natural polymers overcomes a critical barrier to the use of more physiologically relevant tissue models in a variety of biological studies and drug screening applications.
Keywords: tissue engineering, extracellular matrix, hydrogel, viscous fingering, high-throughput
Introduction
The generation of lumen structures through extracellular matrix (ECM) hydrogels is an important step for generating tissue models that mimic the in vivo structure and function of tissues with tubule structures. Such models can contribute to our understanding of the structure–function relationship in both normal and diseased tissue as well as provide more physiologically relevant models for drug screening. To date, several methods have been used to produce ECM hydrogels with lumen structures to successfully model and gain insight into tissues with vessel and ductal structures.1-3 However, the ability to produce 3D tissue models in a cost-effective and high-throughput manner is needed to expand the scope of their use in drug screening assays, organ regeneration studies, and other biological studies.
Nelson et al1 demonstrated the ability to produce arrays of patterned ductal models by using microfluidic stamps to pattern lumen structures into a bottom collagen gel layer and subsequently adding a collagen overlay. Although stamping methods can he used to generate many samples and explore important geometric cues, the models are not physically independent (e.g., compartmentalized) from one another, making studies of multiple conditions in a single array difficult; nor is it straightforward to provide flow into the model. Others have patterned lumens through ECM hydrogels by polymerizing a collagen I hydrogel around needles inserted into a microchannel that are removed following polymerization.2 However, this method is limited to straight-channel geometries and is relatively low throughput. Other methods to pattern cells into vessel-like structures have been developed, but they have not been shown using ECM hydrogels, which would more closely model the in vivo environment.4-7 Several methods have been developed that do pattern cells into vessel-like structures using ECM hydrogels, but they share one or more of the following traits: low throughput, noncompartmentalized, lack of a tubule structure with a lumen, and/or difficulty in generating flow through the lumens.3,8,9 In addition, previous methods generally require specialized apparatus and/or instrumentation presenting an ease of use and cost barrier to widespread adoption.
Here we present viscous finger patterning, a method that uses the principle of viscous fingering to overcome challenges to patterning lumens through ECM hydrogels in a more high-throughput manner. Viscous fingering occurs when a less viscous fluid displaces a more viscous fluid, resulting in finger-like projections of the less viscous fluid.10 Viscous fingering has been studied in porous media, Hele-Shaw cells, and channels experimentally and/or via numerical simulation and has applications to oil recovery.10-15 Here we implement viscous fingering via surface tension-driven pumping (aka passive pumping)16 to flow a less viscous fluid through an unpolymerized ECM hydrogel in a microchannel. The less viscous fluid displaces the hydrogel along the length of a microchannel, leaving behind a continuous lumen after the hydrogel has fully polymerized. A summary of the procedure is shown in Figure 1 as well as the supplementary video. Automated liquid handling systems can be employed to perform all steps required to generate arrays of physically independent lumens in a high-throughput manner.17 Perfusable lumens in multiple different geometries (e.g., straight, curved, branched) as well as lumens surrounded by multiple layers of varying ECM compositions or cell types are demonstrated. Viscous finger patterning provides the ability to pattern lumens through ECM hydrogels using existing infrastructure, overcoming a key practical obstacle toward the high-throughput generation of tissue models with tubule structures.
Figure 1.
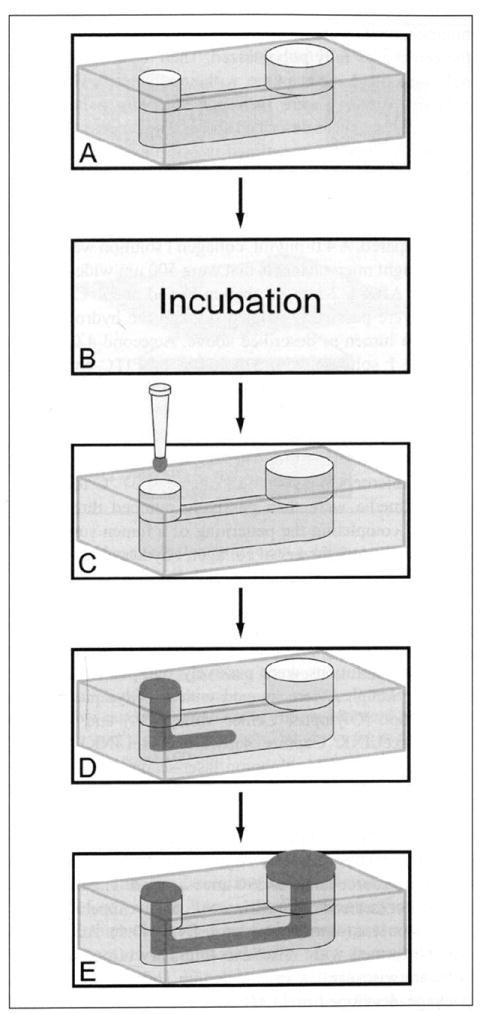
Process used for patterning lumens through an extracellular matrix (ECM) hydrogel via viscous finger patterning. First a microchannel is filled with an ECM hydrogel solution (A). The microchannels are incubated at 37 °C for a period of time depending on desired lumen dimensions, channel geometry, and ECM composition (B). Then passive pumping is performed by placing a droplet of culture media on the inlet (C), flushing out the ECM solution in the center of the microchannel (D), and leaving a continuous lumen through the ECM hydrogel (E).
Materials and Methods
Device Fabrication
Polydimethylsiloxane (PDMS; Sylgard 184 Silicone Elastomer Kit; Dow Corning, Midland, MI) base and cross-linker were mixed at a 10:1 ratio and degassed for 45 to 60 min under vacuum at room temperature. The degassed PDMS was then poured over SU-8 master molds, which were generated using standard soft lithography methods.18 The PDMS was cured at 80 °C for 4 h and allowed to cool to room temperature prior to removal from the master mold. Microchannel geometries used in these experiments varied depending on the experiment.
Preparation of ECM Hydrogels
Collagen I solutions were prepared at final concentrations between 2.0 and 7.0 mg/mL depending on the experiment by neutralizing an acidic collagen solution (9.03 mg/mL; rat tail collagen I; BD Biosciences, Bedford, MA). A basic solution (5 N NaOH) was used to neutralize the collagen I solution. First, 5× phosphate-buffered saline (PBS) was added to a volume of the collagen stock (one-fifth of the total volume) to make 1 × PBS after neutralization. Culture media were added to adjust the final collagen concentration. NaOH solution was then added at a volume equal to 2% of the collagen stock volume added. Prior to channel loading, the final collagen solution was incubated on ice for 5 to 10 min to apply an additional nucleation phase.19 Other ECM hydrogels used included Matrigel (BD Biosciences) diluted to 50% by culture media and a mixture of 50% Matrigel with 1.0 mg/mL collagen I at final concentration. To prepare the 50% Matrigel solution, 100% Matrigel was diluted with culture media in equal parts. To prepare the mixed gel solution, a 2.0 mg/mL collagen I solution was prepared and incubated on ice between 5 and 10 min, and then an equal part of 100% Matrigel was added.
Viscous Finger Patterning
To pattern lumens through collagen I, Matrigel, or a mixed gel, the solution was first loaded into a microchannel via passive pumping. The channels were then incubated at 37 °C for between 0 and 5 min to partially polymerize and increase the viscosity of the solution.20 The incubation time varied depending on ECM composition, channel geometry, and desired lumen dimensions. When using passive pumping to pattern a single, continuous lumen through an ECM hydrogel, a droplet of culture media with a volume about half of the total channel volume was added to the inlet of a microchannel. The lower viscosity fluid displaced the center of the more viscous hydrogel, patterning a lumen through the hydrogel along the length of the microchannel. Incubation at 37 °C was resumed until polymerization was complete. A summary of the procedure is shown in Figure 1 as well as the supplementary video.
Viscous finger patterning was used to pattern lumens through hydrogels in channels with straight or curved geometries. To pattern branching lumens, Y-shaped channels with two inlet ports and one outlet port were used. First, the Y-channels were filled with a 4.5-mg/mL collagen I hydrogel and incubated for 2 min at 37 °C. Culture media were then passively pumped through both inlets, one after the other, toward the common outlet to pattern a branching lumen.
To pattern two parallel lumens through an ECM hydrogel, we designed a device with two 400-μm-tall microchannels controlled by separate inlet and outlet ports and joined by a 200-μm-tall connecting channel. The device was first filled with a hydrogel solution, which was subsequently aspirated from each of the two outlets in sequence using a micropipette. The hydrogel was pinned in the 200-μm chamber but was removed from the side chambers. The microchannels were incubated until the hydrogel pinned in the center was fully polymerized. Then the two side channels were filled one at a time, with unpolymerized hydrogel solution. Lumens were then independently patterned in each side channel as described above. The process for creating parallel lumens is outlined in Supplementary Figure S1.
To demonstrate the ability to pattern multiple concentric ECM layers surrounding a lumen, two collagen I solutions were prepared. A 4.0-mg/mL collagen I solution was loaded into straight microchannels that were 500 μm wide and 500 μm tall. After a 2-min incubation period at 37 °C, culture media were passively pumped through the hydrogel, patterning a lumen as described above. A second 4.0-mg/mL collagen I solution with a solution of FITC-conjugated beads (2.1 μm diameter, 0.1% w/v; Spherotech, Lake Forest, IL) making up 33% of the total volume was prepared. The collagen I/bead solution was then passively pumped into the microchannels, filling the previously formed lumens, and the microchannels were incubated at 37 °C for 2 min. Culture media were then passively pumped through the channel, completing the patterning of a lumen surrounded by two concentric layers of collagen I hydrogels.
Imaging
To visualize lumens through ECM hydrogels, blue or green food color solutions were passively pumped through the lumens. Samples were imaged with an Olympus SZX12 stereoscope (Olympus, Center Valley, PA) and acquired using PixeLINK Capture software (PixeLINK, Ottawa, Ontario, Canada). Multiphoton laser-scanning microscopy (MPLSM) imaging was performed on an Optical Workstation that was constructed around a Nikon Eclipse TE300 (Nikon, Mehlville, NY). A MaiTai Deepsee Ti:sapphire laser (Spectra Physics, Mountain View, CA) excitation source tuned to 890 nm was used. The beam was focused onto the sample with a Nikon 10× SuperFluor air-immersion lens (numerical aperture [NA]=0.5). Acquisition was performed with WiscScan (http://www.loci.wisc.edu/software/wiscscan), a laser-scanning software acquisition package developed at LOCI (Laboratory for Optical and Computational Instrumentation, University of Wisconsin, Madison, WI) described elsewhere.21 For volume-rendered images, stacks were acquired and volume-rendered images were generated using OsiriX imaging software.
Characterization
We characterized lumen width through collagen I hydrogels under various conditions. First, straight microchannels with widths ranging from 200 to 1000 μm, increasing in 200-μm increments, were used to test the effects of channel width on lumen width. Microchannel dimensions were as follows: 3.5 mm in length, with a 1-mm inlet port diameter and 1.5-mm outlet port diameter. Microchannels were filled with a 4.5-mg/mL collagen I solution and incubated for 4 min at 37 °C before viscous finger patterning was performed.
Microchannels were modified to incorporate constrictions adjacent to the inlet and outlet ports to control lumen dimensions (Suppl. Fig. S2). The effects of constriction width and input port diameter on lumen widths were tested. Microchannels were 1 mm wide and had output port diameters of 2 mm, constriction lengths of 0.75 mm adjacent to each port, and total lengths of 5 mm. The effects of constriction width on lumen width were tested with 1-mm channels with constrictions ranging from 300 to 600 μm in 100-μm intervals and had input port diameters of 1.5 mm. The effects of inlet port size on lumen width channels were tested with port diameters ranging from 1 to 2 mm, increasing in 0.25-mm intervals; all had constriction widths of 400 μm. In each case, microchannels were filled with a 4.5-mg/mL collagen I solution and incubated for 4 min at 37 °C before viscous finger patterning was performed.
The effects of incubation time and collagen I concentration on lumen widths were tested. Microchannels were 400 μm tall and 1 mm wide with 400-μm constrictions, with 1.5-mm inlet diameters and 2-mm outlet diameters. The constrictions were 0.75 mm in length with a total channel length of 5 mm. To test the effect of incubation time at 37 °C on lumen width, microchannels were filled with 4.5 mg/mL collagen I solution. Incubation times ranged from 2 to 5 min before viscous finger patterning was performed. To test the effect of collagen I concentration on lumen width, microchannels were filled with collagen solutions between 3.0 and 7.0 mg/mL. All channels were incubated for 4 min at 37 °C before viscous finger patterning was performed.
For each of these experiments, lumens in 5 microchannels for each condition were imaged. Images were taken along the length of the microchannel where the lumen was the widest using MPLSM imaging. The lumen widths were measured from these images in three places along the length of each lumen, resulting in 15 measurements per condition. For microchannels with constrictions, measurements were only taken along the length of the nonconstriction portion of the channel. The averages and standard deviations were calculated for each condition.
Results and Discussion
Passive pumping was used to form continuous lumens through hydrogels in microchannels via viscous finger patterning. Figure 2 shows a representative image of a lumen formed through a collagen I hydrogel in a microchannel. This technique was also used to generate lumens through 50% Matrigel and 50% Matrigel with 1.0 mg/mL collagen I as well as 4.5 mg/mL collagen I in straight microchannels (Suppl. Fig. S3). A characterization of lumens patterned through collagen I hydrogels showed that lumen dimensions depended on channel geometry, incubation period, and hydrogel concentration (Fig. 3).
Figure 2.
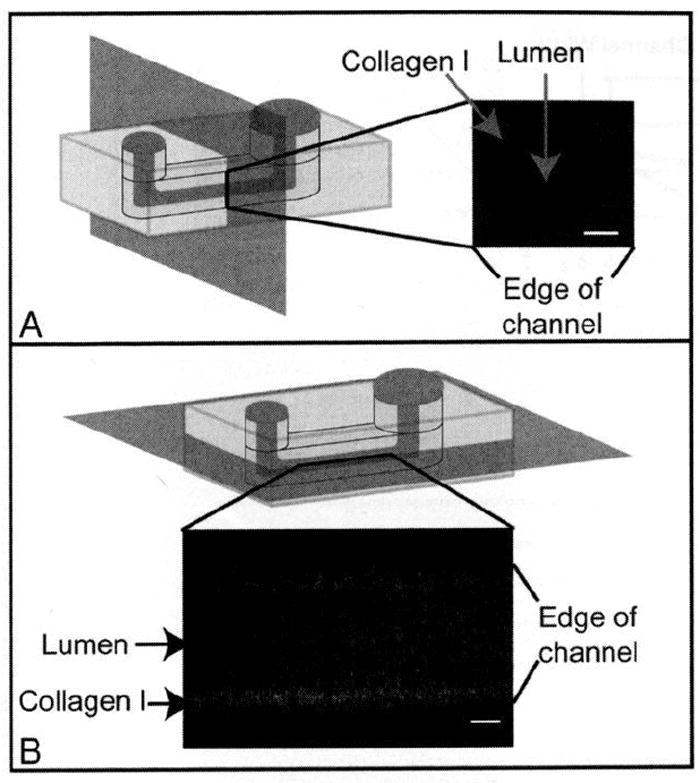
Images of a lumen through a collagen I hydrogel. (A) Volume-rendered cross section. (B) Top view. For this image, a concentration of 4.5 mg/mL collagen I was used for the hydrogel. The microchannel was incubated for 2 min at 37 °C before viscous finger patterning. The microchannel was 500 μm tall, 500 μm wide, and 5 mm long, with inlet and outlet diameters of 1 mm and 1.75 mm, respectively. A z-stack of images was taken using multiphoton laser-scanning microscopy (MPLSM) imaging and volume-rendered using Osirix. Figure 3B represents a slice taken at a height of approximately 250 μm in the microchannel. Scale bars represent 100 μm.
Figure 3.
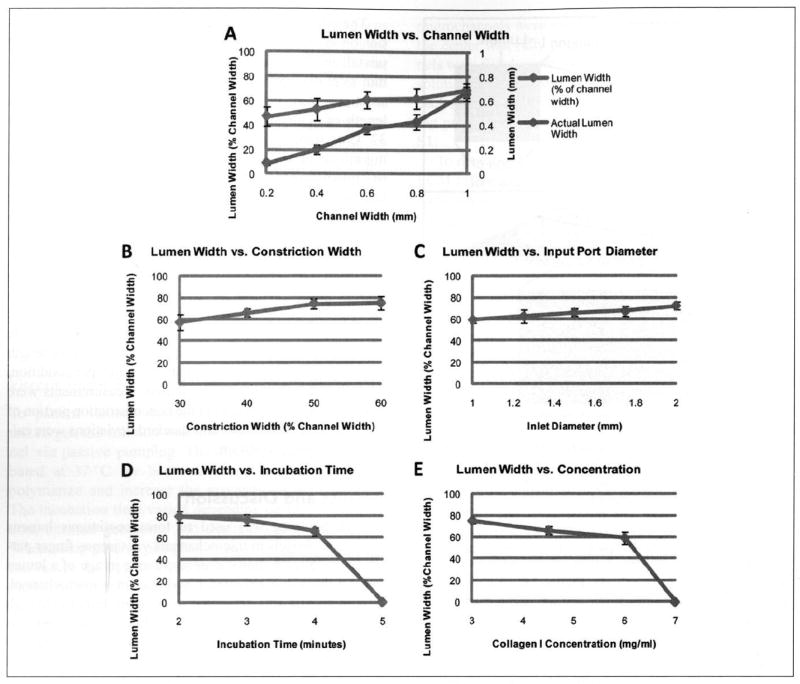
Lumen widths can be controlled by varying channel geometry, incubation time, and hydrogel composition. (A) Lumens were generated through 4.5-mg/mL collagen I hydrogels after a 4-min incubation in straight microchannels of varying widths (no constrictions). (B) Lumens were generated through 4.5-mg/mL collagen I hydrogels after a 4-min incubation in microchannels with constrictions of varying widths adjacent to the inlet and outlet ports. (C) Lumens were generated through 4.5-mg/mL collagen I hydrogels after a 4-min incubation in microchannels with varying inlet port diameters. (D) Lumens were generated through 4.5-mg/mL collagen I hydrogels with varying incubation times. (E) Lumens were generated through collagen I hydrogels with varying concentrations after a 4-min incubation.
Viscous finger patterning was performed through 4.5-mg/mL collagen I hydrogels after incubation for 4 min in microchannels with widths between 200 and 1000 μm (Suppl. Fig. S4). Average lumen widths ranged between 86 and 700 μm as microchannel width was increased (Fig. 3A). As a way to control the diameter of the pumping fluid stream, constrictions were added adjacent to the inlet and outlet ports to control the diameter of the displacing fluid stream. As the constriction width was decreased, the displacing fluid stream was confined to a smaller diameter, resulting in lumens with smaller diameters (Fig. 3B). Inlet port size was varied to control the flow rate. When smaller ports were used, the pumping fluid flowed at a faster rate,22 and lumen widths were decreased (Fig. 3C). This is consistent with numerical models of viscous fingering that predict fingers with smaller diameters when the less viscous fluid is flowed through the more viscous fluid at a faster rate. 10,14,15
Lumen width decreased with increasing incubation time for constant collagen concentration and channel geometry (Fig. 3D). When the incubation time was increased to 5 min, the hydrogel stiffness was increased via polymerization20 to the point where lumen formation did not occur at the concentration of 4.5 mg/mL collagen I. Finally, as the collagen I concentration increased, lumen width decreased for constant incubation time and channel geometry (Fig. 3E). At the highest concentration tested (7 mg/mL), the solution was too stiff (after the 4-min incubation) for lumen formation. However, lumens could be formed through 7-mg/mL collagen I hydrogels after an incubation period of 2 min or less (data not shown). In general, increasing the viscosity of the hydrogel solution by increasing the concentration or incubation time caused the lumen width to decrease. The relationship between viscosity and concentration/incubation time is consistent with numerical models of viscous fingering that predict fingers with smaller diameters when the viscosity difference between the two solutions is increased.10,14,15
In the model presented by Kang et al.,14 a viscosity ratio of 10 resulted in finger widths that were 75.4% of the channel width. The viscosity of a 3- to 5-mg/mL collagen I solution has been shown to increase from 0.1 P to over 100 P during polymerization after an initial lag phase.20 Using 0.1 P as a minimum estimate for the viscosity for a collagen I solution and estimating the viscosity of the culture media to be around 0.01 P, the viscosity ratio between the collagen and media was estimated to be at least 10. Overall, the range of lumen diameters observed here has been near this predicted value (Fig. 3).
Dong et al.15 performed a simulation of viscous fingering in a 2D channel, taking into account viscosity, surface tension, and gravity. The model presented by Dong et al.15 also showed that the height dimensions of viscous fingers became less symmetric as Bond number was increased (i.e., gravitational effects increased relative to surface tension effects). However, Bond number at the microscale is typically very small, so any gravitational effects on the height dimensions of the lumen are negligible. Thus, by symmetry, a similar characterization of lumen height based on channel geometry, incubation time, and concentration is expected to yield similar results to the characterization of lumen width. By varying the channel geometry, incubation time, and hydrogel composition to achieve the desired lumen dimensions, viscous finger patterning can be adapted for a variety of applications.
Other applications of viscous finger patterning include varying the channel geometry to generate lumens in curved (Fig. 4A) or branching (Fig. 4B) geometries. Using the method shown in Supplementary Figure S1, parallel lumens connected by a hydrogel can be patterned independently in a single device (Fig. 4C). Because the lumens can be patterned independently, the diameter of each lumen can be varied for specific applications by altering hydrogel composition and channel geometry for each of the side channels.
Figure 4.
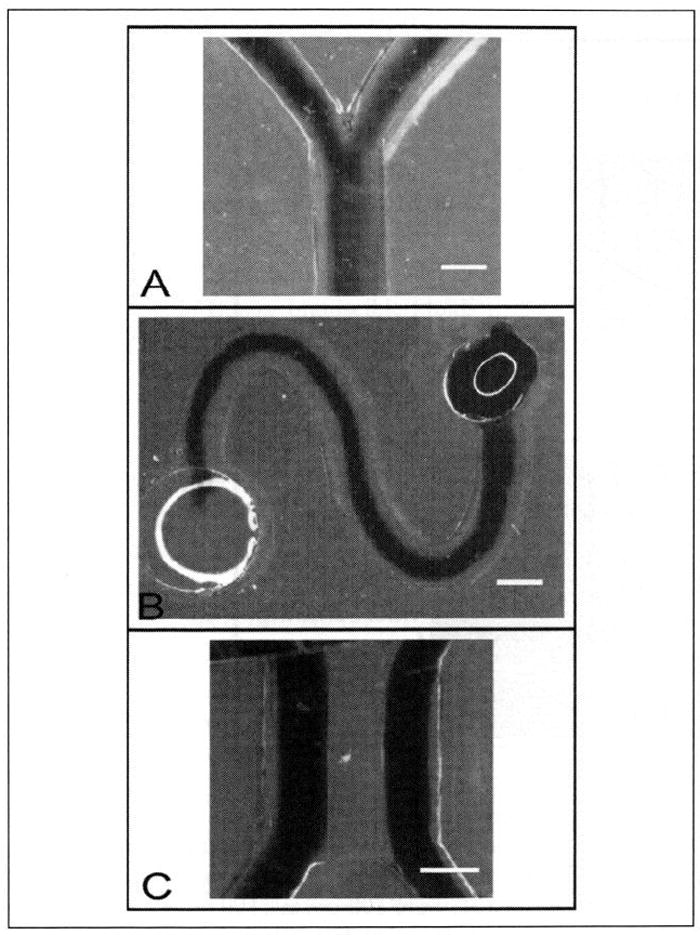
Image of dye flowed through lumens formed through a 4.5-mg/mL collagen I hydrogel in branching (A) or curving (B) microchannels. Microchannels were 300 μm in height with inlet and outlet diameters of 1 mm and 1.75 mm, respectively. The collagen I hydrogel was incubated for 2 min at 37 °C before lumen formation. (C) Dye flowed through two lumens formed through a 4.125-mg/mL collagen I hydrogel after incubation for 2 min. Two 400-μm tall, 500-μm wide chambers with inlet and outlet port diameters of 1 mm were separated by a 200-μm tall, 500-μm wide center chamber. Scale bars represent 500 μm.
It is also possible to use viscous finger patterning to generate lumens surrounded by multiple concentric ECM hydrogel layers (Fig. 5). The ability to create multiple layers extends the potential applications as multiple cell types or various ECM compositions could be layered around a cell-lined lumen for more in vivo–like tissue models. A microscale culture system using layers of different cell types in ECM hydrogels to model blood vessel layers has been developed, but this system did not form a tubular vessel structure.8 Concentric layering patterns have been demonstrated using a sequential assembly method to pattern and assemble concentric rings of synthetic polymer hydrogels laden with different cell types into a tubule structure, but this method has not yet been shown with ECM hydrogel materials.7 We have previously demonstrated similar hydrogel layering via “on-the-fly” polymerization techniques,23,24 but on-the-fly polymerization requires manipulation of hydrogels outside of the microchannels, which is not compatible with passive pumping and would be difficult to use for producing branching structures.
Figure 5.
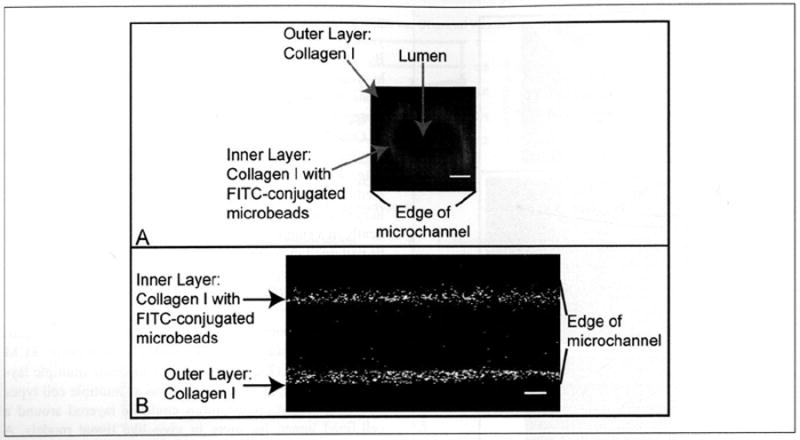
Volume-rendered image (A) and an image taken at the widest portion (B) of a lumen surrounded by multiple collagen I hydrogel layers. First, a lumen was formed through a 4.0-mg/mL collagen I hydrogel after a 2-min incubation. After polymerization was complete, a 4.0-mg/mL collagen I solution with FITC-conjugated microbeads was pumped into the lumen, and the channels were incubated for 2 min. Passive pumping was then used to form a lumen through this second layer. Microchannels were 500 μm tall, 500 μm wide, and 5 mm long, with inlet and outlet diameters of 1 mm and 1.75 mm, respectively. Images were taken using multiphoton laser-scanning microscopy (MPLSM). Scale bar represents 100 μm.
As others have previously shown, tubule structures of patterned cells are useful tools that provide a framework to model tissues with vessel and ductal structures for many biological investigations.1-9 While in vitro models that use ECM hydrogels to better replicate in vivo structure and function are likely to yield more physiologically relevant data, the widespread adoption of these models has been slow. Viscous finger patterning has the potential to increase adoption of these models by providing a cost-effective approach to the generation of lumen structures in ECM hydrogels. In addition, viscous finger patterning can be adapted to generate lumen structures in various hydrogel compositions and geometries based on the desired application. Finally, viscous finger patterning can be easily automated using automated liquid handling systems, which overcomes a key obstacle toward the high-throughput generation of tissue models with tubule structures.
Acknowledgments
The authors thank Edmond Young, Kyung Sung, and Jay Warrick for helpful discussions.
Funding
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by the Korea Research Foundation Grant (KRF-2008-220-D00133), NIH-NCI R33 CA137673. L. L. Bischel is supported by the National Institutes of Health grant T32 HL007889.
Footnotes
Supplementary material for this article is available on the Journal of Laboratory Automation Web site at http://jla.sagepub.com/supplemental.
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D. I. Beebe has an ownership interest in BellBrook Labs, LLC, which has licensed technology presented in this article.
References
- 1.Nelson CM, VanDuijin MM, Inman JL, Fletcher DA, Bissell MJ. Tissue Geometry Determines Sites of Mammary Branching Morphogenesis in Organotypic Cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrobak K, Potter D, Tien J. Formation of Perfused, Functional Microvascular Tubes In Vitro. Microvasc Res. 2006;71(3):185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Golden AP, Tien J. Fabrication of Microfluidic Hydrogels Using Molded Gelatin as a Sacrificial Element. Lab Chip. 2007;7(6):720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 4.Berry SM, Warren SP, Hilgart DA, Schworer AT, Pabba S, Gobin AS, Cohn RW, Keynton RS. Endothelial Cell Scaffolds Generated by 3D Direct Writing of Biodegradable Polymer Microfibers. Biomaterials. 2010;32:1–8. doi: 10.1016/j.biomaterials.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Fiddes LK, Raz N, Srigunapalan S, Tumarkan E, Simmons CA, Wheeler AR, Kumacheva E. A Circular Cross-Section PDMS Microfluidics System for Replication of Cardiovascular Flow Conditions. Biomaterials. 2010;31(13):3459–3464. doi: 10.1016/j.biomaterials.2010.01.082. [DOI] [PubMed] [Google Scholar]
- 6.Sukmana I, Vermette P. Polymer Fibers as Contract Guidance to Orient Microvascularization in a 3D Environment. J Biomat Res. 2010;92A:1587–1597. doi: 10.1002/jbm.a.32479. [DOI] [PubMed] [Google Scholar]
- 7.Du Y, Ghodousi M, Qi H, Haas N, Xiao W, Khademhosseini A. Sequential Assembly of Cell-Laden Hydrogel Constructs to Engineer Vascular-Like Microchannels. Biotechnol Bioeng. 2011;108(7):1693–1703. doi: 10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan W, Desai T. Microscale Multilayer Cocultures for Biomimetic Blood Vessels. J Biomed Mater Res A. 2005;72(2):146–160. doi: 10.1002/jbm.a.30182. [DOI] [PubMed] [Google Scholar]
- 9.Raghavan S, Nelson CM, Baranski JD, Lim E, Chen CS. Geometrically Controlled Endothelial Tubulogenesis in Micropatterned Gels. Tissue Eng Pt A. 2010;16:2255–2263. doi: 10.1089/ten.tea.2009.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin I, Boek E, Coveney P. Lattice Boltzmann Simulation of the Flow of Binary Immiscible Fluids with Different Viscosities Using the Shan-Chen Microscopic Interaction Model. Philos Trans R Soc A. 2002;360(1792):547–558. doi: 10.1098/rsta.2001.0953. [DOI] [PubMed] [Google Scholar]
- 11.Saffman P, Taylor SG. The Penetration of a Fluid into a Porous Medium or Hele-Shaw Cell Containing a More Viscous Liquid. Proc R Soc Lond A. 1958;245:312–329. [Google Scholar]
- 12.Saffman P. Viscous Fingering in Hele-Shaw Cells. J Fluid Mech. 1986;173:73–94. [Google Scholar]
- 13.Homsy G. Viscous Fingering in Porous-Media. Annu Rev Fluid Mech. 1987;19:271–311. [Google Scholar]
- 14.Kang Q, Zhang D, Chen S. Immiscible Displacement in a Channel: Simulations of Fingering in Two Dimensions. Adv Water Resour. 2004;27(1):13–22. [Google Scholar]
- 15.Dong B, Yan YY, Li W, Song Y. Lattice Boltzmann Simulation of Viscous Fingering Phenomenon of Immiscible Fluids Displacement in a Channel. Comput Fluids. 2010;39(5):768–779. [Google Scholar]
- 16.Walker G, Beebe D. A Passive Pumping Method for Microfluidic Devices. Lab Chip. 2002;2(3):131–134. doi: 10.1039/b204381e. [DOI] [PubMed] [Google Scholar]
- 17.Montanez-Sauri SI, Sung KE, Puccinelli JP, Pehlke C, Beebe DJ. Automation of Three-Dimensional Cell Culture in Arrayed Microfluidic Devices. J Lab Autom. 2011;16(3):171–185. doi: 10.1016/j.jala.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jo B, Van Lerberghe L, Motsegood K, Beebe D. Three-Dimensional Micro-Channel Fabrication in Polydimethylsiloxane (PDMS) Elastomer. J Microelectromech S. 2000;9(1):76–81. [Google Scholar]
- 19.Sung KE, Su G, Pehlke C, Trier SM, Eliceiri KW, Keely PJ, Friedl A, Beebe DJ. Control of 3-Dimensional Collagen Matrix Polymerization for Reproducible Human Mammary Fibroblast Cell Culture in Microfluidic Devices. Biomaterials. 2009;30(27):4833–4841. doi: 10.1016/j.biomaterials.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman S, Cloitre M, Allain C, Forgacs G, Beysens D. Viscosity and Elasticity during Collagen Assembly In Vitro: Relevance to Matrix-Driven Translocation. Biopolymers. 1997;41(3):337–347. doi: 10.1002/(SICI)1097-0282(199703)41:3%3C337::AID-BIP9%3E3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Sung KE, Yang N, Pehlke C, Keely PJ, Eliceiri KW, Friedl A, Beebe DJ. Transition to Invasion in Breast Cancer: A Microfluidic In Vitro Model Enables Examination of Spatial and Temporal Effects. Integr Biol. 2010;3:439–450. doi: 10.1039/c0ib00063a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthier E, Beebe DJ. Flow Rate Analysis of a Surface Tension Driven Passive Micropump. Lab Chip. 2007;7(11):1475–1478. doi: 10.1039/b707637a. [DOI] [PubMed] [Google Scholar]
- 23.Jeong W, Kim J, Kim S, Lee S, Mensing G, Beebe D. Hydrodynamic Microfabrication via “On the Fly” Photopolymerization of Microscale Fibers and Tubes. Lab Chip. 2004;4(6):576–580. doi: 10.1039/b411249k. [DOI] [PubMed] [Google Scholar]
- 24.Kang E, Shin SJ, Lee KH, Lee SH. Novel PDMS Cylindrical Channels That Generate Coaxial Flow, and Application to Fabrication of Microfibers and Particles. Lab Chip. 2010;10(14):1856–1861. doi: 10.1039/c002695f. [DOI] [PubMed] [Google Scholar]


