Abstract
OBJECTIVES
We studied the influence of the number of sternotomy mechanical fixation points on deep sternal wound infection (DSWI).
METHODS
Between September 2007 and February 2011, 2672 patients underwent a standard peri-sternal wire closure following a median sternotomy for a first-time cardiac surgery. Data were collected during the study period.
RESULTS
The mean age of the patients was 66 ± 11 and 1978 (74.0%) were male. The mean body mass index (BMI) was 28.9 ± 9.3 and the median of the logistic EuroSCORE was 3.14, with a range of 0.88–54.1. Postoperatively, 40 (1.5%) patients developed DSWI after 14 ± 6 days, of whom 39 (92.5%) had positive deep sternal wound specimen cultures, predominantly Staphylococci (62.5%). The risk of DSWI was significantly increased in patients in whom eight or fewer paired points of sternal wire fixation were used when compared with patients in whom nine or more paired points of fixation were used (P = 0.002). Preoperative myocardial infarction (P = 0.001), elevated BMI (P = 0.046), bilateral internal mammary artery harvest (P < 0.0001), postoperative hypoxia (P < 0.0001), sepsis (P = 0.019) and postoperative inotrope use (P = 0.007) significantly increased the risk of DSWI.
CONCLUSIONS
DSWI is associated with hypoxia, ischaemia, sepsis and mechanical sternal instability. DSWI may be prevented by using nine or more paired fixation points when closing with standard peri-sternal wires.
Keywords: Cardiac, Sternum, Surgery/Complications, Wound dehiscence, Wound infection
INTRODUCTION
Although the incidence of sternal wound dehiscence following cardiac surgery via sternotomy is rare, it blights the success of the operation. Sternal dehiscence can be non-infectious (biomechanical failure occurring in 0.2–5%) or infectious (deep sternal wound infection—DSWI in 0.3–5%) [1–3]. Biomechanical failure is usually due to sternal wire cut-through [4]; that is, sterile dehiscence; whereas DSWI is associated with the signs and symptoms of an infected surgical site. The reported mortality rate of patients suffering from DSWI has remained high and varies from 9.8 to 47% [3]. Patients who have survived following DSWI requiring surgical revision do not regain their quality of life [5]. Sternal wound complications are associated with intensive medical therapy, prolonged in-hospital stay and a high mortality rate [6]. The multitude of independent risk factors influencing the incidence of DSWI implies the complexity of the pathology [1, 2, 7–9]. Prevention of DSWI should be focused on achieving mechanical sternal stability and preventing infection. To our knowledge, there are few studies evaluating the impact of the number of sternal wire fixation points on DSWI [10].
MATERIALS AND METHODS
Patients
Between September 2007 and February 2011, 2672 patients underwent standard peri-sternal stainless steel wire closure following first-time cardiac surgery via median sternotomy. Pre-, peri- and postoperative data were collected and recorded during the study period. Patients with a formal, preoperative diagnosis of osteoporosis (none of whom suffered DSWI) and sternal weave closure were excluded. All sterna were closed by a six- or seven- (light or heavy wires) gauge stainless steel wire; patients in whom other materials had been used to close the sternum were excluded from the study. The standard closure of the median sternotomy involved the use of peri-sternal, single or figure-of-eight sternal wires with a multi-twist closure. The number of fixation points was determined radiographically. The suturing technique and material for both subcuticular and subcutaneous closures were identical in all patients. In all coronary artery bypass graft (CABG) surgery operations, the left internal mammary artery (LIMA) was harvested. The patients who underwent bilateral internal mammary artery (BIMA) harvest had isolated CABG. Preoperatively, all patients received intravenous Cefuroxime 1.5 g as per the institution's protocol. Institutional review board approval was obtained for this study and the ethics approval was waived.
Definitions and techniques
The diagnosis of DSWI was based on the guidelines of the Centres for Disease Control and Prevention [11] when patients developed one or more of the following criteria:
An organism is isolated from the culture of mediastinal tissue or fluid.
Evidence of mediastinitis is seen during sternal re-operation.
One of the following: chest pain, sternal instability, temperature more than 38°C is present and there is either purulent discharge from the mediastinum or an organism isolated from blood culture or culture of drainage of the mediastinal area.
None of the patients with DSWI were managed conservatively. Therefore, additionally a requirement of surgical intervention via the re-opening of the sternum was added to the definition of DSWI. Superficial sternal wound infection (SSWI) was defined as a wound infection limited to the skin and subcutaneous tissue [11], requiring local surgical intervention with regular wound care, accompanied by antibiotic therapy and/or vacuum-assisted closure and/or wire removal.
Statistical analysis
Univariate analysis was performed using the χ2 test. Logistic regression analysis was used to identify the independent risk factors. Continuous variables were expressed as mean ± standard deviation unless stated otherwise. Statistical analysis was performed using PASW software (SPSS Inc., Chicago, IL, USA).
RESULTS
Pre-, peri- and postoperative characteristics are presented in Table 1. The mean age of the patients was 66 ± 11 and 1978 (74.0%) were male .The mean follow-up was 20.5 months with the range of 7–37 months. Forty patients (1.5%) developed DSWI within 14 ± 6 days following cardiac surgery via a median sternotomy. The predominant pathogen isolated from the sternal wound sample was Staphylococcus aureus, with a total of 25 (62.5%) positive samples; of these 25 patients, 17 (42.5%) cultured methicillin-sensitive staphylococci and 8 (20.0%) were methicillin-resistant. Six (15.0%) had coagulase-negative staphylococci, three (7.5%) Streptococcus species, two (5.0%) Bacteroides species, two (5.0%) Candida species and two (5.0%) Escherichia coli. In one patient, the wound culture was inconclusive. Patients with DSWI had an in-hospital stay of 41 ± 31 days, which was, on average, 32 days (95% CI: 22.38–41.62, P < 0.0001) longer than that of patients without DSWI. The surgical intervention for all DSWI patients was the re-opening of the sternotomy wound and debridement with or without sternal re-wiring and/or installation of an irrigation system. Nineteen (47.5%) sternotomies were rewired at the time of debridement, 16 (40.0%) were rewired following at least 1 week of irrigation of the mediastinum using iodine-based anti-septic solution and the remaining 5 (12.5%) underwent major surgical reconstruction of the anterior chest wall. Eight (20.0%) patients died following surgical revision: two (5.0%) patients died during the debridement immediately after the procedure due to the injury to the right ventricle, while six (15.0%) patients died 36 ± 14 days following the initial intervention. DSWI was significantly associated with a high mortality rate compared with non-DSWI patients (odds ratio = 9.87; 95% CI: 4.37–22.25, P < 0.0001).
Table 1:
Patient's characteristics: non-DSWI vs DSWI
| Preoperative | Non-DSWI (n = 2632) | DSWI (n = 40) | Odds ratio | 95% CI | P-value |
|---|---|---|---|---|---|
| Age ≥70 years | 1129 (42.9%) | 22 (55.0%) | 1.62 | 0.86–3.04 | 0.14 |
| Sex (male) | 1948 (74.0%) | 30 (75.0%) | 1.053 | 0.51–2.16 | 0.52 |
| BMI ≥30 | 903 (34.3%) | 25 (62.5%) | 3.19 | 1.67–6.08 | 0.001 |
| Smoking | 478 (18.1%) | 6 (15.0%) | 0.79 | 0.33–1.90 | 0.83 |
| Alcohol consumption (male >21 unit/week, female >14 unit/week) | 598 (22.7%) | 10 (25%) | 1.13 | 0.55–2.33 | 0.70 |
| CCS 2 and 3 | 1006 (38.2%) | 23 (57.5%) | 2.18 | 1.16–4.11 | 0.021 |
| New York Heart Association classification (NYHA) 3 and 4 | 1257 (47.7%) | 23 (57.5%) | 1.48 | 0.78–2.78 | 0.26 |
| Diabetes | 578 (21.9%) | 12 (30.0%) | 1.52 | 0.77–3.01 | 0.24 |
| Hypertension | 1824 (69.3%) | 35 (87.5%) | 3.10 | 1.21–7.94 | 0.014 |
| Endocarditis | 59 (2.2%) | 0 | 0.40 | ||
| Triple CAD | 1306 (49.6%) | 26 (65%) | 1.98 | 1.01–3.62 | 0.049 |
| Left ventricle ejection fraction (<50%) | 521 (19.8%) | 9 (22.5%) | 1.176 | 0.55–2.48 | 0.68 |
| Preoperative MI | 855 (32.4%) | 22 (55.0%) | 2.54 | 1.35–4.76 | 0.004 |
| Antibiotic therapy up to operation | 247 (9.38%) | 6 (15.0%) | 1.70 | 0.70–4.09 | 0.26 |
| PVD | 370 (14.0%) | 9 (22.5%) | 1.77 | 0.83–3.75 | 0.16 |
| Renal failure | 47 (1.7%) | 1 (2.5%) | 1.41 | 0.19–10.48 | 0.51 |
| Cerebrovascular accident (CVA) | 256 (9.7%) | 3 (7.5%) | 0.75 | 0.23–2.4 | 0.44 |
| COPD | 129 (4.9%) | 2 (5.0%) | 1.02 | 0.24–4.28 | 0.59 |
| Asthma | 126 (4.7%) | 3 (7.5%) | 1.61 | 0.49–5.3 | 0.44 |
| IV nitrate infusion up to operation | 101 (3.8%) | 5 (12.5%) | 3.58 | 1.37–9.33 | 0.019 |
| Mechanical ventilation | 18 (0.6%) | 0 | 0.76 | ||
| Inotrope infusion >24 h | 15 (0.5%) | 0 | 0.79 | ||
| IABP | 122 (4.6%) | 0 | 0.25 | ||
| Perioperative | |||||
| Logistic EuroSCORE >5 | 851 (32.3%) | 17 (42.5%) | 1.54 | 0.82–2.91 | 0.17 |
| Emergency operation | 110 (4.1%) | 0 | 0.41 | ||
| LIMA harvest | 1508 (57.2%) | 21 (52.5%) | 0.63 | 0.44–1.54 | 0.32 |
| BIMA harvest | 156 (5.9%) | 11 (27.5%) | 6.02 | 2.9–12.2 | 0.001 |
| Valve operation | 1008 (38.3%) | 12 (30.0%) | 0.69 | 0.35–1.36 | 0.18 |
| Redo-sternotomy | 64 (2.4%) | 2 (5.0%) | 2.11 | 0.49–8.94 | 0.26 |
| Operation time (incision to closure) >270 min | 247 (9.3%) | 8 (20.0%) | 2.41 | 1.10–5.29 | 0.049 |
| Cardiopulmonary bypass | 1888 (71.6%) | 24 (60.0%) | 0.59 | 0.31–1.11 | 0.11 |
| Sternal wires ≤8 | 1708 (64.9%) | 39 (97.5%) | 21.09 | 2.89–153.8 | <0.0001 |
| Heavy sternal wires (gauge 7) | 497 (18.8%) | 6 (18.0%) | 0.75 | 0.31–1.81 | 0.68 |
| Figure-of-eight sternal wiring | 134 (5.0%) | 2 (5.0%) | 0.98 | 0.23–4.11 | 0.97 |
| Postoperative | |||||
| Blood transfusion | 1017 (38.6%) | 20 (50.0%) | 1.58 | 0.85–2.96 | 0.14 |
| Low cardiac output syndrome | 37 (1.4%) | 3 (7.5%) | 5.68 | 1.67–19.27 | 0.021 |
| IABP | 170 (6.4%) | 7 (17.5%) | 3.07 | 1.33–7.04 | 0.014 |
| Inotrope infusion >24 h | 468 | 22 | 5.65 | 3.00–10.62 | <0.0001 |
| Gastrointestinal bleeding | 43 (1.6%) | 3 (7.5%) | 4.88 | 1.44–16.44 | 0.030 |
| CVA | 34 (1.3%) | 2 (5.0%) | 4.02 | 0.93–17.34 | 0.10 |
| Delirium | 301 (11.4%) | 5 (12.5%) | 1.10 | 0.43–2.84 | 0.80 |
| Upper respiratory tract infection | 507 (19.3%) | 15 (37.5%) | 2.50 | 1.31–4.78 | 0.008 |
| Respiratory failure (hypoxia) | 101 (3.8%) | 16 (40.0%) | 16.70 | 8.60–32.42 | <0.0001 |
| Mechanical ventilation >24 h | 307 (11.6%) | 19 (47.5%) | 6.85 | 3.64–12.89 | 0.001 |
| AF (atrial fibrilation) | 640 (24.3%) | 10 (25.0%) | 1.03 | 0.50–2.13 | 0.85 |
| Acute renal failure | 78 (2.9%) | 2 (5.0%) | 1.72 | 0.40–7.27 | 0.33 |
| SSWI | 156 (5.9%) | 1 (2.5%) | 0.40 | 0.56–2.98 | 0.73 |
| Re-opening | 138 (5.2%) | 1 (2.5%) | 0.46 | 0.06–3.39 | 0.72 |
| Sepsis | 30 (1.1%) | 6 (15.0%) | 15.30 | 5.98–39.16 | 0.001 |
| Mortality (during follow-up) | 65 (2.4%) | 8 (20.0%) | 9.87 | 4.37–22.25 | <0.0001 |
Pre-, peri- and postoperative characteristics associated with DSWI were as follows: body mass index (BMI) ≥30, Canadian Cardiovascular Society (CCS) score 2 and 3, hypertension, triple-vessel coronary artery disease (CAD) (flow limiting >50% diameter reduction), myocardial infarction (MI) <3 months prior to operation, intravenous nitrate infusion up to operation, BIMA harvest, operation time (incision to closure) >270 min, number of paired sternal wire fixation point ≤8, low cardiac output syndrome, postoperative intra-aortic balloon pump (IABP) requirement, postoperative inotrope infusion >24 h, postoperative gastrointestinal bleeding, postoperative upper respiratory tract infection, postoperative respiratory failure (hypoxia), post-operative mechanical ventilation >24 h and sepsis (Table 1). Multivariate logistic regression analysis confirmed the significance of the following independent risk factors inducing DSWI (Table 2): sternal wires ≤8 (P = 0.002), intravenous nitrate infusion up to operation (P = 0.031), preoperative MI <3 months to operation (P = 0.001), intravenous nitrate infusion up to operation (P = 0.031), BMI ≥30 (P = 0.046), BIMA harvest (P < 0.0001), post-operative respiratory failure (P < 0.0001), sepsis (P = 0.019) and postoperative inotrope infusion (P = 0.007).
Table 2:
Multivariate logistic regression analysis
| Variable | P-value | Odds ratio | 95% CI |
|---|---|---|---|
| Sternal wires ≤8 | 0.002 | 27.31 | 3.39–219.63 |
| IV nitrate infusion up to operation | 0.031 | 4.05 | 1.14–14.41 |
| Preoperative MI | 0.001 | 4.23 | 1.80–9.93 |
| BMI ≥30 | 0.046 | 2.24 | 1.01–4.98 |
| BIMA harvest | <0.0001 | 12.78 | 4.2–38.81 |
| Postoperative respiratory failure (hypoxia) | <0.0001 | 10.09 | 3.61–28.19 |
| Sepsis | 0.019 | 5.63 | 1.33–23.83 |
| Postoperative inotrope infusion >24 h | 0.007 | 2.983 | 1.35–6.59 |
The use of heavy sternal wire and the figure-of-eight wiring technique was not significantly different in both groups: 9 and more vs 8 or less pairs of fixation points (heavy wires: 161 (17.4%) vs 342 (19.5%) P = 0.17, figure-of-eight: 46 (4.9%) vs 90 (5.1%) P = 0.92). The incidence of SSWI including wound sinus was not associated with the number of wires [46 (4.9%) vs 157 (5.8%), P = 0.16].
DISCUSSION
Our study demonstrated a negative impact of DSWI on patient in-hospital stay and survival, which has been reported previously [2, 6, 9].
In contrast with other studies [2, 7, 9], we did not find age, diabetes, chronic obstructive pulmonary disease (COPD), smoking, peripheral vascular disease (PVD), emergency operation, type of operation, re-opening or postoperative blood transfusion to be significant predictors of DSWI. Our results demonstrated no association between the logistic EuroSCORE and DSWI, which may support the design of an alternative risk model capable of predicting DSWI [7].
Pre- and postoperative characteristics that indicate a potential ongoing ischaemic event such as triple CAD, CCS score of 2 and 3, preoperative MI, intravenous nitrate infusion up to the surgery, postoperative IABP, postoperative inotrope infusion [2], low cardiac output syndrome and gastrointestinal bleeding were associated with an increased incidence of DSWI. An unstable haemodynamic state associated with ischaemia may impair the host immune system and the microcirculation of the tissues. This study also identified a high BMI as an independent risk factor; a high BMI augments the lateral forces separating the two halves of the sternum [4]. Postoperative respiratory failure and upper respiratory tract infection, in which hypoxia and cough are common features, significantly increased DSWI. Harvest of both IMAs, operation time and lower number of paired sternal wire fixation points increased the risk of DSWI. Postoperative sepsis may also increase the risk of DSWI.
Optimal wound healing requires haemostasis, an inflammatory response, cell proliferation and remodelling. Haemostasis and inflammation are the initial events involving multi-cellular chemotaxis followed by proliferation and remodelling phases [12]. Sternal instability may have an adverse impact on wound healing phases by delaying haemostasis and prolonging inflammation as well as disrupting formation of the extra-cellular matrix and epithelialization. Sternal union requires dependable biomechanical fixation. It is recognized that optimal sternal stabilization prevents the incidence of DSWI [13]. An unstable surgical wound, usually attributable to a technical fault, is a recognized risk factor for wound infection [14]. The lack of association of SSWI and DSWI supports the hypothesis that it is the sternal instability per se that is the main driver for the development of DSWI rather than the presence of micro-organisms in the more superficial layers of the wound.
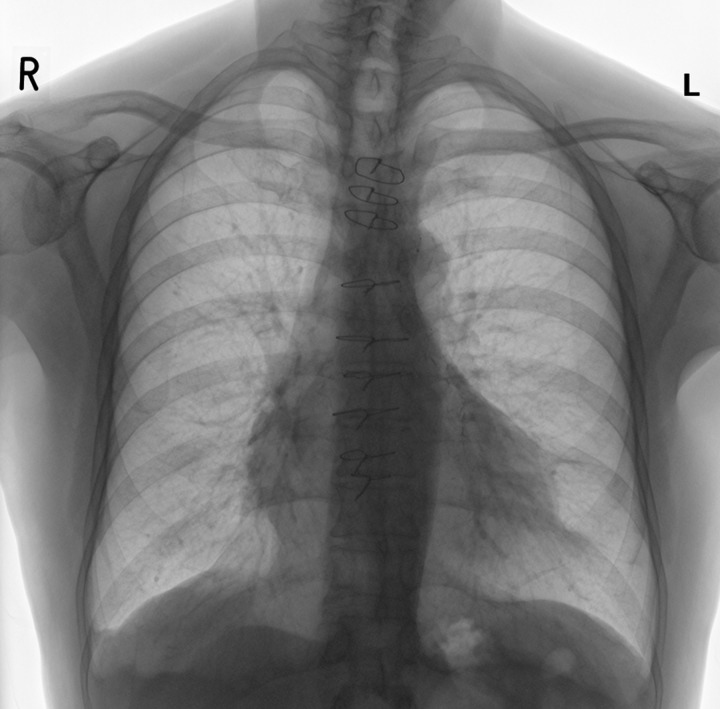
The most popular biomechanical fixation of sternotomy is achieved by stainless steel wiring, which is simple, inexpensive and easy to use [4]. The standard single peri-sternal closure has been previously identified as a reliable strenal closure [15]. A suggested method of wiring is three single intra-manuburium accompanied by six single peri-sternal pairs of fixation point from which three pairs are applied on the one-third distal of the sternum (Fig. 1) as more dehiscence occurs at this area requiring more re-enforcement [4].
Figure 1:
Postoperative reversed chest X-ray demonstrating sternal wiring with nine pairs of fixation point.
DSWI may be reduced by using nine or more paired fixation points when closing with standard peri-sternal stainless steel wires.
STUDY LIMITATIONS
The study is limited in that it is a retrospective, observational study rather than a prospective, randomised trial. However, all data were collected during the study period, and major factors related to sternal dehiscence were demonstrated on the analysis of multiple factors believed to be relevant to the condition. We did not measure the impact of the surgical team on DSWI.
ACKNOWLEDGEMENTS
We gratefully acknowledge Jackie Howlett for her assistance in retrieving the data.
Conflict of interest: none declared.
REFERENCES
- 1.Robicsek F, Fokin A, Cook J, Bhatia D. Sternal instability after midline sternotomy. Thorac Cardiovasc Surg. 2000;48:1–8. doi: 10.1055/s-2000-9945. [DOI] [PubMed] [Google Scholar]
- 2.The Parisian Medistinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective multicentre study. J Thorac Cardiovasc Surg. 1996;111:1200–7. doi: 10.1016/s0022-5223(96)70222-2. [DOI] [PubMed] [Google Scholar]
- 3.El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg. 1996;61:1030–6. doi: 10.1016/0003-4975(95)01035-1. [DOI] [PubMed] [Google Scholar]
- 4.McGregor WE, Trumble DR, Magovern JA. Mechanical analysis of midline sternotomy wound closure. J Thorac Cardiovasc Surg. 1999;117:1144–50. doi: 10.1016/s0022-5223(99)70251-5. [DOI] [PubMed] [Google Scholar]
- 5.Jidéus L, Liss A, Ståhle E. Patients with sternal wound infection after cardiac surgery do not improve their quality of life. Scand Cardiovasc J. 2009;43:194–200. doi: 10.1080/14017430802573098. [DOI] [PubMed] [Google Scholar]
- 6.Toumpoulis IK, Anagnostopoulos CE, Derose JJ, Jr, Swistel DG. The impact of deep sternal wound infection on long-term survival after coronary artery bypass grafting. Chest. 2005;127:464–71. doi: 10.1378/chest.127.2.464. [DOI] [PubMed] [Google Scholar]
- 7.Fowler VG, Brien SMO, Muhlbaier LH, Corey GR, Ferguson TB, Peterson ED. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112:I-358–65. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 8.Olbrecht VA, Barreiro CJ, Bonde PN, Williams JA, Baumgartner WA, Gott VL, et al. Clinical outcomes of non-infectious sternal dehiscence after median sternotomy. Ann Thorac Surg. 2006;82:902–8. doi: 10.1016/j.athoracsur.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 9.Borger MA, Rao V, Weisel RD, Ivanov J, Cohen G, Scully HE, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg. 1998;65:1050–6. doi: 10.1016/s0003-4975(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 10.Friberg O, Dahlin LG, Söderquist B, Källman J, Svedjeholm R. Influence of more than six sternal fixation wires on the incidence of deep sternal wound infection. Thorac Cardiovasc Surg. 2006;54:468–73. doi: 10.1055/s-2006-924437. [DOI] [PubMed] [Google Scholar]
- 11.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–78. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 12.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–6. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 13.Losanoff JE, Collier AD, Wagner-Mann CC, Richman BW, Huff H, Hsieh F, et al. Biomechanical comparison of median sternotomy closures. Ann Thorac Surg. 2004;77:203–9. doi: 10.1016/s0003-4975(03)01468-1. [DOI] [PubMed] [Google Scholar]
- 14.Nichols RL. Surgical wound infection. Am J Med. 1991;91(Suppl 3B):54S–64S. doi: 10.1016/0002-9343(91)90344-w. [DOI] [PubMed] [Google Scholar]
- 15.Cheng W, Cameron DE, Warden KE, Fouger JD, Gott VL. Biomechanical study of sternal closure techniques. Ann Thorac Surg. 1993;55:737–40. doi: 10.1016/0003-4975(93)90285-p. [DOI] [PubMed] [Google Scholar]