Abstract
Bronchopulmonary dysplasia (BPD) continues to be a major pulmonary complication in very low birth weight (VLBW) and extremely low birth weight (ELBW) survivors of neonatal intensive care units (NICUs). Many factors including partial pressures of carbon dioxide (PaCO2) have been implicated as possible causes. Permissive hypercapnia has become a more common practice in ventilated infants, but its effect on BPD is unclear. The hypothesis of this study was that hypercarbia is associated with increased BPD in infants with birth weights of 500–1,499 g. Nine hospitals were involved in this observational cohort study. Maternal and infant information including socio-demographics, antenatal steroids, gender, race, gestational age, birth weight, intubation and ventilator status, physiologic variables and data on therapies were collected by chart abstraction. SNAP scores were assigned. Candidate BPD risk factors, including cumulative exposures derived from blood gas and ventilation data in the first 6 days of life, were identified. Risk models were developed for 425 preterm infants who survived to 36 weeks post-menstrual age. BPD occurrence was associated with the cumulative burden of MAP >0 cm H2O in the first 6 days of life (P < 0.0001). After adjustment for the burden of MAP, the occurrence of hypercarbia (Paco2 >50 torr) was associated with a greater incidence of BPD (P = 0.024). Among 293 intubated, mechanically ventilated infants, those with hypercarbia occurring only when MAP B8 cm H2O, a scenario more comparable to permissive hypercapnia, also had increased BPD incidence compared to infants without hypercarbia (P = 0.0003). Hypercarbia during the first 6 days of life was associated with increased incidence of BPD in these infants. Mechanically ventilated infants with hypercarbia during low MAP also had a significant increase in BPD. Permissive hypercapnia in ventilated infants needs further close review before the practice becomes even more widespread.
Keywords: Bronchopulmonary dysplasia, Hypercapnia, Hypercarbia, Extremely low birth weight and very low birth weight infants, Ventilated
Introduction
Bronchopulmonary Dysplasia (BPD) continues to be a major pulmonary complication in survivors of NICU among VLBW and ELBW infants [1, 2] The incidence of BPD at 36 weeks of post menstrual age of these infants varies from 4.5 to 36% [3]. BPD has been associated with multiple factors including birth weight [3], male gender [3], respiratory distress syndrome [4], mechanical ventilation [5–7] supplemental oxygen therapy [5–7] fluid intake [6] and patent ductus arteriosus [8]. Some studies [9] suggest that a lower Paco2 associated with ventilator strategies may be a BPD risk factor. In 1987, Avery et al. [10] found differences among centers in the incidence of chronic lung disease of prematurity. Since then the ventilatory method least associated with complications for managing RDS in premature infants has been debated. Permissive hypercapnia or hypercarbia (allowing Paco2 above 50–60 torr) became a more accepted approach in clinical practice [11–18].
The hypothesis of this study was that hypercarbia during the first 6 days of life would be associated with increased BPD in infants with birth weights of 500–1,499 g in a multi-center study. This study was a part of a larger protocol investigating the relationship of care practices in NICUs. The Institutional Review Boards of all the participating institutions and the National Institutes of Health approved the protocol.
Methods
As part of the National Institute of Health—District of Columbia (NIH-DC) initiative, nine of the ten NICUs caring for low birth weight infants in DC agreed to collaborate in this study. Eight of the NICUs provided acute care. The ninth provided only chronic care. The acute care units ranged in size from 9 to 40 beds, staffed by 2–9 fulltime neonatologists, and had admission volumes of 180–900 admissions per year. Four units were affiliated with a university, five with residency programs, and three had neonatology fellows as well as residents. Two of the eight acute neonatal units accept out-born infants; six accept only inborn neonates.
Eligibility Criteria
Eligibility criteria for inclusion included the following: (a) live birth, with birth weight from 500 to 1,499 g; (b) birth to a DC resident; (c) care in a participating NICU or intermediate/step-down unit; and (d) date of birth and date of first admission to a participating NICU during the enrollment period. Exclusion criteria included: (a) neonates not expected to survive due to extreme prematurity (<500 g and/or only comfort care given) or lethal malformations; (b) neonates admitted after discharge to home; (c) deaths prior to 2 h of age; and (d) infants transferred from a non-participating hospital after 24 h of age.
Data Collection
Data were abstracted from clinical records from October 1, 1994 through April 30, 1997. Clinical data included maternal information (age, socio-demographics, prenatal care, antenatal steroids, and multi-fetal gestation) and infant data. Birth data collected for infants included gender, race, congenital malformations and gestational age (from medical records). Additional infant data included delivery room status (birth weight, “small for gestational age” (SGA) status, Apgar scores, mode of delivery), transportation status, and physiologic data required to compute the score for neonatal acute physiology (SNAP) (mean blood pressure, heart rate, respiratory rate and temperature, blood gas data, blood chemistries and blood counts, urine output, presence of seizures, apnea, and stool guaiac) [19]. In addition, data on therapies and monitoring were collected for applying the neonatal therapeutic intervention scoring system (NTISS) [20]. SNAP assesses severity of illness using the worst recorded values of more than two dozen physiologic variables during the first 24 h of stay in the NICU. NTISS uses 62 specific therapeutic and monitoring items during the first 24 h of stay. Physiologic and NTISS data were collected daily for 216 consecutive infants; for 306 infants data were collected for every 7th day after the first week. Physiologic measurements in the 2 h before death were excluded from data collected for SNAP scores.
Transfers between participating NICUs were tracked to provide a complete record of birth hospitalization until death or discharge. Infants transferred to nonparticipating hospitals were classified as survivors since these transfers only occurred for non-critical care reasons. Infants reaching “boarder” status (i.e., retained in the hospital for nonmedical reasons) were considered discharged. Infants remaining in hospitals at the end of the study were also considered to have survived hospitalization.
General and site-specific data collection procedures were developed and documented in a detailed operations manual. Each site designated a staff physician and a nurse to work on the study. A team of registered nurses collected maternal and infant data following an in-depth training period. Data were transmitted electronically to the study center each evening. Sample completeness was assessed using NICU and delivery room logs.
Statistical Methods
Logistic regression models were used to assess whether longitudinal factors were associated with BPD, after adjustment for baseline risk and severity of illness. The analyses were restricted to inborn infants. BPD was operationally defined as the requirement for supplemental oxygen at 36 weeks of post-menstrual age among those still alive [21]. Infants born at gestational ages of 36 weeks or greater were excluded. Infants transferred to non-participating hospitals prior to 36 post-menstrual weeks were excluded if they were receiving supplemental oxygen when transferred. Infants discharged prior to 36 weeks were considered not to have BPD, since none were receiving supplemental oxygen at the time of discharge.
Candidate Risk Factors for BPD
Candidate baseline risk factors (measured at birth or within the first 24 h after NICU admission) included birth weight, SNAP, gender, race (black/non-black), antenatal steroids, SGA, and Apgar score <7 at 5 min. Longitudinal factors (measured during the hospital course of the infant prior to 36 weeks post-menstrual age) included: symptomatic patent ductus arteriosus (PDA); use of surfactant (yes/no); use of indomethacin (yes/no); average intravenous fluid intake (ml/day) during the first 6 days after admission; average dose of Intralipid (grams/kilogram/day) up to 36 weeks of post-menstrual age; use of mechanical ventilation at any time during the first 6 days in NICU; and area under (or above) the curve (AUC) for mean airway pressure (MAP); Fio2 >0.21 and >0.40; and Paco2 <35 and >50 torr in blood over the first 6 days after admission. The longitudinal AUCs were computed using the trapezoidal rule [22], with interpolation to replace missing values (see “Appendix” Fig. 2). For MAP, an AUC >0 is equivalent to the use of mechanical ventilation in the first 6 days.
Fig. 2.
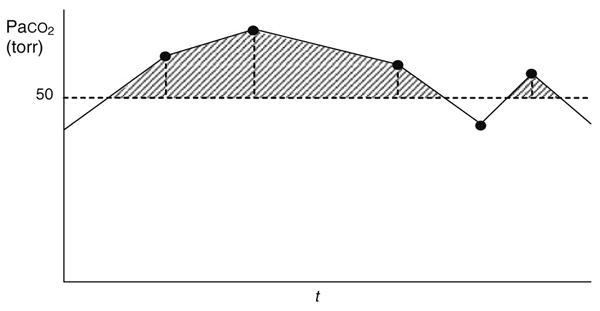
Illustration of longitudinal area under the curve (AUC) computation for Paco2 >50 torr by the trapezoidal rule. Data points for Paco2 are connected by straight lines, and the area under the curve but above PaCO2 = 50 torr is accumulated as the sum of the series of shaded trapezoidal and triangular areas. Thus the AUC for PaCO2 >50 torr provides a measure of the cumulative burden of PaCO2 exceeding 50 torr
Development of Baseline Risk Adjustment for BPD
A baseline risk model for BPD was developed using backward logistic regression, retaining variables with P < 0.15. This liberal retention criterion was adopted to avoid potential under-fitting. Squared terms were examined for birth weight and SNAP and were also included if P < 0.15. The retained variables were birth weight, SNAP (quadratic), gender, and Apgar score <7 at 5 min. The risk model for BPD did not display significant lack of fit (P = 0.51, Hosmer–Lemeshow goodness of fit test) [23, 24]. Discriminatory ability as quantified by the area under the empirical Receiver Operating Characteristic (ROC) curve [25] was 0.766.
Baseline-Adjusted Associations Between Longitudinal Factors and BPD
The baseline-adjusted association between BPD and each longitudinal factor was evaluated using a logistic regression that included the longitudinal factor and the reduced set of baseline factors as predictors.
Stepwise Selection of Longitudinal Factors
Longitudinal factors for which P < 0.15 for the baseline-adjusted association with BPD were included as candidates in stepwise logistic regression modeling adjusted for baseline risk. Although both the AUCs for Fio2 >0.21 and Fio2 >0.40 were significant in adjusted testing, the AUC for Fio2 >0.40 was retained to avoid co-linearity. To limit the number of predictors in stepwise modeling, the baseline predictors were included in the form of a risk score using the linear combination derived from the baseline risk adjustment model. Both forward and backward stepwise approaches were employed, using P < 0.05 as the criterion for entry or retention as appropriate. These approaches yielded identical results. Models used for inference were comprised of the longitudinal variables selected by stepwise modeling plus the five individual terms from the baseline adjustment model.
Descriptive statistics are presented as proportions or means and standard deviations. Unadjusted comparisons between infants with or without BPD used the Pearson chi-square test for categorical measures, Wilcoxon rank sum test for continuous measures other than AUCs, and a 2 degree-of-freedom Wald chi-square for the AUCs. All P values for single parameter hypotheses are two-sided.
Results
The initial cohort consisted of 552 infants. Thirty infants were excluded due to comfort care only, deaths prior to 2 h of life, transfers from non-participating sites, and missing charts, leaving 522 infants in the primary study cohort [26]. Since this analysis was restricted to inborn infants, 29 infants transferred from non-participating hospitals within the first 24 h of life were additionally excluded, leaving 493 infants. All inborn infants were admitted to NICUs within 2.4 h of delivery. Exclusions based on the operational definition of BPD included 64 deaths (13%) prior to 36 weeks post-menstrual age, three infants born after 36 weeks of gestation, and one infant on supplemental oxygen lost to follow-up prior to 36 weeks, leaving 425 infants for the analysis.
Mothers of these 36-week survivors were predominantly unmarried (78.5%), 12.9% were less than 20 years old, and 18.1% had received no prenatal care. Illicit drug use was self-reported in 31.4% of the mothers. Prolonged rupture of membranes was reported for 40.5% of the mothers. Mothers of surviving infants who had been mechanically ventilated in the first 6 days after admission were less likely to have received antenatal steroids than mothers of infants not requiring mechanical ventilation (70.7% vs. 80.6%, P = 0.036). Differences were not significant for any other factors.
The clinical and demographic data for these 425 infants are summarized in Table 1. Infants who were mechanically ventilated in the first 6 days after admission tended to have smaller birth weights and younger gestational ages, but were less likely to be SGA; were more likely to have Apgar score <4 at 1 min and <7 at 5 min; and had higher SNAP and NTISS scores (P = 0.0038 for SGA; otherwise, P < 0.0001 for each). Seventy-four (17.4%) of the 36-week survivors had BPD as defined by the requirement for supplemental oxygen at 36 weeks.
Table 1. Infant factors, for infants surviving to 36 weeks post-menstrual age.
| Variable | All infants | Infant mechanically ventilated at any time in the first 6 days after admission? | P valueb | |
|---|---|---|---|---|
|
|
||||
| N = 425a n (%) |
Yes N = 293a n (%) |
No N = 132a n (%) |
||
| Birth weight (g) | ||||
| Mean ± SD | 1,091 ± 260 | 1,008 ± 250 | 1,276 ± 173 | <0.0001 |
| Gestational age (weeks) | ||||
| Mean ± SD | 28.9 ± 2.5 | 28.0 ± 2.3 | 31.0 ± 1.8 | <0.0001 |
| Small for gestational agec | 81 (19.1) | 45 (15.4) | 36 (27.3) | 0.0038 |
| Male | 191 (44.9) | 135 (46.1) | 56 (42.4) | 0.48 |
| Cesarean section | 204 (48.0) | 140 (47.8) | 64 (48.5) | 0.89 |
| Apgar score at 1 min | ||||
| <4 | 98 (23.4) | 86 (29.9) | 12 (9.2) | <0.0001 |
| Apgar score at 5 min | ||||
| <4 | 11 (2.6) | 10 (3.4) | 1 (0.8) | 0.19 |
| <7 | 80 (19.0) | 77 (26.5) | 3 (2.3) | <0.0001 |
| SNAP | ||||
| Mean ± SD | 11.9 ± 6.7 | 14.1 ± 6.5 | 7.1 ± 3.9 | <0.0001 |
| Range | 1–41 | 3–41 | 1–17 | |
| NTISS score | ||||
| Mean ± SD | 18.4 ± 6.7 | 21.6 ± 5.3 | 11.4 ± 2.9 | <0.0001 |
| Range | 6–42 | 9–42 | 6–23 | |
SNAP score for neonatal acute physiology, NTISS neonatal therapeutic intervention scoring system, SD standard deviation
Denominators vary due to missing values
Pearson chi-square, Fisher's exact (for Apgar score less than 4 at 1 min), or Wilcoxon rank sum test
Birth weight less than 10th percentile for gestational age
Table 2 provides the unadjusted association between BPD and baseline and longitudinal factors considered as potential predictors. BPD infants tended to have smaller birth weights, younger gestational ages, lower Apgar scores, and higher SNAP scores (P < 0.0001). Longitudinal factors, including surfactant use, occurrence of symptomatic PDA, indomethacin use, requirement for mechanical ventilation, use of postnatal steroids, 6-day average fluid intake (ml/kg/day) and Intralipid intake (gm/kg/day), were all significantly (P < 0.0001 for each) associated with BPD infants compared to non BPD infants.
Table 2. Unadjusted associations between BPD and baseline and longitudinal factors.
| Variable | All infants N = 425a n (%) |
BPD N = 74a n (%) |
Non-BPD N = 351a n (%) |
P valueb |
|---|---|---|---|---|
| Baseline factors | ||||
| African-American | 385 (90.6) | 69 (93.2) | 316 (90.0) | 0.39 |
| Male | 191 (44.9) | 40 (54.1) | 151 (43.0) | 0.083 |
| Birth weight (g) | 1,091 ± 260 | 913 ± 236 | 1,129 ± 250 | <0.0001 |
| Gestational age (weeks) | 28.9 ± 2.55 | 27.5 ± 2.10 | 29.2 ± 2.54 | <0.0001 |
| Small for gestational agec | 81 (19.1) | 15 (20.3) | 66 (18.8) | 0.77 |
| 5 min Apgar score <7 | 80 (19.0) | 26 (35.1) | 54 (15.5) | <0.0001 |
| Antenatal steroid use | 311 (74.1) | 54 (74.0) | 257 (74.1) | 0.99 |
| SNAP | ||||
| Mean ± S.D. | 11.9 ± 6.7 | 15.4 ± 6.8 | 11.2 ± 6.4 | <0.0001 |
| Range | (1–41) | (2–41) | (1–41) | |
| Longitudinal factors | ||||
| Surfactant used | 197 (46.4) | 58 (78.4) | 139 (39.6) | <0.0001 |
| Symptomatic PDA | 93 (21.9) | 36 (48.7) | 57 (16.2) | <0.0001 |
| Postnatal indomethacin used | 80 (18.8) | 27 (36.5) | 53 (15.1) | <0.0001 |
| Mechanical ventilation at any time in the first 6 days after admission | 293 (68.9) | 69 (93.2) | 224 (63.8) | <0.0001 |
| Postnatal steroid used | 92 (21.7) | 45 (60.8) | 47 (13.4) | <0.0001 |
| Average fluid intake (ml/kg/day) in the first 6 days after admission | 108.3 ± 43.8 | 136.9 ± 43.8 | 102.3 ± 41.4 | <0.0001 |
| Intralipid intake (gm/kg/day)d | 1.66 ± 0.92 | 2.09 ± 0.63 | 1.58 ± 0.95 | <0.0001 |
SNAP score for neonatal acute physiology, PDA patent ductus arteriosus
Denominators vary due to missing values; continuous variables are reported as mean ± standard deviation
Pearson chi-square or Wilcoxon rank sum test
Birth weight less than 10th percentile for gestational age
Prior to 36 weeks post-menstrual age
Table 3 provides the unadjusted associations between BPD and 6-day longitudinal AUCs for Paco2, Fio2 and MAP. The AUC values for hypocarbia (<35 torr), hypercarbia (>50 torr), Fio2 (>0.21 and >0.40) and MAP were all significantly associated with BPD. The percentages of AUC values = 0 were between 20 and 50%.
Table 3. Unadjusted associations between BPD and longitudinal blood gas and ventilatory factors: 6-day areas under the curve (AUCs) for partial pressures of CO2, inspired O2 concentration, and measured mean airway pressure.
| Variable | All infants (N= 425a) | BPD (N = 74a) | Non-BPD (N= 351a) | P valueb | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Infants with AUC >0 n (%) |
AUC, for infants with AUC >0 Mean ± SD |
Infants with AUC >0 n (%) |
AUC, for infants with AUC >0 Mean ± SD |
Infants with AUC >0 n (%) |
AUC, for infants with AUC >0 Mean ± SD |
||
| Paco2 <35 torr | 325 (76.5) | 162.9 ± 180.8 | 68 (91.9) | 153.8 ± 198.5 | 257 (73.2) | 165.3 ± 176.1 | 0.0051 |
| Paco2 >50 torr | 205 (48.2) | 64.2 ± 108.2 | 60 (81.2) | 87.6 ± 138.9 | 145 (41.3) | 54.6 ± 91.4 | <0.0001 |
| Fio2 >0.21 | 342 (80.5) | 14.2 ± 16.3 | 73 (98.7) | 23.0 ± 20.7 | 269 (76.6) | 11.78 ± 14.0 | <0.0001 |
| Fio2 >0.40 | 247 (58.1) | 6.43 ± 11.10 | 64 (86.5) | 9.94 ± 15.57 | 183 (52.1) | 5.20 ± 8.77 | <0.0001 |
| MAP >0 cm H2O | 290 (68.2) | 639.9 ± 445.0 | 69 (93.2) | 954.1 ± 376.2 | 221 (63.0) | 541.8 ± 419.2 | <0.0001 |
Paco2 partial pressure of carbon dioxide, Fio2 fraction of inspired oxygen, MAP mean airway pressure, SD standard deviation
Units are torr hours for the Paco2 AUCs, and proportion hours for the Fio2 AUCs and cm H2O hours for the MAP AUC
Denominators vary due to missing values
Two degree of freedom Wald chi-square test from logistic model (see “Methods”)
Table 4 presents adjusted tests of association with BPD for each longitudinal factor, controlled for birth weight, SNAP, SNAP squared, gender (male) and Apgar score <7 at 5 min. The use of postnatal indomethacin and the occurrence of hypocarbia were no longer significantly associated with BPD when controlling for these baseline factors. Infants with a positive AUC for hypercarbia (Paco2 >50 torr) had a significantly greater incidence of BPD, and the difference did not depend on the magnitude of the AUC. The risk of BPD significantly increased as the magnitude of the MAP increased.
Table 4. Adjusted associations between longitudinal factors and BPD.
| Longitudinal variable | Odds ratio (95% confidence interval)a | P valueb | |
|---|---|---|---|
| Surfactant usec | 2.71 (1.37, 5.37) | 0.0042 | |
| Symptomatic PDA | 2.32 (1.25, 4.31) | 0.0080 | |
| Postnatal indomethacin usec | 1.28 (0.67, 2.45) | 0.46 | |
| Mechanical ventilation at any time in the first 6 days after admission | 2.70 (0.94, 7.74) | 0.064 | |
| Postnatal steroid usec | 5.56 (2.87, 10.78) | <0.0001 | |
| Average fluid intake (ml/kg/day) in the first 6 days after admission | 1.50 (1.02, 2.20) | 0.038 | |
| Intralipid intake (gm/kg/day)c | 1.37 (0.96, 1.95) | 0.079 | |
| Paco2 <35 torr | |||
| Intercept for AUC >0 (vs. AUC = 0) | 1.62 (0.60, 4.38) | 0.34 | |
| Slope for AUC >0 | 0.85 (0.63, 1.15) | 0.28 | |
| Joint test | 0.43 | ||
| Paco2 >50 torr | |||
| Intercept for AUC >0 (vs. AUC = 0) | 3.37 (1.69, 6.69) | 0.0005 | |
| Slope for AUC >0 | 1.18 (0.86, 1.62) | 0.30 | |
| Joint test | 0.0004 | ||
| Fio2 > 0.21 | |||
| Intercept for AUC >0 (vs. AUC = 0) | 6.15 (0.78, 48.8) | 0.086 | |
| Slope for AUC >0 | 1.78 (1.30, 2.43) | 0.0003 | |
| Joint test | 0.0002 | ||
| Fio2 >0.40 | |||
| Intercept for AUC >0 (vs. AUC = 0) | 2.33 (1.03, 5.24) | 0.041 | |
| Slope for AUC >0 | 1.50 (1.08, 2.10) | 0.017 | |
| Joint test | 0.0023 | 0.0023 | |
| MAP >0 cm H2O | |||
| Intercept for AUC >0 (vs. AUC = 0) | 0.96 (0.29, 3.23) | 0.95 | |
| Slope for AUC >0 | 2.61 (1.78, 3.81) | <0.0001 | |
| Joint test | <0.0001 |
PDA patent ductus arteriosus, AUC area under the curve (for the first 6 days after admission), Paco2 partial pressure of carbon dioxide, Fio2 fraction of inspired oxygen, MAP mean airway pressure
Logistic regression with baseline adjustment terms: birth weight, SNAP, SNAP squared, gender (male), and Apgar score <7 at 5 min; separate logistic regression model for each longitudinal variable; sample sizes vary due to missing values; AUCs are based on the first 6 days after admission
Odds ratios for continuous predictors correspond to the following unit changes: birth weight, 100 g; AUCs (linear terms), one standard deviation computed among those with AUC >0; others, one standard deviation
One degree of freedom Wald chi-square test; joint test for AUCs based on a two degree of freedom Wald chi-square test of the indicator variable for AUC >0 and the linear term for the AUC
Prior to 36 weeks post-menstrual age
Table 5 shows the final model based on selection of longitudinal factors by the stepwise process. Of the longitudinal factors, only the linear term for MAP (P < 0.0001) and the increment in the intercept for hypercarbia AUCs >0 (P = 0.0078) were retained. Of the baseline variables, only birth weight remained significant (P = 0.037) after controlling for the MAP and hypercarbia AUCs.
Table 5. Results for the multivariate logistic regression model of BPD, with terms for the reduced set of baseline predictors and the longitudinal factors selected by the stepwise process.
| Model terms | Odds ratio (95% confidence interval)a | P valueb | |
|---|---|---|---|
| Baseline predictors | |||
| Birth weight | 0.87 (0.76, 0.99) | 0.037 | |
| SNAPc | 0.78 (0.56, 1.10) | 0.15 | |
| SNAP squared | 0.83 (0.37, 1.87) | 0.65 | |
| Male | 1.39 (0.77, 2.51) | 0.27 | |
| Apgar score <7 at 5 min | 1.56 (0.81, 3.01) | 0.18 | |
| Longitudinal predictors | |||
| Paco2 >50 torr | |||
| Intercept for AUC >0 (vs. AUC = 0) | 2.65 (1.29, 5.45) | 0.0078 | |
| AUC (linear) | 1.00 (0.71, 1.40) | 0.99 | |
| Joint test | 0.024 | ||
| MAP >0 cm H2O | |||
| intercept for AUC >0 (vs. AUC = 0) | 1.00 (0.29, 3.43) | 1.00 | |
| AUC (linear) | 2.33 (1.56, 3.47) | <0.0001 | |
| Joint test | <0.0001 |
SNAP score for neonatal acute physiology, AUC area under the curve (for the first 6 days after admission); Paco2 partial pressure of carbon dioxide, MAP mean airway pressure
N = 419 (six infants were excluded for missing Apgar score or MAP AUC); lack of fit was nonsignificant (P = 0.87) by the Hosmer–Lemeshow test; area under the Receiver Operating Characteristic curve was 0.838
Odds ratios are adjusted for the other factors in the model. Odds ratios for continuous predictors correspond to the following unit changes: birth weight, 100 g; AUCs (linear terms), one standard deviation computed among those with AUC >0; others, one standard deviation
One degree of freedom Wald chi-square test; joint test for AUCs based on a two degree of freedom Wald chi-square test of the indicator variable for AUC >0 and the linear term for the AUC
Results for SNAP are from a reduced model with the SNAP squared term excluded
The adjusted odds ratios and 95% confidence intervals in the final model are shown in Fig. 1. After adjusting for baseline variables, only birth weight, AUCs for Paco2 >50 torr and MAP >0 cm H2O were significantly associated with BPD. The linear AUC term for Fio2 >0.21 approached significance (P = 0.059). The AUC for Paco2 <35 torr was not associated with BPD.
Fig. 1.
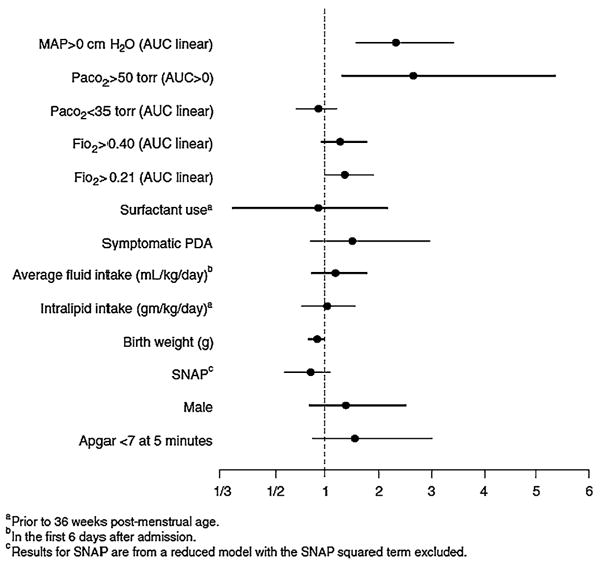
Adjusted odds ratios and 95% confidence limits for predictors of BPD, based on 419 infants (six infants were excluded for missing Apgar score or MAP AUC). Results for the baseline factors and the AUCs for MAP >0 cm H2O and Paco2 >50 torr are based on the model in Table 5 without the nonsignificant linear term for the AUC for Paco2>50 torr. Results for the remaining factors (AUC for Paco2 <35 torr through Intralipid intake) were based on separately adding each factor to the logistic regression model described above. Odds ratios for continuous predictors correspond to the following unit changes: birth weight, 100 g; AUCs (linear terms), one standard deviation computed among those with AUC >0; others, one standard deviation
The foregoing analyses indicated that hypercarbia was a statistically significant risk for BPD even after adjusting for the burden of AUC for MAP >0 cm H2O. However, this analysis did not address whether the occurrence of Paco2 >50 torr was simply a marker for severity of illness in sicker infants. To address this issue, we examined the proportions of ventilated infants with hypercarbia occurring in association with values of MAP above and below the observed median MAP value.
Table 6 gives the incidence of BPD by MAP-specific hypercarbia in ventilated infants. The infants were subdivided into 4 groups according to whether or not hypercarbia occurred at any time within periods of high MAP (>8 cm H2O) and periods of low MAP (≤8 cm H2O) during the first 6 days. The incidence of BPD differed significantly among the four groups (P = 0.0002), with the lowest incidence (11.9%) among infants with no hypercarbia (Group 4). The incidence of BPD was greater when hypercarbia was present, but did not statistically differ by whether the hypercarbia occurred only when MAP was ≤8, or only when MAP was >8, or under both conditions (BPD in 32.8% (Group 3), 34.0% (Group 2), and 36.6% (Group 1); P = 0.92). Group 3, in which hypercarbia occurred only during times when the MAP was ≤8, is similar to permissive hypercapnia. The incidence of BPD (32.8%) in this group was significantly greater than in the group without hypercarbia (P = 0.0003) and similar to the infants who had occurrences of hypercarbia when the MAP was >8. This suggested that hypercarbia may be associated with BPD and was not simply a marker for severity of illness.
Table 6. Incidence of BPD by MAP-specific hypercarbia (Paco2 >50 torr) in ventilated babies.
| Group | Hypercarbia | N | BPD | P valuea | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| At any time when MAP >8 cm H2O | At any time when MAP ≤8 cm H2O | n (%) | ||||||
| 1 | Yes | Yes | 41 | 15 (36.6) |
|
0.92 |
|
0.0002 |
| 2 | Yes | No | 47 | 16 (34.0) | ||||
| 3 | No | Yes | 67 | 22 (32.8) | ||||
| 4 | No | No | 135 | 16 (11.9) | ||||
| Totalb | 290 | 69 (23.8) | ||||||
Pearson chi-square test for differences among groups
Three of the 293 ventilated infants had missing values
Discussion
Many investigators have shown increased incidence and severity of BPD in infants weighing <1,500 g associated with intubation and use of mechanical ventilator [28], low Paco2 (<35 torr) [6, 9], PDA [10, 14] increased fluids intake [5, 9], increased Fio2 [5–7, 9, 28] lower birth weights [3] and male gender [3]. Over the years, permissive hypercapnia came into practice in some centers using nasal CPAP [4]. However, this practice was extended to the babies who are intubated and subjected to positive pressure ventilation [5, 17]. There are very few studies in adults [6, 29] and in infants [12, 31–33] to show the benefit of using permissive hypercapnia to decrease BPD. Published trials neither show benefit nor have the strength to show benefit. Concerns have been raised about the lack of evidence this extended practice of permissive hypercapnia is safe [12, 27, 30]. Our study attempts to analyze the relationship between BPD and CO2 concentrations in blood in the first 6 days of life in infants weighing 500–1,499 g.
The last Cochrane review [12] after considering the available evidence [11, 34] does not find any benefit of permissive hypercapnia compared to normal ventilator strategy. Our study, after considering the variables that are usually implicated with BPD, suggests that hypercarbia actually may be strongly associated with BPD in infants weighing 500–1,499 g. We examined the areas under (or over) the curve for MAP, Paco2 and Fio2 during the first 6 days of life and their association with BPD. After accounting for the other variables, the AUC for MAP >0 (P < 0.0001) and the occurrence of Paco2 >50 torr (P = 0.024) were significantly associated with higher incidence of BPD.
During this study permissive hypercapnia or any standard respiratory care protocols were not necessarily practiced in the study hospitals. To get an idea of the permissive hypercapnia component of the AUCs for Paco2 and MAP, we analyzed MAP-specific hypercarbia among ventilated infants. As shown in Table 6, infants with hypercarbia had a greater risk of BPD compared with those with no hypercarbia, but the increase in risk was similar regardless of whether hypercarbia occurred during periods with higher MAP, lower MAP, or both. This suggests that whether hypercarbia occurs because of increased severity of illness (higher MAP and hypercarbia) or because of a scenario similar to permissive hypercapnia (lower MAP and hypercarbia), the risk of BPD is increased.
The results of this study demonstrate an association between higher Paco2 during the first few days of life and the subsequent incidence of BPD. The understanding of potential etiologic factors associated with BPD has evolved. Vascular factors are now well recognized in the pathology of BPD [35–37]. Pulmonary vascular morbidity was previously assumed to be secondary to the alveolar damage. However, growing evidence supports the hypothesis that vascular injury is the triggering mechanism in the development of BPD [35]. The pathogenesis of hyperoxia-induced BPD might be attributed to the induction of vascular cytokines production and secondary vascular spasm triggering alveolar injury. Such hyperoxia-induced BPD was treated and/or prevented in animal models using vascular endothelial growth factor (VEGF) [38]. Higher carbon dioxide levels and acidosis are both known to induce pulmonary vascular spasm. We speculate based on the recent findings in the literature that hypercarbia during the first few days of life can cause vascular spasm leading to subsequent alveolar maldevelopment and BPD.
Since the practice of permissive hypercapnia has come into vogue without the necessary rigorously controlled clinical trials, its impact needs to be observed as to its potential detrimental effects. This is particularly important in ventilated preterm infants, where potential detrimental effects include BPD [16, 31] neurodevelopmental delay [16, 17, 29, 31] and other outcomes [16, 17, 31]. This study is confined to one geographic area of nine hospitals and has a good sample size. The association of hypercarbia and BPD remained strong after rigorously analyzing the data for confounding variables and not simply as a marker of severity.
This study was limited by the fact that it was an observational study and could not establish hypercarbia as the cause of BPD. Even though permissive hypercapnia was not practiced by design in this study, our results increase concern regarding the current widely practiced permissive hypercapnia in ventilated infants with birth weights of 500-1,499 grams. Our results call for controlled trials to further define the role of permissive hypercapnia in relation to BPD and other neurodevelopmental outcomes before it becomes a standard of practice.
Acknowledgments
We thank all the nurses, doctors and researchers who helped in the study hospitals. We thank all the hospitals for participating in the study. We acknowledge Irina Rapoport for statistical programming. We also thank Vijaya Rao, Dorris Bartel, John Grausz, and Murray Pollack for their early contributions to this protocol. The study was approved by the institutional review boards at all participating institutions. This study was part of the National Institutes of Health (NIH) Washington, DC Initiative to Reduce Infant Mortality in Minority Populations in the District of Columbia (Phase I) and was funded by the NIH Office of Research on Minority Health and the National Institute of Child Health and Human Development (NICHD) (U18-HD30447, U18-HD30458, U18-HD30450, U18-HD30445, U18-HD31919, U18-HD30454, and U18-HD31206).
Abbreviations
- PDA
Patent ductus arteriosus
- SNAP
Score for neonatal acute physiology
- AUC
Area under the curve
- Paco2
Partial pressure of carbon dioxide in blood
- Fio2
Fraction of inspired oxygen
- MAP
Mean airway pressure
- NTISS
Neonatal therapeutic intervention scoring system
- SGA
Small for gestational age
Appendix
The computation of the longitudinal AUC variables is illustrated in Fig. 2 for Paco2 >50 torr. Other longitudinal AUCs were derived analogously.
AUC variables exhibited appreciable numbers of observations with values of zero. Because the group of infants with a value of zero for a particular AUC may be heterogeneous, there may be a discontinuity between the risk of BPD among those with AUC = 0 and those with positive but very small values of AUC. Consequently, for each AUC variable we employed two terms in the logistic regression modeling: (1) an indicator variable, with a value of 1 when AUC >0 and a value of 0 when AUC = 0; and (2) a linear regression term for the AUC. The test of the significance of each AUC variable was based on a Wald chi-square, which tested the joint significance of the indicator and linear terms. This approach was followed throughout the analysis, including unadjusted tests, adjusted tests, and tests performed as part of the stepwise selection.
For each AUC the logistic model (without covariates) was:
where π is the population proportion of infants with BPD; α is the logit of the population proportion of BPD among infants with AUC = 0; α + β0 is the intercept of the regression line for infants with AUC> 0; and β1 is the slope of the regression line on AUC. Thus, β0 is the difference between the logit for infants with AUC = 0 and the logit for infants with very small but positive values of the AUC (Fig. 3a). If β1, the population slope, is zero, then β0 becomes the difference between the group of infants with AUC = 0 and the entire group with AUC >0 (Fig. 3b).
Fig. 3.
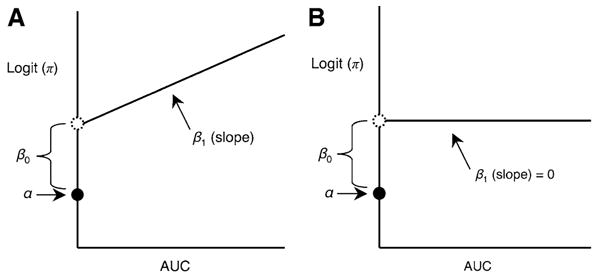
Model parameters for AUC variables. a The general case. b tHe case where β1, the population slope, is zero
Contributor Information
Siva Subramanian, Email: sivasubk@georgetown.edu, doc4baby@aol.com, Neonatal Perinatal Medicine, Department of Pediatrics, Georgetown University Hospital, 3800 Reservoir Rd, NW, #M3400, Washington, DC 20007, USA.
Ayman El-Mohandes, Email: Aelmohandes@unmc.edu, College of Public Health, University of Nebraska Medical Center, WH 5030, Omaha, NE 68198, USA.
Ramasubbareddy Dhanireddy, Email: rdhanire@uthsc.edu, Department of Pediatrics, Division of Neonatology, University of Tennessee Health Science Center, 853 Jefferson Avenue, Suite 201, Memphis, TN 38163, USA.
Matthew A. Koch, Email: dmakoc@rti.org, Statistics and Epidemiology Division, RTI International, 3040 Cornwallis Road, Cox 305, P.O. Box 12194, Research Triangle Park, NC 27709-2194, USA
References
- 1.Bhuta T, Henderson-Smart DJ. Elective high frequency jet ventilation versus conventional ventilation for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews. 2000;(2):CD000328. doi: 10.1002/14651858.CD000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northway WH., Jr Bronchopulmonary dysplasia thirty three years later. Pediatric Pulmonology. 2001;26(S23):5–7. [PubMed] [Google Scholar]
- 3.Stevenson DK, Wright LL, Lemons JA, Oh W, Korones SB, Papile LA, et al. Very low birth weight outcomes of the NICHD Neonatal Research Network, January 1993 to December 1994. American Journal of Obstetrics and Gynecology. 1998;179(6):1632–1639. doi: 10.1016/s0002-9378(98)70037-7. [DOI] [PubMed] [Google Scholar]
- 4.Wung JT, James LS, Kilchevsky E, James E. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985;76(4):488–494. [PubMed] [Google Scholar]
- 5.Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: A prospective study. Critical Care Medicine. 1994;22(10):1568–1578. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- 6.Tammela OK, Koivisto ME. Fluid restriction for preventing bronchopulmonary dysplasia? Reduced fluid intake during the first weeks of life improves the outcome of low-birth-weight infants. Acta Paediatrica. 1992;81(3):207–212. doi: 10.1111/j.1651-2227.1992.tb12205.x. [DOI] [PubMed] [Google Scholar]
- 7.Abman SH. Bronchopulmonary dysplasia “a vascular hypothesis”. American Journal of Respiratory and Critical Care Medicine. 2001;164(10 Pt 1):1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 8.Yu VY, Orgill AA, Lim SB, Bajuk B, Astbury J. Bronchopulmonary dysplasia in very low birthweight infants. Australian Paediatric Journal. 1983;19(4):233–236. doi: 10.1111/j.1440-1754.1983.tb02109.x. [DOI] [PubMed] [Google Scholar]
- 9.Erickson SJ, Grauaug A, Gurrin L, Swaminathan M. Hypocarbia in the ventilated preterm infant and its effect on intraventricular haemorrhage and bronchopulmonary dysplasia. Journal of Paediatrics and Child Health. 2002;38(6):560–562. doi: 10.1046/j.1440-1754.2002.00041.x. [DOI] [PubMed] [Google Scholar]
- 10.Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics. 1987;79(1):26–30. [PubMed] [Google Scholar]
- 11.Dries DJ. Permissive hypercapnia. Journal of Trauma. 1995;39(5):984–989. doi: 10.1097/00005373-199511000-00028. [DOI] [PubMed] [Google Scholar]
- 12.Woodgate PG, Davies MW. Permissive hypercapnia for the prevention of morbidity and mortality in mechanically ventilated newborn infants. Cochrane Database of Systematic Reviews. 2009;2001(2):CD002061. doi: 10.1002/14651858.CD002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laffey JG, Kavanagh BP. Biological effects of hypercapnia. Intensive Care Medicine. 2000;26(1):133–138. doi: 10.1007/s001340050027. [DOI] [PubMed] [Google Scholar]
- 14.Tooley WH. Epidemiology of bronchopulmonary dysplasia. Journal of Pediatrics. 1979;95(5 Pt 2):851–858. doi: 10.1016/s0022-3476(79)80451-5. [DOI] [PubMed] [Google Scholar]
- 15.Plavka R, Kopecky P, Sebron V, Svihovec P, Zlatohlavkova B, Janus V. A prospective randomized comparison of conventional mechanical ventilation and very early high frequency oscillatory ventilation in extremely premature newborns with respiratory distress syndrome. Intensive Care Medicine. 1999;25(1):68–75. doi: 10.1007/s001340050789. [DOI] [PubMed] [Google Scholar]
- 16.Vannucci RC, Towfighi J, Heitjan DF, Brucklacher RM. Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: An experimental study in the immature rat. Pediatrics. 1995;95(6):868–874. [PubMed] [Google Scholar]
- 17.Ambalavanan N, Carlo WA. Hypocapnia and hypercapnia in respiratory management of newborn infants. Clinics in Perinatology. 2001;28(3):517–531. doi: 10.1016/s0095-5108(05)70104-4. [DOI] [PubMed] [Google Scholar]
- 18.Bigatello LM, Patroniti N, Sangalli F. Permissive hypercapnia. Current Opinion in Critical Care. 2001;7(1):34–40. doi: 10.1097/00075198-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for neonatal acute physiology: A physiologic severity index for neonatal intensive care. Pediatrics. 1993;91(3):617–623. [PubMed] [Google Scholar]
- 20.Gray JE, Richardson DK, McCormick MC, Workman-Daniels K, Goldmann DA. Neonatal therapeutic intervention scoring system: A therapy-based severity-of-illness index. Pediatrics. 1992;90(4):561–567. [PubMed] [Google Scholar]
- 21.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: Prediction from oxygen requirement in the neonatal period. Pediatrics. 1992;82(4):527–532. 1988. [PubMed] [Google Scholar]
- 22.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SAS Institute. SAS/STAT software changes and enhancements through release 6.12. Cary, NC: SAS Institute; 1997. [Google Scholar]
- 24.Hosmer DW, Lemeshow S. Applied logistic regression. New York: Wiley; 1989. [Google Scholar]
- 25.Ruttimann UE. Statistical approaches to development and validation of predictive instruments. Critical Care Clinics. 1994;10(1):19–35. [PubMed] [Google Scholar]
- 26.Pollack MM, Koch MA. Association of outcomes with organizational characteristics of neonatal intensive care units. Critical Care Medicine. 2003;31(6):1620–1629. doi: 10.1097/01.CCM.0000063302.76602.86. [DOI] [PubMed] [Google Scholar]
- 27.Levene MI, Fawer CL, Lamont RF. Risk factors in the development of intraventricular haemorrhage in the preterm neonate. Archives of Disease in Childhood. 1982;57(6):410–417. doi: 10.1136/adc.57.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. New England Journal of Medicine. 1967;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 29.Bidani A, Tzouanakis AE, Cardenas VJ, Jr, Zwischenberger JB. Permissive hypercapnia in acute respiratory failure. JAMA. 1994;272(12):957–962. [PubMed] [Google Scholar]
- 30.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. New England Journal of Medicine. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 31.Mariani G, Cifuentes J, Carlo WA. Randomized trial of permissive hypercapnia in preterm infants. Pediatrics. 1999;104(5 Pt 1):1082–1088. doi: 10.1542/peds.104.5.1082. [DOI] [PubMed] [Google Scholar]
- 32.Mammel MC, De Regnier RA. Barotrauma, oxygen toxicity, and chronic lung disease. Pediatrics. 2001;108(2):525. doi: 10.1542/peds.108.2.525. [DOI] [PubMed] [Google Scholar]
- 33.Thome UH, Carlo WA. Permissive hypercapnia. Seminars in Neonatology. 2002;7(5):409–419. doi: 10.1053/siny.2002.0135. [DOI] [PubMed] [Google Scholar]
- 34.Feihl F, Perret C. Permissive hypercapnia. How permissive should we be? American Journal of Respiratory and Critical Care Medicine. 1994;150(6 Pt 1):1722–1737. doi: 10.1164/ajrccm.150.6.7952641. [DOI] [PubMed] [Google Scholar]
- 35.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. American Journal of Respiratory and Critical Care Medicine. 2007;175(10):978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenmark KR, Abman SH. Lung vascular development: Implications for the pathogenesis of bronchopulmonary dysplasia. Annual Review of Physiology. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 37.Chess PR, D'Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Seminars in Perinatology. 2006;30(4):171–178. doi: 10.1053/j.semperi.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. American Journal of Physiology Lung Cellular and Molecular Physiology. 2005;289(4):L529–L535. doi: 10.1152/ajplung.00336.2004. [DOI] [PubMed] [Google Scholar]


