Abstract
Hepatocellular carcinoma (HCC) is the most rapidly increasing cause of cancer death in the United States. Although many risk factors for HCC, including hepatitis B (HBV), hepatitis C (HCV), and alcohol are well-defined, most series show that 5-30% of HCC patients lack a readily-identifiable risk factor for their cancer. The majority of “cryptogenic” HCC in the U.S. is attributed to non-alcoholic fatty liver disease (NAFLD), a hepatic manifestation of the metabolic syndrome. Metabolic syndrome is a constellation of problems including insulin resistance, obesity, hypertension, and hyperlipidemia. Increasingly, components of the metabolic syndrome are being linked to various forms of cancer with respect to both increased risk of disease and worsened outcome. In this review, we focus on the relationship between metabolic syndrome and hepatocellular carcinoma. We discuss the increased risks of HCC in those with features of metabolic syndrome, potentially worsened cancer outcomes in these patients, possible pathogenic mechanisms to explain these relationships, and treatment options for those with NAFLD and its progressive counterpart, non-alcoholic steatohepatitis (NASH). Metabolic syndrome is predicted to lead to large increases in the incidence of HCC over the next decades. A better understanding of the relationship between these two diseases should ultimately lead to improved screening and treatment options for those with HCC.
Keywords: Hepatocellular carcinoma, HCC, metabolic syndrome, NASH
Introduction
Hepatocellular carcinoma (HCC) is the most rapidly increasing cause of cancer death in the United States.1 Although many risk factors for HCC, including hepatitis B (HBV), hepatitis C (HCV), and alcohol are well-defined, most series show that 5-30% of HCC patients lack a readily-identifiable risk factor for their cancer.2 The majority of “cryptogenic” HCC in the U.S. is attributed to non-alcoholic fatty liver disease (NAFLD),3 a hepatic manifestation of the metabolic syndrome (Figure 1).
Figure 1.
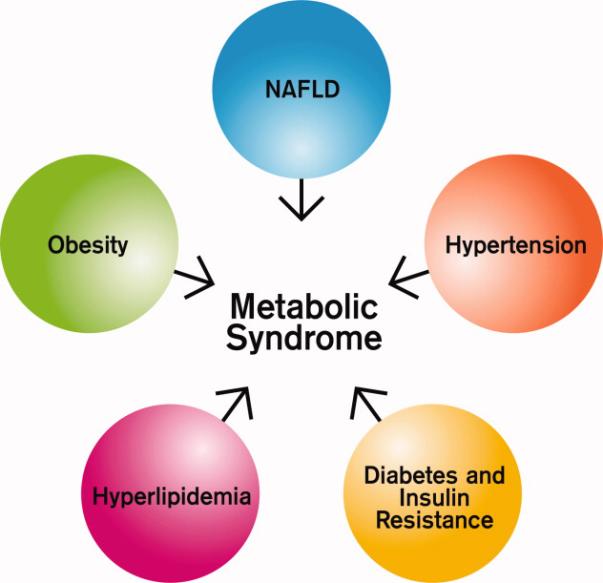
Components of the Metabolic Syndrome
Metabolic syndrome is a constellation of problems including obesity, dyslipidemia, diabetes, and insulin resistance.4 The prevalence of metabolic syndrome is increasing, paralleling the obesity epidemic in this country. Nearly one quarter of the U.S. population meet criteria for the metabolic syndrome,5 and U.S. obesity rates (BMI >30 kg/m2) now also exceed 25% in most regions of the country. Up to three quarters of obese adults will develop fatty liver disease. Unlike the HCV epidemic, which is estimated to peak in 2010, the obesity/metabolic syndrome epidemic shows no signs of abating.6
NAFLD comprises a spectrum of disorders, from fatty liver disease to progressive inflammation and cirrhosis. The prevalence of NAFLD varies widely depending on the method of assessment. About 2-5% of the U.S. population have “cryptogenic” elevated liver enzymes consistent with NAFLD,7 and up to 90% of those with obesity have some degree of fatty liver disease.8 Ultrasound and MRI studies from the U.S. and other Western countries suggest that 20-30% of the population have evidence of fatty liver disease attributed to NAFLD.9, 10, 11 About 10% of patients with NAFLD progress to nonalcoholic steatohepatitis (NASH), and 8-26% of those with NASH progress to cirrhosis.12
Retrospective data suggest that after cirrhosis develops, 4%-27% cases of NASH transform to HCC.13 These figures lead to theoretical HCC incidences which range from 0.6/100,000 to 210/100,000. (Figure 2) The obesity/metabolic syndrome epidemic is relatively recent, and it is likely that several decades are required before NASH develops into cirrhosis. Thus, the NASH-HCC “epidemic” may not have fully established itself yet. Currently, those with NASH but no underlying cirrhosis are not routinely screened for HCC at most centers due to the very low risk of HCC development. About 10-25% of those with NASH go on to develop cirrhosis, but it is not yet clear what predisposes to this progression of disease. The ability to better determine which patients will progress to cirrhosis will have important screening implications for the future.
Figure 2.
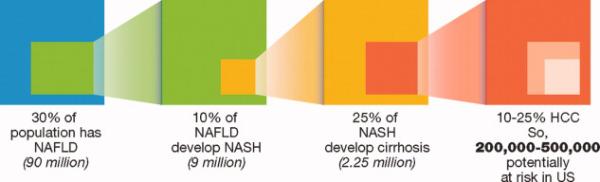
Relationship between NAFLD, NASH, and HCC
Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis
The gold standard for the diagnosis of both NAFLD and NASH is tissue biopsy. NAFLD is characterized by hepatic steatosis in the absence of a history of significant alcohol use or other known liver disease. Alcohol intake as low as 20 grams/day in females and 30 grams/day in males may be sufficient to cause alcohol-induced liver disease; 12 oz of beer (350 ml), 4 oz (120 ml) of wine, and 1.5 oz (45ml) of hard liquor each contain 10g of alcohol.14 NASH is a progressive form of NAFLD, and includes inflammatory components on pathology. The NASH Clinical Research Network designed and validated a pathological scoring system for NASH which is the sum of measures of steatosis, lobular inflammation, and hepatocellular ballooning (a feature of cellular injury characterized by large hepatocytes).15 Sampling error may be a problem using biopsy samples to diagnose NAFLD and NASH.16 In addition, once cirrhosis has developed, NASH pathology may be difficult to evaluate, because fat often disappears as NASH transforms into cirrhosis.17 Thus, accurate non-pathologic markers which distinguish NASH from other underlying disorders would be helpful to study this entity, but none has been found that is diagnostic. Imaging has also not been a useful tool thus far for the diagnosis of NASH.18
Most cryptogenic cirrhosis in the United States is thought to be related to risk factors associated with NASH and the metabolic syndrome.19 Several studies have now shown that patients with cryptogenic cirrhosis are more likely to be obese and up to four times as likely to be diabetic than those with other forms of cirrhosis.20, 21 Hispanics have the highest rates of both cryptogenic cirrhosis and NASH in many studies, and they also have the most rapidly increasing incidence of HCC in the United States.21, 22 One study defining rates of the metabolic syndrome in the U.S. by ethnicity found that Mexican Americans have the highest rates with 32%, while 24% of European-Americans and 22% of African Americans meet criteria for the metabolic syndrome.23
It is not clear why African Americans may have lower rates of NASH than other racial/ethnic groups, despite a high prevalence of diabetes and obesity. Some investigators have suggested that African American have less insulin resistance, despite other risk factors of the metabolic syndrome. For instance, African Americans with adult onset diabetes have human leukocyte antigen DQ differences which may lead to beta cell dysfunction, rather than insulin resistance. In addition, adiponectin, a cytokine negatively related to obesity and diabetes, does not seem to correlate with insulin resistance in African Americans. Others have suggested differences in body fat distribution among African Americans. 24, 25,19, 21, 26
It is also currently unclear whether diabetes, obesity and the metabolic syndrome are risk factors for HCC independently of the presence of NAFLD. However, it seems likely that NAFLD usually mediates the relationship between metabolic syndrome and HCC based on the high correlation between features of metabolic syndrome and NAFLD. Up to 70% of those with type II diabetes, and up to 90% of obese patients have some degree of fatty liver disease. 8 Although NAFLD can occur in the absence of metabolic syndrome, this appears to be relatively uncommon. Marchesini and colleagues assessed the prevalence of the metabolic syndrome in 304 patients with NAFLD, but no overt diabetes. 67% of those with NAFLD and obesity had metabolic syndrome, compared with only 18% of normal-weight patients. 27 Similarly, it is likely that the majority of those with metabolic syndrome who develop HCC also have cirrhosis prior to their diagnosis, although this is also unproven. Case reports have described patients with NASH who developed cirrhosis and then HCC, 17, 28 and animal models have shown clear progression from NASH, to cirrhosis, to cancer. 29 Large, prospective studies are needed to answer both of these questions more definitively.
Confounding risks
Metabolic syndrome risk factors may also be modified by other underlying liver diseases with respect to HCC risk. For instance, diabetes appears to be synergistic with both virally-mediated and alcohol-related HCC.30 In a large prospective cohort study from Taiwan, obesity led to a four-fold overall increased risk of HCC in those with hepatitis C. In those without underlying viral infection, there was about a two-fold increased risk, while for those with hepatitis B, BMI was not associated with HCC risk. Diabetes led to a two to three- fold increase in HCC risk no matter what the underlying viral etiology was, and also had a synergistic effect with obesity, leading to a greater than 100-fold increased cancer risk.31 Interestingly, these authors showed an inverse relationship between serum levels of HBV DNA and triglyceride levels. They note that in culture models the HBV X protein inhibits the secretion of apolipoprotein B, which is a component of very low density lipoprotein (VLDL).32
Yu and colleagues recently published a prospective study of 2,903 male HBV surface-antigen positive government employees from Taiwan. With mean follow-up of almost 15 years, they found that the hazard ratio for overweight men for incident HCC was 1.48 (95% CI 1.04-2.12), while that for obese men was 1.96 (95% CI=0.72-5.38).33 These authors found no significant effect of diabetes on the development of HCC after adjusting for quartiles of BMI and other confounders, but noted that only 2.5% of their sample reported diabetes at enrollment. Those with higher BMI were significantly more likely to have underlying fatty liver disease (assessed by ultrasound) as well as cirrhosis, leading to the question of whether there might have been confounding by ascites in the higher BMI patients. However, as the authors note, the presence of chronic HBV may provide a synergistic effect with obesity on hepatic lipid accumulation and the development of steatosis.
Thus, cancer risks related to underlying liver disease from either HBV/HCV or alcohol may be worsened by features of metabolic syndrome. In addition, patients with insulin resistance and NASH often have hepatic iron deposition which is distinct from hemochromatosis. 34 Further, those with NASH-related cirrhosis who have more stored iron in their livers (and do not have hemochromatosis) seem to have a higher risk of cancer in a large retrospective study.35 Given the heterogeneity of underlying risk factors in those with HCC, more study is necessary to dissect out the relationships between these and the influence on HCC risk.
Obesity and Metabolic Syndrome
Metabolic syndrome may be defined as 1) increased waist circumference, or BMI> 30 kg/m2 and any two of the following: 1) Triglycerides >150 mg/dL or treatment for elevated triglycerides 2) HDL cholesterol <40 mg/dL in men or <50 mg/dL in women, or treatment for low HDL 3) Systolic blood pressure >130, diastolic blood pressure >85, or treatment for hypertension 4) Fasting plasma glucose >100 mg/dL, or previously diagnosed type 2 diabetes.36 Obesity, a major component of the metabolic syndrome, can also be defined in several ways. Body mass index, or BMI, is an individual's weight in kilograms divided by the square of height in meters. Both the World Health Organization and the U.S. Department of Health and Human Services define “overweight” as a BMI of 25 kg/m2-29.9 kg/m2, “obese” as a BMI>30 kg/m2, and “normal” as between 18.5 and 24.9 kg/m2.37, 38 Others have defined important correlates of obesity such as truncal obesity (measuring either waist circumference or waist/hip ratio), or visceral fat (often measured by CT or MRI). These anthropomorphic measurements may each be more sensitive measures of sequelae of obesity such as metabolic syndrome and NASH than BMI. 39 These definitions have their own complexity since different ethnic groups have different amounts of visceral fat accumulation for given amounts of total body fat 40, 41 and women and older people also tend to have higher percentages of body fat than men or younger people with the same BMI because of differences in body composition.42 Similarly, the normal BMI range for Asians is lower than for other racial/ethnic groups, with 23 kg/m2 considered to be an “action point” for public health interventions in Asian patients. 43, 44
The Center for Disease Control (CDC)'s National Center for Health Statistics has conducted a series of cross-sectional surveys, known as National Health and Nutrition Surveys (NHANES). Beginning in 1960, these surveys recorded height and weight in nationally representative samples of Americans. In 2003-2004, NHANES data showed that 33% of adults age 20-74 were obese, compared to 11% of men and 16% of women in the 1960's, with much of the increase occurring after 1980.45 There were also differences in age, with older people, African Americans and Hispanic women and children more likely to be obese than the general population.45 Obesity (BMI of 30 or more) increases the risk for many types of health problems, including diabetes, hypertension, and cardiovascular disease, as well as the overall risk of death.46, 47
Overall mortality is clearly related to obesity, but many have suggested that the relationship may be “U-shaped” with optimal survivals at intermediate BMIs,48, 49 or “J-shaped,” with thin patients having higher mortality than those in the middle.50 This may be because smokers and those with chronic illness tend to be thin, and both tend to have high mortality rates.
Obesity and Cancer Risk
Several studies suggest that patients with obesity are also at increased risk for several types of cancer, both in the United States and in other countries. 51, 52-54 A large meta-analysis published in the Lancet showed that increased BMI was strongly associated with risk of esophageal, thyroid, colon, and renal cell carcinomas in men. In women, endometrial, gallbladder, esophageal, and renal cell carcinomas were increased in those with a 5 kg/m2 increase in BMI.54 The Million Woman study, a large prospective cohort of women in England and Scotland, also reported significant increases in several cancers in women with increasing BMI, including postmenopausal breast cancer, pancreatic and ovarian cancers, and several hematologic malignancies.55
Explanations for these increases in cancer incidence have focused on the relationship between metabolic syndrome, adipokines, and hormone levels. For instance, post-menopausal breast cancer and endometrial cancer risk in obese women may be mediated by increased estrogen levels. In addition, the insulin-growth factor axis has been implicated in risk of several types of cancer in both men and women. High levels of peripheral insulin-like growth factor-1 (IGF-1) have been associated with increased risk of developing several types of cancer including prostate, colon and breast. 56-58 Adipokines related to obesity, such as leptin, also may mediate cancer risk via their effects on angiogenesis.59
Further, increasing evidence suggests that obesity may lead to a state of chronic inflammation. Excess consumption of fatty acids and glucose can then lead to the increased expression of several signaling molecules known to be important in carcinogenesis, including NF-kB, EGF, and FGF.60, 61
Obesity and cancer outcome
Individuals with features of metabolic syndrome, such as obesity, may have worsened outcomes from many different types of cancer, particularly HCC. An article by Calle and colleagues in the New England Journal of Medicine showed that obesity is associated with significantly increased cancer death rates, particularly from HCC (Figure 3).52
Figure 3.
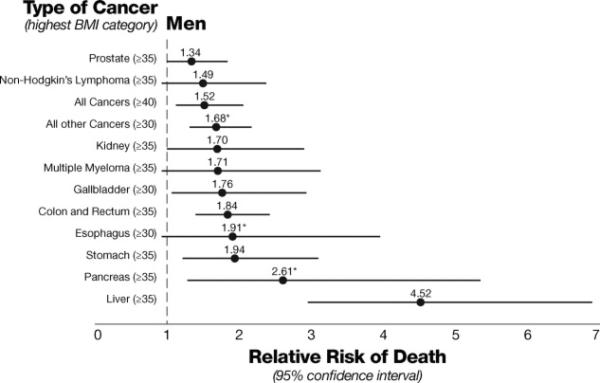
BMI category and relative risk of cancer death in men 52
These findings were not replicated in a study of male Korean government employees and teachers published by Park and colleagues. In this report, 14,578 subjects were followed from 1996 until 2004. Unlike the Calle study, here a BMI of 25 kg/m2 or greater did not lead to a statistically significant increase in mortality for HCC (HR=1.03, (0.92-1.14). This is much less than the cutoff used in the Calle study of 40 or greater for the highest BMI, which may explain the discordant findings 62
Reasons for these possibly worsened outcomes remain unclear. These may include comorbidities such as coronary artery disease, and/or underdosing of chemotherapy in some types of cancers in those who are obese.63 Unfavorable tumor characteristics may also be associated with obesity due to delayed screening, or biological characteristics of the tumor itself. However, several studies have suggested that the increased mortality seen with obesity is not related to later diagnosis, since differences often persist after adjusting for stage and tumor size.64, 65
Another possible mechanism connecting obesity to worsened clinical outcome may be related to dysregulated angiogenesis. Adipose tissue induces expression of VEGF and other adipokines in both human and animal models.66 In 58 patients with renal cell carcinoma, high serum leptin levels were significantly associated with venous invasion in pathology samples and aggressive clinical features.67 Adipose tissue induces expression of leptin, a hormone that regulates body mass. Leptin, in turn, has been shown to promote angiogenesis and mediate the progression of NASH to HCC in animal models.68 Leptin also upregulates signal transduction pathways involved in cancer progression such as JAK/STAT, AKT and ERK in HCC cells.69 These relationships suggest a possible association between the metabolic syndrome and worsened clinical outcomes which may be mediated by adipokines which lead to increased vascular invasion.
Metabolic syndrome and HCC: Risk, Outcome, and NASH management
The mechanism mediating the interaction between NAFLD, NASH and HCC is not completely elucidated. Some of the hypothetical mechanisms are illustrated in Figure 4. For HCC, the relationship with obesity seems to be primarily mediated by factors related to metabolic syndrome, NAFLD, and NASH. Insulin modulates intracellular signaling through the tyrosine kinase activity of the insulin receptor. Defects in these signaling pathways are thought to contribute to insulin resistance, which can then lead to hepatic fat accumulation by lipolysis.14 Hepatic fat accumulation can then produce inflammatory changes in the liver.14 In particular, free fatty acids may lead to hepatic inflammation through production of cytokines such as TNF-α. Mitochondrial dysfunction is also thought to lead to free radical production and oxidative stress, which may provide the “second hit” which allows progression from steatosis to steatohepatitis and cirrhosis.14,70 Moreover, leptin levels have been demonstrated to be increased in patients with NASH,71 pointing to a possible role for increased angiogenesis and vascular invasiveness in HCC in the setting of metabolic syndrome.
Figure 4.
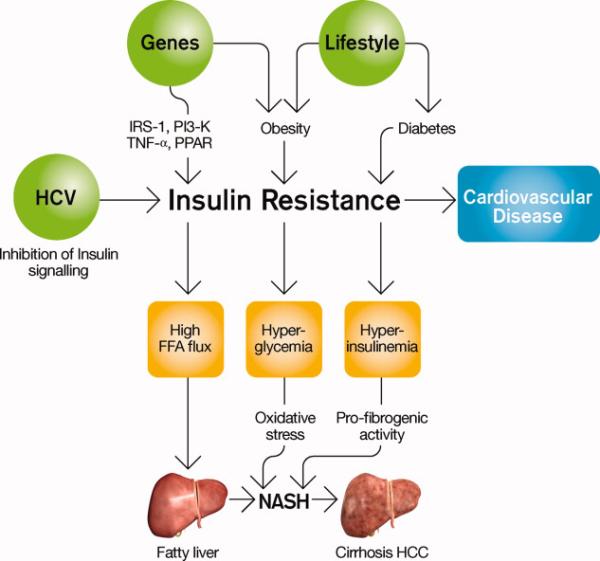
NAFLD pathogenesis 100
With respect to obesity as a risk factor for HCC, the exact relationship between HCC and risk is still being defined. Nair and colleagues used the United Network of Organ Sharing, a database on all liver transplantations performed in the United States, and showed that obesity was an independent predictor of HCC in patients with alcoholic cirrhosis, and cryptogenic cirrhosis, but not for those with cirrhosis of other etiologies. 72A meta-analysis of 11 cohort studies conducted in Europe, the United States, and Asia showed that those who were overweight had a significantly increased relative risk of developing HCC (1.07 (95% CI=1.01-1.15), while for those who were obese, the relative risk was even higher at 1.85 (95% CI=1.44-2.37). This analysis excluded studies which included cirrhotics in order to avoid confounding by ascites.73
While earlier studies did not show a clear relationship between diabetes and HCC risk, more recent epidemiologic data suggest that diabetes is likely associated with a 2-4-fold increased risk of HCC. 74-76 It is not known whether insulin resistance causes NASH. Patients with cirrhosis of all types may become insulin resistant within the liver because insulin is not cleared properly.77,78 However, peripheral insulin resistance is thought to be “primary,” leading to hepatic steatosis, which can then contribute to both peripheral and hepatic insulin resistance.79 Steatosis, or fatty liver, can also be seen with hepatitis C infection, and those with both hepatitis C and fatty liver changes have a greater risk of HCC than those with hepatitis C alone.80 Thus, mechanisms of carcinogenesis may relate at least partially to the intermediate steps of fatty change in the liver and insulin resistance rather than the “final outcome” of cirrhosis. Data are available which suggest that diabetes precedes liver disease and subsequently increases HCC risk. 76 Using data from a VA population, and excluding those with chronic liver disease at baseline. Dr. El-Serag showed that the incidence of HCC was doubled among patients with diabetes, and was higher among those with longer follow up. Similar findings were recently reported in a Japanese cohort study.76, 81 These studies strongly suggest that the insulin resistance precedes the cirrhosis and HCC.
We performed a preliminary retrospective analysis of patients undergoing surgery for HCC, and found a dose-response relationship between increasing BMI and the percentage of patients having microvascular invasion in their specimens, 82 supporting the idea that the obese milieu may contribute to angiogenesis. As noted above, those with other etiologies of fatty liver disease may also be at higher risk of cancer: a study of 99 patients undergoing liver transplantation with underlying hepatitis C showed that those with steatosis had a higher risk of having underlying HCC.80
Given the likely relationships between metabolic syndrome and HCC risk, several strategies have been attempted in the management of NASH. The most straightforward are interventions which lead to weight loss. Bariatric surgery has shown clear improvements in liver histology.83 In children, a two-year lifestyle intervention including diet and 45 minutes per day of aerobic exercise improved metabolic parameters and liver histology.84
One pharmacologic intervention which has received attention as a potential treatment for NASH is pioglitazone, a thiazolidinedione which improves insulin resistance and has anti-inflammatory effects in patients with type II diabetes. In a proof-of-concept study, 55 patients with impaired glucose tolerance or type 2 diabetes received either a hypocaloric diet plus pioglitazone (45 mg daily), or hypocaloric diet plus placebo. Patients assigned to the diet plus pioglitazone group showed improved glycemic control, but also showed reduced necroinflammation on pathology after 6 months of treatment.85 Unfortunately, these results seem to reverse quickly when the medication is stopped, and weight gain is a major side effect of the drug.86 Other insulin-sensitizing drugs, such as metformin, have been used, with improvements seen in metabolic parameters.87
Because oxidative stress is thought to mediate the progression of simple steatosis to steatohepatitis, antioxidants have also been tried in those with fatty liver disease. In the lifestyle intervention in children described above, the addition of alpha-tocepherol (600 IU/day) and ascorbic acid (500 mg/day) did not improve results compared with placebo (all children received the exercise and lifestyle intervention).84 In adults, a randomized trial of 49 patients randomized to a combination of alpha-tocepherol and ascorbic acid or placebo led to improvements in fibrosis after 6 months in the treatment group, the placebo group unexpectedly did as well, making the interpretation of results unclear. 88,89A pilot study of pentoxifylline, a drug which inhibits TNF alpha, also led to improvements in biochemical markers, but the drug led to nausea in many subjects.90
Outcomes of those with metabolic syndrome and HCC are not well-studied. For instance, little is known about how those with underlying NASH respond to systemic treatments for their HCC. Leung and colleagues evaluated 149 patients treated with PIAF (cisplatin, interferon-alpha, doxorubicin and 5-Fluorouracil) and found that the presence of hepatitis C serology was an independent predictor of a worsened response rate. It is difficult to conclude that these results are definitive since only 4 patients were HCV positive. Further, since almost all the remaining patients had underlying HBV, the authors note that it is impossible to determine outcomes in those with other etiologies of liver disease. The presence of cirrhosis also led to worsened overall survival in patients treated with this regimen, similar to results seen in past trials with single agent doxorubicin, and data from the recently published Phase II trial of sorafenib in HCC. 92-94 Neither the SHARP trial nor the recently-published Asia-pacific trial of HCC using sorafenib comment on outcomes of those with non-viral disease.95, 96 In the SHARP trial, those with vascular invasion showed a suggestion of a better response to sorafenib, although this was not statistically significant. Thus, data showing a higher degree of vascular invasion in tumors of those with NASH may have implications for response to anti-angiogenic agents like sorafenib.
Outcomes in obese patients with HCC after surgery may also be worse. One Japanese study showed no effect on overall survival or disease recurrence for initial resection, but a significantly worsened overall survival and disease recurrence in obese patients undergoing repeat hepatectomy.97 Patients with hepatic steatosis may be at increased risk for tumor recurrence after resection.98 Finally, diabetics also may have an increased risk of HCC recurrence. Patients who underwent resection for HCC with hepatitis C and were diabetic had significantly worsened survivals compared with those who were not diabetic. On multiple regression analysis, receiving insulin was an independent predictor of recurrence, underscoring the possibility that insulin may have carcinogenic properties in vivo. 99
Summary and future directions
Obesity and the metabolic syndrome are growing epidemics in the United States and worldwide. These diseases are associated with both increased risk for, and worsened outcomes of many types of cancer. In the liver, inflammatory and angiogenic changes due to underlying insulin resistance and fatty liver disease will likely lead to increased numbers of patients with HCC in the near future. Much work needs to be done to define more clearly the risks for development of HCC in those with underlying metabolic syndrome, the best methods of screening those at risk, and ultimately, the best treatments targeting the underlying mechanisms of pathogenesis.
Table 1.
Selected therapeutic strategies for non-alcoholic steatohepatitis
| Treatment | Strategy | Selected references: |
|---|---|---|
| Lifestyle intervention (diet +/− exercise) | Weight loss | Huang et al, 101, Nobili et al 84 |
| Bariatric surgery | Weight loss | Klein et al, 102 Mathurin et al 103 |
| Tetrahydrolipstatin (Orlistat) | Weight loss | Harrison et al, 104 Assy et al 105 |
| HMG-CoA reductase inhibitor (Atorvostatin) | Lipid lowering | Hyogo et al, 106 |
| Thiazolidinedione (Pioglitazone) | Insulin sensitizer | Belfort et al, 85 Aithal et al 107 |
| Biguanide (Metformin) | Insulin sensitizer | Bugianesi et al, 100 Marchesini et al 108 |
| ATII inhibitor (Losartan) | Antifibrotic | Yokohama, 2004 109 |
| Antioxidant (Vitamin E) | ROS scavenger | Harrison et al, 89Yakaryilmaz et al, 2007 110 |
| Anti-inflammatory (Pentoxifyllin) | TNF-α blockade | Adams et al, 2004 |
Acknowledgments
Supported in part by a K12 award from the National Institutes of Health and the Steven J. Levinson Medical Research Foundation.
Footnotes
Neither Dr. Siegel nor Dr. Zhu has financial disclosures relevant to this manuscript.
References
- 1.Ries L, Melbert D, Krapcho M, Mariotto A, Miller B, Feuer E, et al. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute; Bethesda, MD: 2007. [Google Scholar]
- 2.Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29(3):664–9. doi: 10.1002/hep.510290347. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10051466. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lindor KD. Nonalcoholic fatty liver disease. Ann Epidemiol. 2007;17(11):863–9. doi: 10.1016/j.annepidem.2007.05.013. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17728149. [DOI] [PubMed] [Google Scholar]
- 4.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. doi: 10.1002/hep.20973. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16447287. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004;27(10):2444–9. doi: 10.2337/diacare.27.10.2444. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15451914. [DOI] [PubMed] [Google Scholar]
- 6.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90(10):1562–9. doi: 10.2105/ajph.90.10.1562. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11029989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98(5):960–7. doi: 10.1111/j.1572-0241.2003.07486.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12809815. [DOI] [PubMed] [Google Scholar]
- 8.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202–19. doi: 10.1053/jhep.2003.50193. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12717402. [DOI] [PubMed] [Google Scholar]
- 9.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15565570. [DOI] [PubMed] [Google Scholar]
- 10.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26(7):856–63. doi: 10.1111/j.1478-3231.2006.01311.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16911469. [DOI] [PubMed] [Google Scholar]
- 11.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15895401. [DOI] [PubMed] [Google Scholar]
- 12.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9. doi: 10.1016/s0016-5085(99)70506-8. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10348825. [DOI] [PubMed] [Google Scholar]
- 13.Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35(6):1485–93. doi: 10.1053/jhep.2002.33324. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12029634. [DOI] [PubMed] [Google Scholar]
- 14.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31. doi: 10.1056/NEJMra011775. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11961152. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. doi: 10.1002/hep.20701. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15915461. [DOI] [PubMed] [Google Scholar]
- 16.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–906. doi: 10.1053/j.gastro.2005.03.084. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15940625. [DOI] [PubMed] [Google Scholar]
- 17.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80. doi: 10.1002/hep.1840110114. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2295475. [DOI] [PubMed] [Google Scholar]
- 18.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–50. doi: 10.1053/gast.2002.35354. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12198701. [DOI] [PubMed] [Google Scholar]
- 19.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am J Gastroenterol. 2002;97(6):1496–500. doi: 10.1111/j.1572-0241.2002.05795.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12094872. [DOI] [PubMed] [Google Scholar]
- 20.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32(4 Pt 1):689–92. doi: 10.1053/jhep.2000.17894. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11003611. [DOI] [PubMed] [Google Scholar]
- 21.Browning JD, Kumar KS, Saboorian MH, Thiele DL. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99(2):292–8. doi: 10.1111/j.1572-0241.2004.04059.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15046220. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167(18):1983–9. doi: 10.1001/archinte.167.18.1983. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17923599. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287(3):356–9. doi: 10.1001/jama.287.3.356. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11790215. [DOI] [PubMed] [Google Scholar]
- 24.Perry AC, Applegate EB, Jackson ML, Deprima S, Goldberg RB, Ross R, et al. Racial differences in visceral adipose tissue but not anthropometric markers of health-related variables. J Appl Physiol. 2000;89(2):636–43. doi: 10.1152/jappl.2000.89.2.636. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10926648. [DOI] [PubMed] [Google Scholar]
- 25.Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA. Ethnic differences in adiponectin levels. Metabolism. 2004;53(1):1–3. doi: 10.1016/j.metabol.2003.07.002. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14681833. [DOI] [PubMed] [Google Scholar]
- 26.Banerji MA, Norin AJ, Chaiken RL, Lebovitz HE. HLA-DQ associations distinguish insulin-resistant and insulin-sensitive variants of NIDDM in black Americans. Diabetes Care. 1993;16(2):429–33. doi: 10.2337/diacare.16.2.429. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8432213. [DOI] [PubMed] [Google Scholar]
- 27.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23. doi: 10.1053/jhep.2003.50161. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12668987. [DOI] [PubMed] [Google Scholar]
- 28.Cotrim HP, Parana R, Braga E, Lyra L. Nonalcoholic steatohepatitis and hepatocellular carcinoma: natural history? Am J Gastroenterol. 2000;95(10):3018–9. doi: 10.1111/j.1572-0241.2000.03241.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11051414. [DOI] [PubMed] [Google Scholar]
- 29.de Lima VM, Oliveira CP, Alves VA, Chammas MC, Oliveira EP, Stefano JT, et al. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol. 2008;49(6):1055–61. doi: 10.1016/j.jhep.2008.07.024. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18929425. [DOI] [PubMed] [Google Scholar]
- 30.Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36(5):1206–13. doi: 10.1053/jhep.2002.36780. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12395331. [DOI] [PubMed] [Google Scholar]
- 31.Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135(1):111–21. doi: 10.1053/j.gastro.2008.03.073. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18505690. [DOI] [PubMed] [Google Scholar]
- 32.Kang SK, Chung TW, Lee JY, Lee YC, Morton RE, Kim CH. The hepatitis B virus X protein inhibits secretion of apolipoprotein B by enhancing the expression of N-acetylglucosaminyltransferase III. J Biol Chem. 2004;279(27):28106–12. doi: 10.1074/jbc.M403176200. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15123606. [DOI] [PubMed] [Google Scholar]
- 33.Yu MW, Shih WL, Lin CL, Liu CJ, Jian JW, Tsai KS, et al. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J Clin Oncol. 2008;26(34):5576–82. doi: 10.1200/JCO.2008.16.1075. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18955457. [DOI] [PubMed] [Google Scholar]
- 34.George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, et al. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114(2):311–8. doi: 10.1016/s0016-5085(98)70482-2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9453491. [DOI] [PubMed] [Google Scholar]
- 35.Sorrentino P, D'Angelo S, Ferbo U, Micheli P, Bracigliano A, Vecchione R. Liver iron excess in patients with hepatocellular carcinoma developed on non-alcoholic steato-hepatitis. J Hepatol. 2008 doi: 10.1016/j.jhep.2008.09.011. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19070395. [DOI] [PubMed]
- 36.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16182882. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization The global epidemic of obesity. 1997.
- 38.US Department of Health and Human Services Dietary Guidelines for Americans. 2005.
- 39.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165(7):777–83. doi: 10.1001/archinte.165.7.777. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15824297. [DOI] [PubMed] [Google Scholar]
- 40.van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, et al. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology. 2008;48(2):449–57. doi: 10.1002/hep.22350. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18627003. [DOI] [PubMed] [Google Scholar]
- 41.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–7. doi: 10.1038/nature05488. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17167477. [DOI] [PubMed] [Google Scholar]
- 42.Baumgartner RN, Heymsfield SB, Roche AF. Human body composition and the epidemiology of chronic disease. Obes Res. 1995;3(1):73–95. doi: 10.1002/j.1550-8528.1995.tb00124.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7712363. [DOI] [PubMed] [Google Scholar]
- 43.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14726171. [DOI] [PubMed] [Google Scholar]
- 44.Samaha FF. New international measuring stick for defining obesity in non-Europeans. Circulation. 2007;115(16):2089–90. doi: 10.1161/CIRCULATIONAHA.107.696260. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17452616. [DOI] [PubMed] [Google Scholar]
- 45.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–102. doi: 10.1053/j.gastro.2007.03.052. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17498505. [DOI] [PubMed] [Google Scholar]
- 46.Katzmarzyk PT, Janssen I, Ardern CI. Physical inactivity, excess adiposity and premature mortality. Obes Rev. 2003;4(4):257–90. doi: 10.1046/j.1467-789x.2003.00120.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14649376. [DOI] [PubMed] [Google Scholar]
- 47.Parikh NI, Pencina MJ, Wang TJ, Lanier KJ, Fox CS, D'Agostino RB, et al. Increasing trends in incidence of overweight and obesity over 5 decades. Am J Med. 2007;120(3):242–50. doi: 10.1016/j.amjmed.2006.06.004. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17349447. [DOI] [PubMed] [Google Scholar]
- 48.Engeland A, Bjorge T, Selmer RM, Tverdal A. Height and body mass index in relation to total mortality. Epidemiology. 2003;14(3):293–9. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12859029. [PubMed] [Google Scholar]
- 49.Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–78. doi: 10.1056/NEJMoa055643. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16926275. [DOI] [PubMed] [Google Scholar]
- 50.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. N Engl J Med. 1995;333(11):677–85. doi: 10.1056/NEJM199509143331101. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7637744. [DOI] [PubMed] [Google Scholar]
- 51.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565–74. doi: 10.1016/s1470-2045(02)00849-5. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12217794. [DOI] [PubMed] [Google Scholar]
- 52.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12711737. [DOI] [PubMed] [Google Scholar]
- 53.Song YM, Sung J, Ha M. Obesity and risk of cancer in postmenopausal Korean women. J Clin Oncol. 2008;26(20):3395–402. doi: 10.1200/JCO.2007.15.7867. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18612154. [DOI] [PubMed] [Google Scholar]
- 54.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–78. doi: 10.1016/S0140-6736(08)60269-X. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18280327. [DOI] [PubMed] [Google Scholar]
- 55.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Bmj. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17986716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, et al. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–6. doi: 10.1126/science.279.5350.563. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9438850. [DOI] [PubMed] [Google Scholar]
- 57.Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351(9113):1393–6. doi: 10.1016/S0140-6736(97)10384-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9593409. [DOI] [PubMed] [Google Scholar]
- 58.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91(7):620–5. doi: 10.1093/jnci/91.7.620. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10203281. [DOI] [PubMed] [Google Scholar]
- 59.Hsing AW, Chua S, Jr., Gao YT, Gentzschein E, Chang L, Deng J, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001;93(10):783–9. doi: 10.1093/jnci/93.10.783. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11353789. [DOI] [PubMed] [Google Scholar]
- 60.Nathan C. Epidemic inflammation: pondering obesity. Mol Med. 2008;14(7-8):485–92. doi: 10.2119/2008-00038.Nathan. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18431463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11229684. [DOI] [PubMed] [Google Scholar]
- 62.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24(31):5017–24. doi: 10.1200/JCO.2006.07.0243. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17075121. [DOI] [PubMed] [Google Scholar]
- 63.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med. 2005;165(11):1267–73. doi: 10.1001/archinte.165.11.1267. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15956006. [DOI] [PubMed] [Google Scholar]
- 64.Abrahamson PE, Gammon MD, Lund MJ, Flagg EW, Porter PL, Stevens J, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1871–7. doi: 10.1158/1055-9965.EPI-06-0356. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17035393. [DOI] [PubMed] [Google Scholar]
- 65.Chagpar AB, McMasters KM, Saul J, Nurko J, Martin RC, 2nd, Scoggins CR, et al. Body mass index influences palpability but not stage of breast cancer at diagnosis. Am Surg. 2007;73(6):555–60. discussion 60. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17658091. [PubMed] [Google Scholar]
- 66.Rega G, Kaun C, Demyanets S, Pfaffenberger S, Rychli K, Hohensinner PJ, et al. Vascular Endothelial Growth Factor Is Induced by the Inflammatory Cytokines Interleukin-6 and Oncostatin M in Human Adipose Tissue In Vitro and in Murine Adipose Tissue In Vivo. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.143081. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17525365. [DOI] [PubMed]
- 67.Horiguchi A, Sumitomo M, Asakuma J, Asano T, Zheng R, Asano T, et al. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol. 2006;176(4 Pt 1):1631–5. doi: 10.1016/j.juro.2006.06.039. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16952705. [DOI] [PubMed] [Google Scholar]
- 68.Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, et al. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122(5):1399–410. doi: 10.1053/gast.2002.32995. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11984526. [DOI] [PubMed] [Google Scholar]
- 69.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67(6):2497–507. doi: 10.1158/0008-5472.CAN-06-3075. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17363567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11(2):119–32. doi: 10.1016/j.ccr.2006.12.016. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17292824. [DOI] [PubMed] [Google Scholar]
- 71.Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, et al. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36(2):403–9. doi: 10.1053/jhep.2002.34738. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12143049. [DOI] [PubMed] [Google Scholar]
- 72.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36(1):150–5. doi: 10.1053/jhep.2002.33713. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12085359. [DOI] [PubMed] [Google Scholar]
- 73.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97(7):1005–8. doi: 10.1038/sj.bjc.6603932. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17700568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu SN, Lin TM, Chen CJ, Chen JS, Liaw YF, Chang WY, et al. A case-control study of primary hepatocellular carcinoma in Taiwan. Cancer. 1988;62(9):2051–5. doi: 10.1002/1097-0142(19881101)62:9<2051::aid-cncr2820620930>3.0.co;2-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2844388. [DOI] [PubMed] [Google Scholar]
- 75.Adami HO, Chow WH, Nyren O, Berne C, Linet MS, Ekbom A, et al. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88(20):1472–7. doi: 10.1093/jnci/88.20.1472. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8841022. [DOI] [PubMed] [Google Scholar]
- 76.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126(2):460–8. doi: 10.1053/j.gastro.2003.10.065. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14762783. [DOI] [PubMed] [Google Scholar]
- 77.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21(6):1135–9. doi: 10.1016/s0168-8278(05)80631-2. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7699240. [DOI] [PubMed] [Google Scholar]
- 78.Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, et al. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77(5):703–10. doi: 10.1097/01.tp.0000114283.04840.3a. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15021833. [DOI] [PubMed] [Google Scholar]
- 79.Smedile A, Bugianesi E. Steatosis and hepatocellular carcinoma risk. Eur Rev Med Pharmacol Sci. 2005;9(5):291–3. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16231592. [PubMed] [Google Scholar]
- 80.Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007 doi: 10.1002/cncr.22701. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17487861. [DOI] [PubMed]
- 81.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. Jama. 2005;293(2):194–202. doi: 10.1001/jama.293.2.194. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15644546. [DOI] [PubMed] [Google Scholar]
- 82.Siegel AB, Wang S, Jacobson JS, Yu J, Lim E, Ferrante L, et al. Obesity and increased microvascular invasion in hepatocellular carcinoma. Proc ASCO. 2008 doi: 10.3109/07357907.2010.483500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Furuya CK, Jr., de Oliveira CP, de Mello ES, Faintuch J, Raskovski A, Matsuda M, et al. Effects of bariatric surgery on nonalcoholic fatty liver disease: preliminary findings after 2 years. J Gastroenterol Hepatol. 2007;22(4):510–4. doi: 10.1111/j.1440-1746.2007.04833.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17376042. [DOI] [PubMed] [Google Scholar]
- 84.Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48(1):119–28. doi: 10.1002/hep.22336. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18537181. [DOI] [PubMed] [Google Scholar]
- 85.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355(22):2297–307. doi: 10.1056/NEJMoa060326. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17135584. [DOI] [PubMed] [Google Scholar]
- 86.Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, et al. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46(2):424–9. doi: 10.1002/hep.21661. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17559148. [DOI] [PubMed] [Google Scholar]
- 87.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100(5):1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15842582. [DOI] [PubMed] [Google Scholar]
- 88.Adams LA, Angulo P. Vitamins E and C for the treatment of NASH: duplication of results but lack of demonstration of efficacy. Am J Gastroenterol. 2003;98(11):2348–50. doi: 10.1111/j.1572-0241.2003.08695.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14638333. [DOI] [PubMed] [Google Scholar]
- 89.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98(11):2485–90. doi: 10.1111/j.1572-0241.2003.08699.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14638353. [DOI] [PubMed] [Google Scholar]
- 90.Adams LA, Zein CO, Angulo P, Lindor KD. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99(12):2365–8. doi: 10.1111/j.1572-0241.2004.40064.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15571584. [DOI] [PubMed] [Google Scholar]
- 91.El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins Are Associated With a Reduced Risk of Hepatocellular Carcinoma in a Large Cohort of Patients With Diabetes. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.01.053. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19208359. [DOI] [PMC free article] [PubMed]
- 92.Leung TW, Tang AM, Zee B, Yu SC, Lai PB, Lau WY, et al. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94(2):421–7. doi: 10.1002/cncr.10236. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11905412. [DOI] [PubMed] [Google Scholar]
- 93.Johnson PJ, Alexopoulos A, Johnson RD, Williams R. Significance of serum bilirubin level in response of hepatocellular carcinoma to doxorubicin. J Hepatol. 1986;3(2):149–53. doi: 10.1016/s0168-8278(86)80020-4. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3025287. [DOI] [PubMed] [Google Scholar]
- 94.Abou-Alfa GK, Amadori D, Santoro A, Figer A, Greve JD, Lathia C, et al. Is sorafenib (S) safe and effective in patients (pts) with hepatocellular carcinoma (HCC) and Child-Pugh B (CPB) cirrhosis? ASCO. 2008 abstr 4518. [Google Scholar]
- 95.Llovet J, Ricci S, Mazzaferro V, Hilgard P, Raoul J, Zeuzem S, et al. Sorafenib improves survival in advanced Hepatocellular Carcinoma (HCC): Results of a Phase III randomized placebo-controlled trial (SHARP trial). Proc ASCO. 2007;25(18S) (June 20 Supplement), 2007. [Google Scholar]
- 96.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2008 doi: 10.1016/S1470-2045(08)70285-7. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19095497. [DOI] [PubMed]
- 97.Utsunomiya T, Okamoto M, Kameyama T, Matsuyama A, Yamamoto M, Fujiwara M, et al. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14(10):1553–8. doi: 10.3748/wjg.14.1553. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18330947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takuma Y, Nouso K, Makino Y, Saito S, Takayama H, Takahara M, et al. Hepatic steatosis correlates with the postoperative recurrence of hepatitis C virus-associated hepatocellular carcinoma. Liver Int. 2007;27(5):620–6. doi: 10.1111/j.1478-3231.2007.01462.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17498246. [DOI] [PubMed] [Google Scholar]
- 99.Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102(9):1939–46. doi: 10.1111/j.1572-0241.2007.01354.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17573788. [DOI] [PubMed] [Google Scholar]
- 100.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42(5):987–1000. doi: 10.1002/hep.20920. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16250043. [DOI] [PubMed] [Google Scholar]
- 101.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100(5):1072–81. doi: 10.1111/j.1572-0241.2005.41334.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15842581. [DOI] [PubMed] [Google Scholar]
- 102.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130(6):1564–72. doi: 10.1053/j.gastro.2006.01.042. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16697719. [DOI] [PubMed] [Google Scholar]
- 103.Mathurin P, Gonzalez F, Kerdraon O, Leteurtre E, Arnalsteen L, Hollebecque A, et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130(6):1617–24. doi: 10.1053/j.gastro.2006.02.024. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&dbPubMed&dopt=Citation&list_uids=16697725. [DOI] [PubMed] [Google Scholar]
- 104.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49(1):80–6. doi: 10.1002/hep.22575. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19053049. [DOI] [PubMed] [Google Scholar]
- 105.Assy N, Hussein O, Abassi Z. Weight loss induced by orlistat reverses fatty infiltration and improves hepatic fibrosis in obese patients with non-alcoholic steatohepatitis. Gut. 2007;56(3):443–4. doi: 10.1136/gut.2006.106021. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17339254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hyogo H, Tazuma S, Arihiro K, Iwamoto K, Nabeshima Y, Inoue M, et al. Efficacy of atorvastatin for the treatment of nonalcoholic steatohepatitis with dyslipidemia. Metabolism. 2008;57(12):1711–8. doi: 10.1016/j.metabol.2008.07.030. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19013295. [DOI] [PubMed] [Google Scholar]
- 107.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135(4):1176–84. doi: 10.1053/j.gastro.2008.06.047. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18718471. [DOI] [PubMed] [Google Scholar]
- 108.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358(9285):893–4. doi: 10.1016/s0140-6736(01)06042-1. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11567710. [DOI] [PubMed] [Google Scholar]
- 109.Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40(5):1222–5. doi: 10.1002/hep.20420. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15382153. [DOI] [PubMed] [Google Scholar]
- 110.Yakaryilmaz F, Guliter S, Savas B, Erdem O, Ersoy R, Erden E, et al. Effects of vitamin E treatment on peroxisome proliferator-activated receptor-alpha expression and insulin resistance in patients with non-alcoholic steatohepatitis: results of a pilot study. Intern Med J. 2007;37(4):229–35. doi: 10.1111/j.1445-5994.2006.01295.x. Available from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17388862. [DOI] [PubMed] [Google Scholar]


