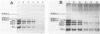Abstract
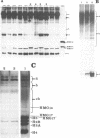
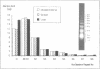
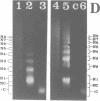
We report the preparation of HMG17-containing oligonucleosomes from chicken embryos and from liver and oviduct of laying hens. Monoclonal antibodies against HMG17 were used for their isolation. An unusual size distribution with respect to their repeat number was observed. The oligonucleosomes of repeat number up to N6 were highly enriched for DNA of the vitellogenin II gene (liver) and for DNA of the ovalbumin and lysozyme genes (oviduct).
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellard M., Dretzen G., Bellard F., Oudet P., Chambon P. Disruption of the typical chromatin structure in a 2500 base-pair region at the 5' end of the actively transcribed ovalbumin gene. EMBO J. 1982;1(2):223–230. doi: 10.1002/j.1460-2075.1982.tb01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Cartwright I. L., Abmayr S. M., Fleischmann G., Lowenhaupt K., Elgin S. C., Keene M. A., Howard G. C. Chromatin structure and gene activity: the role of nonhistone chromosomal proteins. CRC Crit Rev Biochem. 1982;13(1):1–86. doi: 10.3109/10409238209108709. [DOI] [PubMed] [Google Scholar]
- Geiser M., Mattaj I. W., Wilks A. F., Seldran M., Jost J. P. Structure and sequence of the promoter area and of a 5' upstream demethylation site of the estrogen-regulated chicken vitellogenin ii gene. J Biol Chem. 1983 Jul 25;258(14):9024–9030. [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Cockerill P. N., Zavou S., Wright C. A. The effect of salt extraction on the structure of transcriptionally active genes; evidence for a DNAseI-sensitive structure which could be dependent on chromatin structure at levels higher than the 30 nm fibre. Nucleic Acids Res. 1985 May 24;13(10):3561–3579. doi: 10.1093/nar/13.10.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie S., Sezaki M., Kato Y. A faithful double stain of proteins in the polyacrylamide gels with Coomassie blue and silver. Anal Biochem. 1982 Nov 1;126(2):350–354. doi: 10.1016/0003-2697(82)90526-7. [DOI] [PubMed] [Google Scholar]
- Karpov V. L., Preobrazhenskaya O. V., Mirzabekov A. D. Chromatin structure of hsp 70 genes, activated by heat shock: selective removal of histones from the coding region and their absence from the 5' region. Cell. 1984 Feb;36(2):423–431. doi: 10.1016/0092-8674(84)90235-6. [DOI] [PubMed] [Google Scholar]
- Malik N., Smulson M., Bustin M. Enrichment of acetylated histones in polynucleosomes containing high mobility group protein 17 revealed by immunoaffinity chromatography. J Biol Chem. 1984 Jan 25;259(2):699–702. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandeen G., Wood W. I., Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980 Sep 11;8(17):3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shick V. V., Belyavsky A. V., Mirzabekov A. D. Primary organization of nucleosomes. Interaction of non-histone high mobility group proteins 14 and 17 with nucleosomes, as revealed by DNA-protein crosslinking and immunoaffinity isolation. J Mol Biol. 1985 Sep 20;185(2):329–339. doi: 10.1016/0022-2836(85)90407-3. [DOI] [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of actively transcribed nucleosomes using immobilized HMG 14 and 17 and an analysis of alpha-globin chromatin. Cell. 1981 Feb;23(2):391–400. doi: 10.1016/0092-8674(81)90134-3. [DOI] [PubMed] [Google Scholar]
- Wittig B., Wittig S. Nucleosome mono, di, tri-, and tetramers from chicken embryo chromatin. Nucleic Acids Res. 1977 Nov;4(11):3901–3917. doi: 10.1093/nar/4.11.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig S., Wittig B. Function of a tRNA gene promoter depends on nucleosome position. Nature. 1982 May 6;297(5861):31–38. doi: 10.1038/297031a0. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Yau P., Imai B. S., Thorne A. W., Goodwin G. H., Bradbury E. M. Effect of HMG protein 17 on the thermal stability of control and acetylated HeLa oligonucleosomes. Nucleic Acids Res. 1983 May 11;11(9):2651–2664. doi: 10.1093/nar/11.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]