Abstract
Purpose
Gemcitabine plus cisplatin is active in malignant mesothelioma (MM), although single-arm phase II trials have reported variable outcomes. Vascular endothelial growth factor (VEGF) inhibitors have activity against MM in preclinical models. We added the anti-VEGF antibody bevacizumab to gemcitabine/cisplatin in a multicenter, double-blind, placebo-controlled randomized phase II trial in patients with previously untreated, unresectable MM.
Patients and Methods
Eligible patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1 and no thrombosis, bleeding, or major blood vessel invasion. The primary end point was progression-free survival (PFS). Patients were stratified by ECOG performance status (0 v 1) and histologic subtype (epithelial v other). Patients received gemcitabine 1,250 mg/m2 on days 1 and 8 every 21 days, cisplatin 75 mg/m2 every 21 days, and bevacizumab 15 mg/kg or placebo every 21 days for six cycles, and then bevacizumab or placebo every 21 days until progression.
Results
One hundred fifteen patients were enrolled at 11 sites; 108 patients were evaluable. Median PFS time was 6.9 months for the bevacizumab arm and 6.0 months for the placebo arm (P = .88). Median overall survival (OS) times were 15.6 and 14.7 months in the bevacizumab and placebo arms, respectively (P = .91). Partial response rates were similar (24.5% for bevacizumab v 21.8% for placebo; P = .74). A higher pretreatment plasma VEGF concentration (n = 56) was associated with shorter PFS (P = .02) and OS (P = .0066), independent of treatment arm. There were no statistically significant differences in toxicity of grade 3 or greater.
Conclusion
The addition of bevacizumab to gemcitabine/cisplatin in this trial did not significantly improve PFS or OS in patients with advanced MM.
INTRODUCTION
Malignant mesothelioma (MM) is an uncommon malignancy, affecting about 2,500 Americans annually.1 Pemetrexed plus cisplatin, the current benchmark chemotherapy regimen, yields a median overall survival (OS) time of 12.1 months and a median time to progression of 5.7 months.2 Before US Food and Drug Administration approval of this combination in 2003, gemcitabine plus cisplatin was widely used.3 Retrospective data suggest similar activity for gemcitabine and pemetrexed platinum doublets in MM.4
In 1999, an Australian trial of gemcitabine/cisplatin in MM reported a 48% response rate and a median OS of 9.5 months.5 Variable activity has been observed in subsequent phase II studies of this combination (response rate, 12% to 33%; median OS, 9.6 to 12 months; median progression-free survival [PFS], 4 to 8 months).6–10 These disparate results are likely a result of small sample sizes, heterogeneity in patient prognostic factors, and variations in methods of response assessment.1
Vascular endothelial growth factor (VEGF) signaling plays a key role in MM biology.11,12 In preclinical models, VEGF increases MM proliferation; antibodies against VEGF and its receptors inhibit MM growth.13 Patients with MM have significantly higher serum VEGF levels than patients with other cancers.14 Several VEGF inhibitors, including cediranib, sorafenib, sunitinib, SU5416, thalidomide, and vatalanib, have modest single-agent activity in patients with MM.15–20 Bevacizumab (Avastin; Genentech, South San Francisco, CA) is a recombinant humanized monoclonal antibody against VEGF-A.21 This article reports the results of a National Cancer Institute (NCI) –sponsored, multicenter randomized phase II trial evaluating the addition of bevacizumab to gemcitabine/cisplatin in patients with MM.
PATIENTS AND METHODS
Patients
Eligible patients had histologically or cytologically confirmed MM not amenable to curative intent surgery. An opinion from an MM-experienced surgeon was required for potentially resectable patients. International Mesothelioma Interest Group stage II or greater was required for patients with pleural MM. No prior systemic cytotoxic chemotherapy was permitted; prior intrapleural cytotoxic agents were allowed. Measurable disease; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1; age greater than 18 years; life expectancy of more than 3 months; and adequate bone marrow (leukocytes ≥ 3,000/μL, granulocytes ≥ 1,500/μL, and platelets ≥ 100,000/μL), renal (creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 60 mL/min and urine protein < 1+ or < 1,500 mg/dL per 24 hours), hepatic (total bilirubin within normal institutional limits and AST and ALT ≤ 2.5× upper limit of normal), and coagulation (prothrombin time international normalized ratio ≤ 1.5) function were required. Prior radiation was allowed if completed more than 4 weeks prior and there was measurable disease outside the radiation port. Patients were excluded for a nonhealing wound; major surgery within 6 weeks; bleeding diathesis; pulmonary embolus; deep venous thrombosis; clinically significant cardiac, peripheral vascular, or CNS disease; currently active second malignancy; uncontrolled intercurrent illness; or computed tomography (CT) scan documentation of invasion of adjacent organs.
This protocol (ClinicalTrials.gov identifier: NCT00027703) was reviewed by the institutional review board of each participating center. All patients provided written informed consent according to federal and institutional guidelines.
Treatment
Gemcitabine 1,250 mg/m2 was given intravenously on days 1 and 8 of a 21-day cycle, followed by cisplatin 75 mg/m2 intravenously on day 1, for six cycles. Bevacizumab 15 mg/kg or placebo was administered intravenously after cisplatin on day 1 of each cycle. Bevacizumab or placebo was continued every 21 days until disease progression, unacceptable adverse events, or patient withdrawal of consent.
Dose Adjustments
Adverse effects were graded according to NCI Common Terminology Criteria for Adverse Events (version 3.0). A cycle was not started until the absolute neutrophil count (ANC) was more than 1.5 × 109/L and the platelet count was more than 100 × 109/L. Neutropenic fever requiring antibiotics or bleeding associated with thrombocytopenia required a 25% dose reduction of cisplatin and gemcitabine in subsequent cycles.
On day 8 of a cycle, 75% of the full dose of gemcitabine was given for an ANC between 0.5 and 0.99 × 109/L or platelet count between 50 and 74 × 109/L; gemcitabine was held for an ANC less than 0.5 × 109/L or platelet count less than 50 × 109/L. For grade 3 nonhematologic toxicities other than nausea/vomiting, patients received either 75% of the gemcitabine dose or no treatment at the treating physician's discretion. Gemcitabine was held for grade 4 nonhematologic toxicities.
Cisplatin was reduced by 25% for serum creatinine of 1.6 to 2.0 mg/dL and held for creatinine ≥ 2.0 mg/dL. Cisplatin was reduced by 25% for grade 3 nonhematologic toxicities other than nausea/vomiting and held for grade 4 nonhematologic toxicities.
There were no dose modifications of bevacizumab or placebo. At the beginning of a cycle, if both gemcitabine and cisplatin were held, bevacizumab or placebo was held until chemotherapy could be given. If bevacizumab or placebo was held for toxicity, gemcitabine and cisplatin could be administered. Bevacizumab or placebo was held for bilirubin or hepatic transaminase elevations ≥ grade 3 and not resumed until ≤ grade 1. Bevacizumab or placebo was held for grade 2 proteinuria or grade 3 hemorrhage or thrombosis. Bevacizumab or placebo was held for persistent or symptomatic hypertension and was discontinued if not controlled within 6 weeks by oral medication or if grade 4 hypertension developed. Bevacizumab or placebo was discontinued for arterial thrombotic events or for any grade 4 toxicity attributable to bevacizumab.
Study Evaluations
Pretreatment evaluation included a medical history and physical examination, CBC count and differential, chemistry panel, prothrombin time/partial thromboplastin time, urinalysis, and CT scan. Pretreatment plasma VEGF concentrations were determined using a quantitative sandwich enzyme immunoassay (Human VEGF Immunoassay; R&D Systems, Minneapolis, MN).
A history and physical examination were performed every 21 days. CBC and chemistry panel were performed weekly. Urinalysis was performed every 21 days. CT scans were obtained every two cycles.
Patients were evaluated for response every two cycles according to RECIST.22 Confirmatory scans were obtained at least 4 weeks after initial documentation of response. Scans were not centrally reviewed.
Statistics
This was a double-blind, randomized, placebo-controlled, phase II trial to compare the safety and efficacy of treatment with gemcitabine, cisplatin, and bevacizumab (GCB) versus gemcitabine, cisplatin, and placebo (GCP). Random assignment was stratified by histology (epithelial v sarcomatoid, mixed, or other subtypes) and ECOG PS (0 v 1). Treatment assignment sequences were produced by the study statistician using the random number generator in SAS (SAS Institute, Cary, NC). The method of permuted blocks was used, with blocks of size 6, within each stratum. An e-mail containing the patient identification number and stratum information was sent to the study statistician when a patient required random assignment, and patients were assigned to the next treatment on the list. This information was transmitted electronically to the NCI, which shipped blinded drug to the site.
The primary end point was PFS (ie, the time from random assignment to disease progression or death from any cause). A sample size of 106 patients (53 per arm) provided 90% power to detect a hazard ratio (HR) of 1.75, using a one-sided α = .10. Kaplan-Meier23 curves were generated for PFS and OS. Median survival times were estimated for each arm, and 95% CIs were derived.24 PFS and OS were compared between treatment groups using both an unadjusted and a stratified (by histology and ECOG PS) log-rank test. Cox25 proportional hazards regression models were fit to assess the effects of treatment, histology, PS, and other potential prognostic factors, including baseline VEGF levels, on PFS and OS. Interactions between treatment and stratification factors, as well as treatment and VEGF, were explored using the Cox model. Response rates and toxicities were compared between groups using χ2 or Fisher's exact tests. All P values reported are two sided, and P ≤ .05 is regarded as statistically significant, except for the primary PFS end point, which was evaluated using a one-sided test and a P = .10 threshold for significance.
Because bleeding, thrombosis, and proteinuria were potential concerns with bevacizumab, a sequential stopping guideline26 was used in the GCB arm, and interim, pairwise comparisons of toxicity rates between treatment groups were performed to monitor these selected adverse events. A composite adverse event was defined as the occurrence of any bleeding, thrombosis, or proteinuria ≥ grade 3 or grade 4 hypertension. Early stopping would be considered if there was evidence that the true rate of toxicity for this composite event in the bevacizumab group exceeded 10%. The results of these interim analyses were presented to the University of Chicago High Risk Protocol Committee.
RESULTS
Patient Characteristics
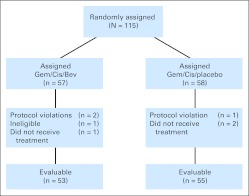
Between November 2001 and July 2005, 115 patients at 11 centers were randomly assigned (57 patients to GCB and 58 patients to GCP). Seven patients (four on GCB and three on GCP) were not evaluable. Three patients withdrew before receiving any treatment. One patient had a GI stromal tumor, not MM, and after being deemed ineligible was no longer observed. Three patients (two on GCB and one on GCP) at two different centers mistakenly received a dose of open-label bevacizumab instead of blinded study medication. Per communication with the NCI, these patients were withdrawn from the protocol and continued on open-label treatment under a special exception protocol. The analyses that follow are based on the 108 remaining evaluable patients (GCB, n = 53; GCP, n = 55; Fig 1).
Fig 1.
CONSORT diagram. Bev, bevacizumab; Cis, cisplatin; Gem, gemcitabine.
Patient characteristics are listed in Table 1. As expected in this occupationally related cancer, most patients were men (74% and 84% for GCB and GCP, respectively). Approximately half had a PS of 0. More than 90% had disease of pleural origin, and more than two thirds had epithelial histology. Leukocytosis and thrombocytosis, which are well-recognized poor prognostic factors in MM,27,28 were similar in both arms.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | Gemcitabine, Cisplatin, Bevacizumab(n = 53) |
Gemcitabine, Cisplatin, and Placebo(n = 55) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Male | 39 | 73.6 | 46 | 83.6 |
| Race | ||||
| White | 47 | 88.7 | 50 | 90 |
| Hispanic | 4 | 7.5 | 2 | 3.6 |
| African American | 1 | 1.9 | 2 | 3.6 |
| Asian | 1 | 1.9 | 1 | 1.8 |
| Age, years | ||||
| Median | 62 | 65 | ||
| Range | 44-78 | 20-84 | ||
| Mean | 62.4 | 62.6 | ||
| SD | 9.0 | 12.1 | ||
| WBC, × 109/L | ||||
| > 8.3, × 109/L | 28 | 52.8 | 29 | 47.3 |
| Median | 8.5 | 8.1 | ||
| Range | 3.5-24.5 | 4.4-17.5 | ||
| Mean | 9.0 | 8.7 | ||
| SD | 3.6 | 2.7 | ||
| Platelets, × 109/L | ||||
| > 400 × 109/L | 21 | 39.6 | 22 | 40 |
| Median | 353 | 347 | ||
| Range | 133-677 | 133-1,061 | ||
| Mean | 385.1 | 385.1 | ||
| SD | 139.0 | 158.3 | ||
| Pretreatment plasma VEGF, pg/mL | ||||
| No. of patients | 28 | 28 | ||
| Median | 131 | 154 | ||
| Range | 31-1,760 | 5-1,786 | ||
| Mean | 235 | 268 | ||
| SD | 238 | 349 | ||
| Primary site of disease | ||||
| Pleural | 49 | 92.5 | 50 | 90.9 |
| Peritoneal | 4 | 7.5 | 5 | 9.1 |
| Histology | ||||
| Epithelioid | 39 | 73.6 | 37 | 67.3 |
| Nonepithelioid | 14 | 26.4 | 18 | 32.7 |
| ECOG PS | ||||
| 0 | 24 | 45.3 | 29 | 52.7 |
| 1 | 29 | 54.7 | 26 | 47.3 |
| Stratum | ||||
| Epithelioid, PS = 0 | 18 | 34.0 | 21 | 38.2 |
| Epithelioid, PS = 1 | 21 | 39.6 | 16 | 29.1 |
| Nonepithelioid, PS = 0 | 6 | 11.3 | 8 | 14.6 |
| Nonepithelioid, PS = 1 | 8 | 15.1 | 10 | 18.2 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; SD, standard deviation; VEGF, vascular endothelial growth factor.
Although the random assignment was stratified by histology and PS, in 39 patients, the information provided at random assignment was incorrect. Twenty-six of the discrepancies in PS were a result of a systematic error in which a Karnofsky performance score of 90 was mapped into an ECOG PS of 1 rather than 0. The data in Table 1 show the correct classifications determined on audit and rereview. The consequence of these errors is that the guaranteed balance of the treatment arms with respect to these factors was lost; however, the validity of the random assignment is not affected.
Response and Survival
There were no complete responses. The partial response rate was similar in the GCB and GCP arms (24.5% v 21.8%, respectively; P = .74). Stable disease occurred in 51% and 60% of patients on GCB and GCP, respectively.
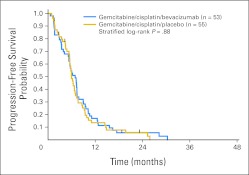
Kaplan-Meier curves for PFS and OS by treatment arm are shown in Figures 2 and 3, respectively. The estimated median PFS is 6.9 months (95% CI, 5.3 to 7.3 months) for GCB and 6.0 months (95% CI, 5.5 to 7.0 months) for GCP. The overall PFS curves are not significantly different (HR, 0.90; 95% CI, 0.61 to 1.34; log-rank P = .62 unadjusted; log-rank P = .88 stratified by PS and histology).
Fig 2.
Progression-free survival by treatment arm.
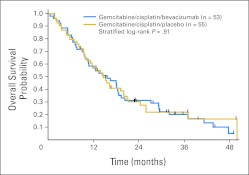
Fig 3.
Overall survival by treatment arm.
The estimated median OS time is 15.6 months (95% CI, 10.6 to 18.7 months) for GCB and 14.7 months (95% CI, 10.3 to 20.0 months) for GCP. The OS curves are not significantly different (HR, 1.04; 95% CI, 0.68 to 1.59; log-rank P = .87 unadjusted; log-rank P = .91 stratified by PS and histology). One-year OS rates for GCB and GCP are 58.5% and 57.0%, respectively; 2-year OS rates are 31.3% and 30.2%, respectively.
Cox regression analyses of PFS and OS were conducted to examine the effects of baseline variables. Treatment and stratification variables (histology and PS) were retained in all models. Candidate covariates included age, race, sex, log WBC count, platelet count, and site of origin (peritoneal v pleural). The only statistically significant covariate detected for both PFS and OS was platelet count. The HR for disease progression increased by a factor of 1.18 for every 100-unit increase in platelet count (P = .023). The death rate increased by a factor of 1.41 per 100-unit increase in platelet count (P < .001).
Adverse Events
Grade 3 and 4 adverse events are listed in Table 2. There were no statistically significant differences in the rates of grade 3 or greater toxicity between treatment groups. Venous thrombosis developed in 17% of patients on GCB and 9% on GCP (P = .26). Statistically significant differences in the rates of any grade of alopecia, epistaxis, hypertension, infection without neutropenia, proteinuria, and stomatitis were also observed. No unique toxicities were observed in this patient population.
Table 2.
Grade 3 and 4 Toxicities per Patient by Common Terminology Criteria for Adverse Events (version 3.0)
| Toxicity | % of Patients |
|
|---|---|---|
| Gemcitabine, Cisplatin,and Bevacizumab (n = 53) | Gemcitabine, Cisplatin,and Placebo (n = 55) | |
| Neutropenia | 42 | 40 |
| Anemia | 4 | 15 |
| Thrombocytopenia | 38 | 25 |
| Febrile neutropenia | 4 | 2 |
| Cerebrovascular accident | 2 | 0 |
| Epistaxis | 8 | 2 |
| Hypertension | 23 | 9 |
| Infection without neutropenia | 6 | 2 |
| Proteinuria | 6 | 2 |
| Venous thrombosis | 17 | 9 |
| Visceral perforation | 0 | 0 |
The monitoring boundary was not crossed during the study. All charts were rereviewed after closure of accrual, and three additional composite adverse events were uncovered for GCB; on the basis of this rereview, the boundary was crossed after eight events occurred in 36 patients. If this information had been available while the trial was accruing, according to the prespecified guidelines, we might have considered closing the study once this boundary was crossed. The composite event occurred in 26.4% of patients on GCB, nearly twice the rate in the control arm (14.6%); this difference does not reach statistical significance (P = .15). The rates of bleeding, thrombosis, and proteinuria were also higher in the GCB arm, but the differences were not statistically significant (Table 3).
Table 3.
Monitored Toxicities
| Toxicity | Gemcitabine, Cisplatin,and Bevacizumab(n = 53) |
Gemcitabine, Cisplatin, and Placebo(n = 55) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| ≥ Grade 3 bleeding | 4 | 7.6 | 1 | 1.8 |
| ≥ Grade 3 thrombosis | 9 | 17 | 5 | 9.1 |
| ≥ Grade 3 proteinuria | 3 | 5.7 | 1 | 1.8 |
| Grade 4 hypertension | 0 | 0 | 1 | 1.8 |
| Any of the above | 14 | 26.4 | 8 | 14.6 |
The median number of cycles was seven (range, one to 42 cycles) for GCB and six (range, two to 39 cycles) for GCP. Ten GCB patients and eight GCP patients discontinued treatment because of toxicity, a nonsignificant difference (P = .61).
Plasma VEGF Concentrations
Baseline VEGF values were obtained in 56 patients; values from two replicate assays were averaged for each patient. The baseline characteristics of patients with missing VEGF data were not significantly different, nor were response rates, PFS, or OS. The median VEGF levels in the GCB and GCP arms (131 and 154 pg/mL, respectively) were not significantly different.
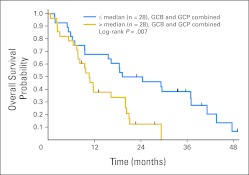
The mean log VEGF level was not significantly different between responders and nonresponders (mean ± SE, 3.58 ± 0.43 pg/mL v 4.80 ± 0.14 pg/mL, respectively; P = .27). Higher baseline log VEGF levels were significantly associated with worse PFS (P = .049 adjusted for treatment and stratification factors) and OS (P = .014 adjusted). For PFS, a two-fold increase in the baseline VEGF level was associated with a 1.30-fold increase in the hazard rate. For OS, the death rate increased by a factor of 1.37 for each doubling of the VEGF level. The association of poorer OS with higher VEGF is illustrated in Figure 4, which presents the OS curves for patients above and below the median VEGF level of 144 pg/mL, pooled over the two treatment groups.
Fig 4.
Overall survival by baseline vascular endothelial growth factor level (pooled over the two treatment arms). GCB, gemcitabine, cisplatin, and bevacizumab; GCP, gemcitabine, cisplatin, and placebo.
In an exploratory analysis, there was a significant treatment-by-VEGF interaction for PFS (P = .030) and a marginally significant interaction for OS (P = .063). In patients with baseline VEGF levels at or below the median, PFS (P = .043) and OS (P = .028) were significantly better for GCB than for GCP. In the high VEGF strata, there were no significant differences in PFS (P = .24) or OS (P = .90) between treatment arms.
DISCUSSION
Cross-trial comparisons have been challenging in MM because of the heterogeneity of this disease and the difficulty of reproducibly measuring tumor response.1 In a malignancy with three major pathologic subtypes (epithelial, sarcomatoid, and biphasic), four sites of disease origin (pleura, peritoneum, pericardium, and tunica vaginalis), and several other key prognostic factors that can substantially affect outcomes,27,28 it should not be surprising that six small phase II trials evaluating the gemcitabine/cisplatin combination in MM produced widely discordant results.5–10 Therefore, to discern the impact of the addition of a novel agent to this chemotherapy backbone, we designed what we believe is the first randomized phase II trial ever performed in patients with MM. It demonstrates that the addition of bevacizumab to gemcitabine/cisplatin does not improve PFS in this disease.
We observed a median OS of 15.7 months for the GCB arm, which is better than most other multicenter studies in MM.1,2,29 Had we performed a single-arm trial, we might have erroneously concluded that this was an active regimen. The apparent better performance of both arms of this study may reflect patient selection, treatment experience at specialized centers, and the impact of subsequent therapies. When this trial began, the safety of using bevacizumab with anticoagulation had not been established30; therefore, patients on anticoagulation were excluded. Because patients with cancer who develop venous thromboembolism tend to have a shorter survival,31 this may have introduced a selection bias. Although we did not collect data on second-line therapies, given the US Food and Drug Administration approval of pemetrexed midway through this trial,2 it is likely that many of the patients who experienced progression on this study subsequently received pemetrexed. This study also confirms the data from prior investigators regarding the significant activity of the gemcitabine/cisplatin combination in patients with MM.3–10
The addition of bevacizumab to chemotherapy improves outcomes in cancers of the breast, colon, lung, and kidney.32–35 Given preclinical data that supported a key role for the VEGF pathway in MM biology12–14 and the multiple phase II trials that suggested modest activity for other VEGF inhibitors in patients with MM,11,15–20 it was plausible to assume that adding bevacizumab to systemic chemotherapy in patients with MM would replicate the results observed in these other cancers. Because pemetrexed was not commercially available when we initiated this trial, we selected the widely used gemcitabine/cisplatin combination. Subsequent studies have shown that adding bevacizumab to a gemcitabine backbone does not improve survival in either pancreatic or lung cancer,36,37 and preclinical data suggest a negative interaction between bevacizumab and gemcitabine.38 Some cytotoxic agents, but not gemcitabine, stimulate angiogenesis and tumor regrowth by mobilizing circulating endothelial progenitors from bone marrow. VEGF inhibitors may augment chemotherapy by blunting this effect. According to this hypothesis, for optimal activity, bevacizumab should be combined with agents other than gemcitabine.38 Thus, an ongoing French randomized phase II/III study evaluating the addition of bevacizumab to pemetrexed/cisplatin in MM seems reasonable,39 despite the negative results of our trial.
Our data support the observation of other investigators that patients with MM have higher VEGF levels than patients with other solid tumors.14 The median plasma VEGF concentrations reported in this trial (144 pg/mL) were significantly higher than those observed in phase III trials in non–small-cell lung cancer (38.7 pg/mL)37 or colorectal cancer (44 pg/mL).40 Pretreatment VEGF levels correlated with PFS and OS, suggesting the potential utility of VEGF as a prognostic factor. Bevacizumab-treated patients with lower pretreatment VEGF levels had a longer PFS and OS. These treatment-by-VEGF interactions, although intriguing, should be viewed cautiously because of the relatively small sample sizes.
In conclusion, we observed that the addition of bevacizumab to gemcitabine/cisplatin does not improve PFS or OS in patients with MM. Given the heterogeneity of MM, our experience supports the use of randomized phase II screening designs41,42 to evaluate novel agents in this disease.
Supplementary Material
Footnotes
Supported by National Cancer Institute Grants No. N01-CM-17102 and N01-CM-62209.
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, May 13-17, 2005, Orlando, FL; the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL; the 11th World Conference on Lung Cancer, July 3-6, 2005, Barcelona, Spain; and the 12th World Conference on Lung Cancer, September 2-6, 2007, Seoul, Korea.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00027703.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Hedy L. Kindler, Genentech/Roche (C); David R. Gandara, Genentech/Roche (C); Lee M. Krug, Genentech (C); James P. Stevenson, Genentech (C), Eli Lilly (C); Pasi A. Jänne, Genentech/Roche (C); David I. Quinn, Genentech (C); Marianna N. Koczywas, Genentech (C), Eli Lilly (C); Julie R. Brahmer, Genentech (C); Kathy S. Albain, Genentech/Roche (C); Walter M. Stadler, Genentech (C); Everett E. Vokes, Genentech (C) Stock Ownership: None Honoraria: James P. Stevenson, Genentech, Eli Lilly; Marianna N. Koczywas, Genentech, Eli Lilly; Julie R. Brahmer, Genentech; Nicholas J. Vogelzang, Genentech/Roche, Eli Lilly Research Funding: Hedy L. Kindler, Genentech, Eli Lilly; David R. Gandara, Genentech/Roche, Eli Lilly; Lee M. Krug, Eli Lilly; James P. Stevenson, Genentech, Eli Lilly; Kathy S. Albain, Eli Lilly; Nicholas J. Vogelzang, Genentech/Roche, Eli Lilly; Walter M. Stadler, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hedy L. Kindler, Theodore G. Karrison, Nicholas J. Vogelzang, Helen X. Chen, Everett E. Vokes
Administrative support: Helen X. Chen, Walter M. Stadler,Everett E. Vokes
Provision of study materials or patients: Hedy L. Kindler, David R. Gandara, Charles Lu, Lee M. Krug, James P. Stevenson, Pasi A. Jänne, David I. Quinn, Marianna N. Koczywas, Julie R. Brahmer, Kathy S. Albain, David A. Taber, Nicholas J. Vogelzang
Collection and assembly of data: Hedy L. Kindler, Theodore G. Karrison, David R. Gandara, Lee M. Krug, James P. Stevenson, Pasi A. Jänne, Julie R. Brahmer, Kathy S. Albain, David A. Taber,Nicholas J. Vogelzang
Data analysis and interpretation: Hedy L. Kindler, Theodore G. Karrison, David R. Gandara, Charles Lu, Lee M. Krug, Pasi A. Jänne, David I. Quinn, Marianna N. Koczywas, Samuel G. Armato III, Nicholas J. Vogelzang, Walter M. Stadler, Everett E. Vokes
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Campbell NP, Kindler HL. Update on malignant pleural mesothelioma. Semin Respir Crit Care Med. 2011;32:102–110. doi: 10.1055/s-0031-1272874. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Kindler HL, van Meerbeeck JP. The role of gemcitabine in the treatment of malignant mesothelioma. Semin Oncol. 2002;29:70–76. doi: 10.1053/sonc.2002.30232. [DOI] [PubMed] [Google Scholar]
- 4.Lee CW, Murray N, Anderson H, et al. Outcomes with first-line platinum-based combination chemotherapy for malignant pleural mesothelioma: A review of practice in British Columbia. Lung Cancer. 2009;64:308–313. doi: 10.1016/j.lungcan.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: A phase II study. J Clin Oncol. 1999;17:25–30. doi: 10.1200/JCO.1999.17.1.25. [DOI] [PubMed] [Google Scholar]
- 6.van Haarst JM, Baas P, Manegold CH, et al. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer. 2002;86:342–345. doi: 10.1038/sj.bjc.6600118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer. 2002;87:491–496. doi: 10.1038/sj.bjc.6600505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castagneto B, Zai S, Dongiovanni D, et al. Cisplatin and gemcitabine in malignant pleural mesothelioma: A phase II study. Am J Clin Oncol. 2005;28:223–226. doi: 10.1097/01.coc.0000144852.75613.56. [DOI] [PubMed] [Google Scholar]
- 9.Kalmadi SR, Rankin C, Kraut MJ, et al. Gemcitabine and cisplatin in unresectable malignant mesothelioma of the pleura: A phase II study of the Southwest Oncology Group (SWOG 9810) Lung Cancer. 2008;60:259–263. doi: 10.1016/j.lungcan.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utkan G, Buyukcelik A, Yalcin B, et al. Divided dose of cisplatin combined with gemcitabine in malignant mesothelioma. Lung Cancer. 2006;53:367–374. doi: 10.1016/j.lungcan.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Dowell JE, Kindler HL. Antiangiogenic therapies for mesothelioma. Hematol Oncol Clin North Am. 2005;19:1137–1145. doi: 10.1016/j.hoc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Ohta Y, Shridhar V, Bright RK, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer. 1999;81:54–61. doi: 10.1038/sj.bjc.6690650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strizzi L, Catalano A, Vianale G, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193:468–475. doi: 10.1002/path.824. [DOI] [PubMed] [Google Scholar]
- 14.Linder C, Linder S, Munck-Wikland E, et al. Independent expression of serum vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in patients with carcinoma and sarcoma. Anticancer Res. 1998;18:2063–2068. [PubMed] [Google Scholar]
- 15.Garland LL, Chansky K, Wozniak A, et al. SWOG S0509: A phase II study of novel oral antiangiogenic agent AZD2171 (NSC-732208) in malignant pleural mesothelioma. J Clin Oncol. 2009;27(suppl 15S; abstr 7511):384s. [Google Scholar]
- 16.Dubey S, Janne PA, Krug L, et al. Phase II study of sorafenib in malignant mesothelioma: Results of Cancer and Leukemia Group B 30307. J Thorac Oncol. 2010;5:1655–1661. doi: 10.1097/JTO.0b013e3181ec18db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak AK, Millward M, Francis RJ, et al. Final results of a phase II study of sunitinib as second-line therapy in malignant pleural mesothelioma (MPM) J Clin Oncol. 2010;28(suppl 15S; abstr 7036):523s. [Google Scholar]
- 18.Kindler HL, Vogelzang NJ, Chien K, et al. SU5416 in malignant mesothelioma: A University of Chicago Phase II Consortium Study. Proc Am Soc Clin Oncol. 2001;20(suppl; abstr 341a):43. [Google Scholar]
- 19.Baas P, Boogerd W, Dalesio O, et al. Thalidomide in patients with malignant pleural mesothelioma. Lung Cancer. 2005;48:291–296. doi: 10.1016/j.lungcan.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Jahan TM, Gu L, Wang X, et al. Vatalanib in malignant mesothelioma: A phase II study by the Cancer and Leukemia Group B (CALGB 30107) J Clin Oncol. 2006;24(suppl; abstr 7081):384s. doi: 10.1016/j.lungcan.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 25.Cox DR. Regression models for life tables. J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 26.Goldman AI. Issues in designing sequential stopping rules for monitoring side effects in clinical trials. Control Clin Trials. 1987;8:327–337. doi: 10.1016/0197-2456(87)90153-x. [DOI] [PubMed] [Google Scholar]
- 27.Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113:723–731. doi: 10.1378/chest.113.3.723. [DOI] [PubMed] [Google Scholar]
- 28.Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J Clin Oncol. 1998;16:145–152. doi: 10.1200/JCO.1998.16.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–2090. doi: 10.1200/JCO.2008.19.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leighl NB, Bennouna J, Yi J, et al. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br J Cancer. 2011;104:413–418. doi: 10.1038/sj.bjc.6606074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sørensen HT, Mellemkjaer L, Olsen JH, et al. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 32.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 33.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 34.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 35.Rini BI, Halabo S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26:5422–5428. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for non-squamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAIL). Ann Oncol. 2010;21:1804–1809. doi: 10.1093/annonc/mdq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: Implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zalcman G, Margery J, Scherpereel A, et al. IFCT-GFPC-0701 MAPS trial, a multicenter randomized phase II/III trial of pemetrexed-cisplatin with or without bevacizumab in patients with malignant pleural mesothelioma. J Clin Oncol. 2010;28(suppl 15S; abstr 7020):519s. [Google Scholar]
- 40.Holden SN, Ryan S, Kearns A, et al. Benefit from bevacizumab is independent of pretreatment plasma vascular endothelial growth factor-A in patients with metastatic colorectal cancer. J Clin Oncol. 2005;23(suppl 17S; abstr 3555):159s. [Google Scholar]
- 41.Cannistra SA. Phase II trials in Journal of Clinical Oncology. J Clin Oncol. 2009;27:3073–3076. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 42.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23:7199–7206. doi: 10.1200/JCO.2005.01.149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.