Abstract
Purpose
To evaluate cardiopulmonary function (as measured by peak oxygen consumption [VO2peak]) across the breast cancer continuum and its prognostic significance in women with metastatic disease.
Patients and Methods
Patients with breast cancer representing four cross-sectional cohorts—that is, (1) before, (2) during, and (3) after adjuvant therapy for nonmetastatic disease, and (4) during therapy in metastatic disease—were studied. A cardiopulmonary exercise test (CPET) with expired gas analysis was used to assess VO2peak. A Cox proportional hazards model was used to estimate the risk of death according to VO2peak category (< 15.4 v ≥ 15.4 mL · kg−1 · min−1) with adjustment for clinical factors.
Results
A total of 248 women (age, 55 ± 8 years) completed a CPET. Mean VO2peak was 17.8 ± a standard deviation of 4.3 mL · kg−1 · min−1, the equivalent of 27% ± 17% below age-matched healthy sedentary women. For the entire cohort, 32% had a VO2peak less than 15.4 mL · kg−1 · min−1—the VO2peak required for functional independence. VO2peak was significantly different across breast cancer cohorts for relative (mL · kg−1 · min−1) and absolute (L · min−1) VO2peak (P = .017 and P < .001, respectively); VO2peak was lowest in women with metastatic disease. In patients with metastatic disease (n = 52), compared with patients achieving a VO2peak ≤ 1.09 L · min−1, the adjusted hazard ratio for death was 0.32 (95% CI, 0.16 to 0.67, P = .002) for a VO2peak more than 1.09 L · min−1.
Conclusion
Patients with breast cancer have marked impairment in VO2peak across the entire survivorship continuum. VO2peak may be an independent predictor of survival in metastatic disease.
INTRODUCTION
Patients with breast cancer are subject to the effects of normal aging, age- and/or disease-related comorbid conditions, and deconditioning that adversely affect cardiopulmonary function. However, in contrast to women from the general population, women with breast cancer are also subjected to the use of prolonged and aggressive multimodal anticancer therapy,1 which together are suspected to cause further impairments in cardiopulmonary function predisposing to serious health conditions (eg, cardiovascular disease).2
In current practice, patient cardiopulmonary function is almost exclusively evaluated through the determination of cardiac function via a resting measurement of left ventricular ejection fraction (LVEF). Although resting LVEF may have utility in prediction and/or assessment of therapy-induced cardiac toxicity,3 it does not provide a measure of global cardiovascular function and reserve.4 Indeed, cardiac function is only one organ component that contributes to the integrative capacity of the cardiovascular and musculoskeletal system to transport and use oxygen (O2) for adenosine triphosphate resynthesis.5 The efficiency of O2 transport and use determines an individual's cardiopulmonary function (or aerobic capacity). An incremental cardiopulmonary exercise test (CPET) with gas exchange measurement, to assess peak oxygen consumption (VO2peak), provides the gold standard assessment of aerobic capacity.6 VO2peak is inversely correlated with cardiovascular and all-cause mortality in a broad range of adult populations.7–11 Accordingly, formalized exercise testing is widely used in numerous clinical settings and provides a wealth of diagnostic, prognostic, and decision-making information.6
In contrast, exercise testing is not routinely performed at any stage of during breast cancer treatment or recovery. However, in clinical research, a growing number of studies are using exercise testing to determine the efficacy of exercise training interventions in patients with breast cancer both during and after adjuvant therapy.12 However, little is known about the level of exercise tolerance in patients with breast cancer or how this may differ during treatment and recovery. We used data from our prior work13–18 to evaluate VO2peak across the entire breast cancer survivorship continuum (ie, diagnosis to metastatic disease). Secondary objectives were to compare age-related declines in VO2peak between patients with breast cancer relative to age- and sex-matched healthy women, evaluate the proportion of patients falling below the VO2peak required for functional independence (ie, 15.4 mL · kg −1 · min−1), and explore the prognostic significance of VO2peak in metastatic disease.
PATIENTS AND METHODS
Patient Cohorts and Setting
Women with histologically confirmed operable (stage I through IIIC) or metastatic (stage IV) breast adenocarcinoma at Duke University Medical Center, Durham, NC, or the Cross Cancer Institute, Edmonton, Alberta, Canada were studied. Specifically, data were pooled from our prior exercise intervention studies (baseline data only) or cross-sectional studies that were categorized into four cross-sectional cohorts—that is, (1) before (n = 20), (2) during (n = 46),13,14 and (3) after adjuvant therapy (n = 130),15–17 for nonmetastatic disease, and (4) during therapy for metastatic disease (n = 52).14,18 The before therapy cohort constituted newly diagnosed untreated patients (ie, before receipt of surgery and any adjuvant therapy); the during therapy cohort included postsurgical patients after completion of at least two cycles of primary adjuvant chemotherapy; the after therapy cohort included patients 6 months to ∼3 years after the completion of primary adjuvant therapy including radiotherapy. Finally, the metastatic cohort included patients with stage IV disease receiving some form of cytotoxic chemotherapy. Institutional review board approval and written informed consent were obtained in all studies.
Cardiopulmonary Function (VO2peak)
To determine VO2peak, a CPET with 12-lead ECG monitoring (Mac 5000, GE Healthcare, Waukesha, WI) was performed by certified exercise physiologists. The specific protocol for this test has previously been reported in detail.18 In brief, all tests were performed on an electronically braked cycle ergometer (Lode, Groningen, Netherlands) with breath-by-breath expired gas analysis. Three minutes of resting metabolic data were collected before participants began cycling at 20 W. Workloads were then increased 5 to 20 W/min until volitional exhaustion or until a symptom limitation was achieved. All cardiopulmonary exercise testing data were recorded as the highest 30-second value elicited during the exercise test. Age-matched normative VO2max data for healthy women without a history of breast cancer were obtained from Fitzgerald et al.19 It is important to note that our assessment of cardiopulmonary function was determined at peak exercise (ie, peak Vo2) as recommended for clinical populations,6 as opposed to maximum Vo2 (ie, oxygen consumption reaches a plateau despite increasing workload20) in the comparison data by Fitzgerald et al.19
Clinical Parameters and Performance Status
Medical characteristics were abstracted from medical records. Performance status was assessed using the Karnofsky performance scale and was assessed at the time of study enrollment by the attending oncologist. Hemoglobin was assessed by an automated CBC. Left ventricular function was determined using multiple gated acquisition scan or echocardiography to assess LVEF using standardized procedures. Exercise behavior was assessed by the Godin Leisure Time Exercise Questionnaire.21
Statistical Analysis
For continuous data, we performed a series of one-way analysis of variance to examine overall differences between the four cross-sectional cohorts with adjustment for age and study site (Edmonton v Durham). We conducted an overall F-test to account for multiple comparisons with post hoc (Tukey-Kramer) analysis as appropriate to control for varying sample sizes between groups. For categorical data, a χ2 test was used to examine overall differences between the four cross-sectional cohorts. Linear regression was used to examine the relationship between VO2peak and age in the total population of patients with breast cancer and after adjuvant therapy group only (this analysis was not performed on the other individual groups as a result of small sample size). Expected mean VO2peak values at a given age for patients with breast cancer were compared with those of healthy, sedentary women.19 The Cox proportional hazards model was used to explore the association between VO2peak and survival in the subset of patients with metastatic disease (n = 52). VO2peak was categorized using the dichotomous classification for functional dependence (< 15.4 v ≥ 15.4 mL · kg−1 · min−1) and a median split for L · min−1 (< 1.09 v ≥ 1.09 L · min−1). Survival was defined as the time between assessment of VO2peak and death; for patients remaining alive, survival was censored at the time of last follow-up. The prognostic value of VO2peak was examined individually with and without adjustment for the following covariates: age, months since diagnosis, and performance status. Statistical significance was set at P < .05 (two-tailed) for all analyses.
RESULTS
Details regarding the profiles of the participants are described in Table 1.
Table 1.
Characteristics of the Participants
| Variable | Overall |
Nonmetastatic Disease |
Advanced Metastatic Disease |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Adjuvant Therapy |
During Adjuvant Therapy |
After Adjuvant Therapy |
||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 248 | 100 | 20 | 8 | 46 | 19 | 130 | 52 | 52 | 21 |
| Site | ||||||||||
| Canada | 201 | 81 | 20 | 100 | 19 | 41 | 130 | 100 | 52 | 100 |
| United States | 47 | 19 | — | 27 | 59 | — | — | |||
| Age, years | ||||||||||
| Mean | 55 | 49 | 53 | 57 | 55 | |||||
| SD | 8 | 9 | 7 | 8 | 10 | |||||
| Range | 30-74 | 30-61 | 40-70 | 31-73 | 36-74 | |||||
| Weight, kg | ||||||||||
| Mean | 76 | 78 | 75 | 77 | 70 | |||||
| SD | 17 | 19 | 16 | 17 | 16 | |||||
| BMI, kg/m2 | ||||||||||
| Mean | 28 | 29 | 28 | 29 | 27 | |||||
| SD | 6 | 7 | 6 | 6 | 5 | |||||
| Range | 16-50 | 18-46 | 18-40 | 16-50 | 18-45 | |||||
| KPS | ||||||||||
| 70 | 3 | 1 | — | — | — | 3 | 6 | |||
| 75 | 1 | < 1 | — | — | — | 1 | 2 | |||
| 80 | 6 | 2 | — | — | — | 6 | 12 | |||
| 90 | 43 | 17 | — | 13 | 28 | — | 30 | 60 | ||
| 100 | 193 | 78 | 20 | 100 | 33 | 72 | 130 | 100 | 10 | 20 |
| Exercise behavior, min·wk−1 | ||||||||||
| Mean | 222 | 135 | 199 | 270 | 172 | |||||
| SD | 269 | 171 | 275 | 307 | 192 | |||||
| Meeting ACSM guidelines* | 49 | 25 | 5 | 25 | 3 | 11 | 32 | 31 | 9 | 18 |
| Time from primary diagnosis, months | ||||||||||
| Mean | 21 | 1 | 7 | 27 | 25 | |||||
| SD | 19 | 0.4 | 6 | 16 | 26 | |||||
| Anatomic stage | ||||||||||
| IA | 37 | 15 | 1 | 5 | 6 | 13 | 30 | 23 | — | |
| IB | 37 | 15 | 4 | 20 | 3 | 7 | 30 | 23 | — | |
| IIA | 52 | 21 | 12 | 60 | 18 | 39 | 22 | 17 | — | |
| IIB | 43 | 17 | 2 | 10 | 11 | 24 | 30 | 23 | — | |
| IIIA | 25 | 10 | 1 | 5 | 7 | 15 | 17 | 13 | — | |
| IIIB | 1 | < 1 | — | 1 | 2 | — | — | |||
| IIIC | 1 | < 1 | — | — | 1 | 1 | — | |||
| IV | 51 | 21 | — | — | — | 52 | 100 | |||
| Current cytotoxic therapy | NA | NA | ||||||||
| Chemotherapy | 63 | 64 | 29 | 63 | 34 | 65 | ||||
| Anthracycline-containing regimen | 43 | 68 | 29 | 100 | 14 | 41 | ||||
| Trastuzumab | 29 | 30 | 17 | 37 | 12 | 23 | ||||
| Radiation | 20 | 20 | 0 | 0 | 20 | 38 | ||||
| Prior primary cytotoxic therapy | NA | NA | ||||||||
| Chemotherapy | 128 | 70 | 96 | 74 | 32 | 62 | ||||
| Anthracycline-containing regimen | 79 | 62 | 62 | 65 | 17 | 53 | ||||
| Trastuzumab | 18 | 10 | 18 | 14 | 0 | 0 | ||||
| Radiation | 127 | 70 | 102 | 78 | 25 | 48 | ||||
Abbreviations: ACSM, American College of Sports Medicine; BMI, body mass index; KPS, Karnofsky performance status; NA, not applicable; SD, standard deviation.
ASCM guidelines defined as percentage of patients reporting ≥ 150 minutes · wk−1 of at least moderate and/or strenuous exercise.
Cardiopulmonary Function Across the Breast Cancer Trajectory
Resting Data.
For the overall sample, mean resting heart rate was 89 ± 16 beats · min−1; 27% of patients presented with resting tachycardia (heart rate ≥ 100 beats · min−1; Table 2). Mean (± standard deviation) LVEF and hemoglobin were 62% ± 7% and 12.7 ± 1.6 g/dL, respectively. Resting heart rate was significantly higher in the during adjuvant therapy group relative to the after therapy group and significantly lower relative to the metastatic group. Incidences of tachycardia were significantly higher in the during adjuvant therapy group relative to all other groups (P < .05). Systolic blood pressure was significantly higher in the after adjuvant therapy group relative to all other groups, whereas diastolic blood pressure was significantly lower in the before adjuvant therapy group, relative to all other groups. Resting LVEF was not different across groups (P > .05). Finally, hemoglobin concentration was significantly lower in the during adjuvant group relative to all other groups (P < .05).
Table 2.
Cardiopulmonary Data Across the Breast Cancer Continuuma
| Variable | Overall | Nonmetastatic Disease |
Advanced Metastatic Disease | Overall Adjusted Pa | ||
|---|---|---|---|---|---|---|
| Prior to Adjuvant Therapy | During Adjuvant Therapy | After Adjuvant Therapy | ||||
| Patients | ||||||
| No. | 248 | 20 | 46 | 130 | 52 | |
| % | 100 | 8 | 19 | 52 | 21 | |
| Resting data | ||||||
| HR, beats·min−1 | < .001 | |||||
| Mean | 89 | 74 | 91b | 89 | 92 | |
| SD | 16 | 10 | 17 | 16 | 17 | |
| Tachycardia (HR ≥ 100 beats·min−1), % | .001 | |||||
| No. | 67 | 1c | 17d | 31 | 18 | |
| % | 27 | 5 | 37 | 24 | 35 | |
| Systolic blood pressure, mmHg | < .001 | |||||
| Mean | 126 | 121 | 116 | 133 | 123 | |
| SD | 18 | 13 | 14e | 19d | 14 | |
| Diastolic blood pressure, mmHg | < .001 | |||||
| Mean | 86 | 78 | 96 | 85 | 80 | |
| SD | 18 | 8d | 34c | 12 | 8 | |
| RPP | .555 | |||||
| Mean | 11,258 | 9,002 | 10,476 | 11,872 | 11,307 | |
| SD | 2,705 | 1,418 | 1,939 | 2,993 | 2,299 | |
| LVEF, %f | .172 | |||||
| Mean | 62 | 62 | 62 | 62 | 59 | |
| SD | 7 | 6 | 6 | 7 | 7 | |
| Hemoglobin, g/dLg | < .001 | |||||
| Mean | 12.7 | 13.3 | 11.5 | 13.5 | 12.6 | |
| SD | 1.6 | 1.2 | 1.3d | 1.0h | 1.7 | |
| Peak exercise data | ||||||
| HR, beats·min−1 | .007 | |||||
| Mean | 158 | 158 | 164 | 157 | 153 | |
| SD | 18 | 23 | 14 | 17 | 18 | |
| HR, beats·min−1, predicted % | ||||||
| Mean | −4 | −8 | −2 | −3 | −8 | |
| SD | 10 | 11 | 8 | 10 | 10 | |
| Cardiac reserve, beats·min−1 | .001 | |||||
| Mean | 68 | 83 | 72 | 68 | 60 | |
| SD | 21 | 22 | 22 | 20i | 18 | |
| Systolic blood pressure, mmHg | .008 | |||||
| Mean | 165 | 154 | 162 | 170 | 160 | |
| SD | 31 | 23 | 25 | 35j | 23 | |
| Diastolic blood pressure, mmHg | .026 | |||||
| Mean | 86 | 86 | 83 | 87 | 87 | |
| SD | 11 | 8 | 10b | 11 | 11 | |
| VO2peak, mL·kg−1·min−1 | < .001 | |||||
| Mean | 17.8 | 18.5 | 17.4 | 18.4 | 16.3 | |
| SD | 4.3 | 6.3 | 4.3 | 4.1i | 3.5 | |
| Range | 7.3-31.2 | 7.3-30.2 | 10.2-28.2 | 9.9-31.2 | 8.8-27.0 | |
| VO2peak, mL·kg−1·min−1, predicted % | ||||||
| Mean | −27 | −31 | −31 | −22 | −33 | |
| SD | 17 | 20 | 18 | 16 | 16 | |
| Range | −73-25 | −73-0 | −59-25 | −58-25 | −62-12 | |
| VO2peak, L·min−1 | < .001 | |||||
| Mean | 1.32 | 1.38 | 1.28 | 1.41 | 1.13 | |
| SD | 0.33 | 0.38 | 0.33 | 0.30d | 0.28 | |
| Workload, Watts | < .001 | |||||
| Mean | 92 | 96 | 90 | 98 | 80 | |
| SD | 26 | 37 | 24 | 23d | 26 | |
| O2 pulse, LO2/beat | .181 | |||||
| Mean | 0.10 | 0.09 | 0.10 | 0.11 | 0.11 | |
| SD | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 | |
| RERk | < .001 | |||||
| Mean | 1.18 | 1.09 | 1.22 | 1.15 | 1.22 | |
| SD | 0.10 | 0.07 | 0.12 | 0.09i | 0.10 | |
| RER ≥ 1.10 | ||||||
| No. | 143 | 12 | 24 | 63 | 48 | |
| % | 78 | 60 | 83 | 76 | 92 | |
Abbreviations: HR, heart rate; LVEF, left ventricular ejection fraction; O2, oxygen; RER, respiratory exchange ratio; RPP, rate pressure product (calculated as [resting heart rate × resting systolic blood pressure]/1,000; cardiac reserve calculated as peak heart rate − resting heart rate); SD, standard deviation; VO2peak, peak oxygen consumption.
Adjusted for age and site.
Significantly different from after adjuvant therapy and metastatic disease groups.
Significantly different from after adjuvant therapy group.
Significantly different from all other groups.
Significantly different from metastatic disease group.
Data available on 143 participants.
Data available on 174 participants.
Significantly different from prior adjuvant therapy and metastatic disease groups.
Significantly different from during adjuvant therapy and metastatic disease groups.
Significantly different from prior and during adjuvant therapy groups.
Data available on 184 participants.
Peak Data.
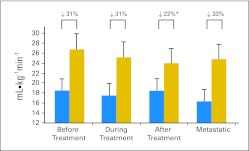
Peak cardiopulmonary data are presented in Table 2. For the overall sample, VO2peak averaged 17.8 ± 4.3 mL · kg−1 · min−1 (range, 7.3 to 31.2 mL · kg−1 · min−1), the equivalent to 27% ± 17% below age- and sex-predicted sedentary values (range, −73% to 25%) or 5.1 ± 1.2 metabolic equivalents. Peak heart rate was 158 ± 18 beats · min−1 or 96% of age-predicted maximum, whereas peak workload averaged 92 ± 26 W. Of these tests, 78% were considered to be of adequate effort given that a respiratory exchange ratio of ≥ 1.10 was achieved. Of the 22% of patients who did not achieve a respiratory exchange ratio ≥ 1.10, the tests were terminated prematurely due to other symptoms (eg, breathing or leg fatigue). For mL · kg−1 · min−1, post hoc tests revealed that VO2peak was significantly lower in the metastatic group (mean difference, −2.1 mL · kg−1 · min−1; P < .05; Fig 1) and the during adjuvant therapy group (mean difference, −1.0 mL · kg−1 · min−1; P < .05; Fig 1) relative to the after adjuvant therapy group. For L · min−1, VO2peak was significantly lower in the metastatic group (mean difference, −0.28 L · min−1; P < .05), the before adjuvant therapy group (mean difference, −0.03 L · min−1; P < .05), and the during adjuvant therapy group (mean difference, −0.13 L · min−1; P < .05) relative to the after adjuvant therapy group.
Fig 1.
Differences in peak oxygen consumption (mL · kg−1min−1; gray bars represent age-sex predicted value) in operable patients with breast cancer before (n = 20), during (n = 46), and after (n = 130) adjuvant therapy, and with metastatic disease (n = 52). Statistical tests: (*) Significantly different from during adjuvant therapy and metastatic disease groups.
Age-Related Functional Dependence and VO2peak Decline
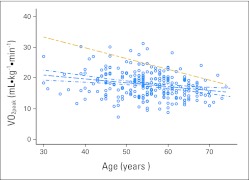
In healthy older women, the minimal aerobic reserve capacity required for functional independence is a VO2peak rate of 15.4 mL · kg−1 · min−1.22 For the overall sample, 32% had a VO2peak less than 15.4 mL · kg−1 · min−1 (Fig 2). The corresponding values for the four cross-sectional cohorts were as follows: 35% for before adjuvant therapy, 37% for during adjuvant therapy, 25% for after adjuvant therapy, and 44% for metastatic disease.
Fig 2.
The linear relationship between peak oxygen consumption (VO2peak) and age for patients with breast cancer (scatterplot and blue regression line with 95% CI; regression equation: VO2peak [mL · kg−1 · min−1] = 24.701 – [0.1251 × age]), and healthy, sedentary adult women (gold dotted regression line; regression equation: VO2peak [mL · kg−1 · min−1] = 46.82 – [0.35 × age]).
We also descriptively compared the age-related decline in VO2peak of patients with breast cancer relative to age- and sex-predicted sedentary healthy women (n = 106 groups). For the entire breast cohort, the regression equation was as follows: VO2peak (mL · kg−1 · min−1) = 24.701 − (0.1251 × age), relative to the reference relationship of VO2peak (mL · kg−1 · min−1) = 46.82 − (0.35 × age) for sedentary, healthy women (Fig 2). For the after adjuvant therapy group, the regression equation was as follows: VO2peak (mL · kg−1 · min−1) = 27.201 − (0.1538 × age). In both circumstances, the regression lines did not overlap at any point across the entire age continuum. The expected mean VO2peak across decades 40 to 70 years between patients with breast cancer and sedentary, healthy women revealed differences across all decades. For instance, the mean VO2peak for patients with breast cancer is 34% less than that for healthy, sedentary women at age 40 years, 30% less at age 50 years, 25% less at 60 years, and 17% less at age 70 years. The mean VO2peak of 19.7 mL · kg−1 · min−1 for a 40-year old patient with breast cancer is of the same magnitude as that of a 70-year old healthy, sedentary woman (19.3 mL · kg−1 · min−1).
VO2peak and Survival in Metastatic Disease
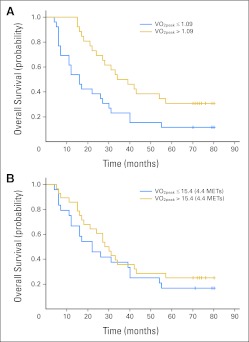
Median follow-up was 122 months (95% CI, 119 to 127 months) after testing, and 41 deaths were recorded (79% of the total sample). The median time from VO2peak assessment to death was 27 months (95% CI, 17 to 34 months). For L · min−1, the adjusted hazard ratio for death was 0.32 (95% CI, 0.16 to 0.67; P = .002) for a VO2peak of more than 1.09 L · min−1 compared with a VO2peak of ≤ 1.09 L · min−1 (Table 3; Fig 3A). Median survival was 16 months (95% CI, 7 to 27 months) for those reporting ≤ 1.09 L · min−1 compared with 36 months (95% CI, 24 to 57 months) for those reporting more than 1.09 L · min−1. Compared with a VO2peak less than 15.4 mL · kg−1 · min−1, the adjusted hazard ratio for death was 0.59 (95% CI, 0.29 to 1.19; P = .141) for a VO2peak of ≥ 15.4 mL · kg−1 · min−1 (Table 3; Fig 3B).
Table 3.
Association Between VO2peak (L · min−1 and mL · kg−1 · min−1) and Survival in Women With Metastatic Disease
| Analysis | VO2peak (L·min−1) |
P | |
|---|---|---|---|
| ≤ 1.09 | > 1.09 | ||
| No. of events | 23 | 18 | |
| No. at risk | 26 | 26 | |
| Survival, months | |||
| Median | 16 | 36 | |
| Range | 7-27 | 24-57 | |
| Adjusted* | .002 | ||
| HR | Referent | 0.32 | |
| 95% CI | 0.16 to 0.67 | ||
| Analysis | VO2peak (L·min−1) |
P | |
|---|---|---|---|
| ≤ 1.09 | > 1.09 | ||
| No. of events | 20 | 21 | |
| No. at risk | 24 | 28 | |
| Survival, months | |||
| Median | 22 | 29 | |
| Range | 12-40 | 18-42 | |
| Adjusted* | 0.141 | ||
| HR | Referent | 0.59 | |
| 95% CI | 0.29 to 1.19 | ||
Abbreviations: HR, hazard ratio; VO2peak, peak oxygen consumption.
Adjusted for age, months since diagnosis, and Karnofsky performance status.
Fig 3.
Association between peak oxygen consumption (VO2peak) and survival for (A) VO2peak (L · min−1) and (B) VO2peak (mL · kg−1 · min−1) in women with metastatic disease (n = 52). METs, metabolic equivalents.
DISCUSSION
Here we show that despite normal cardiac function (ie, LVEF ≥ 50%), women with breast cancer have significant and marked impairments in cardiopulmonary function. On average, VO2peak was 27% less than that of age-matched sedentary but otherwise healthy women without a history of breast cancer. The impairments in VO2peak were particularly striking during primary adjuvant chemotherapy and in those patients with metastatic disease, with VO2peak 31% and 33% less than that of healthy sedentary women, respectively. Remarkably, we found that patients with breast cancer reach a predicted VO2peak for a particular age group (eg, 40 years) approximately 20 to 30 years earlier than healthy women without a history of breast cancer. Sweeney et al23 found that elderly cancer survivors (of mixed diagnoses) were less likely to be able to do heavy household tasks, walk half a mile, or walk up and down stairs, compared with women without a history of cancer. Our findings indicate that approximately one third of patients with breast cancer have a VO2peak less than the functional independence threshold and, by definition, are unlikely to be able to perform such tasks. However, the present results must be interpreted with caution given potential unaccounted differences between patients and controls, including the prevalence of comorbidities and Vo2 measurement (peak Vo2 v maximum Vo2, respectively). These differences may have resulted in an overestimation of the marked differences in cardiopulmonary function between patients and controls. Follow-up prospective studies with appropriate control groups are warranted.
We contend that such VO2peak impairment is a consequence of the direct as well as the indirect (ie, lifestyle perturbations) effects of anticancer therapy (ie, anticancer therapy refers to any type of cancer treatment) that simultaneously impair the reserve capacity of organ systems that govern VO2peak (ie, pulmonary, cardiac, blood-vascular, and skeletal muscle function).2 For example, it is well-established that both locoregional radiotherapy, particularly left-sided radiation,24 as well as anthracycline25,26 and/or trastuzumab-containing27 chemotherapy, can cause acute and late-occurring cardiac injury manifest as impaired global pump function leading to a reduction in O2 delivery and diminished exercise tolerance. Anemia is also a frequent complication of various anticancer therapies,28 an effect that impairs convective oxygen delivery. Finally, preclinical data suggest that chemotherapy (eg, doxorubicin) and antiangiogenic therapy may cause significant alterations in skeletal muscle capillarity, glycolysis, and fatty acid oxidation and reductions in muscle capillary density, respectively29,30; both would directly impair diffusive oxygen transport and oxygen use, resulting in decreased exercise tolerance. The relative contributions of central (ie, cardiac) and peripheral (ie, circulation and skeletal muscle O2 extraction) factors to exercise tolerance have long been debated,31 although the role of these factors in explaining impaired cardiopulmonary function in patients with cancer has received scant attention. In the present study, given the relatively large proportion of patients receiving and/or treated with doxorubicin, one may suspect that symptomatic or asymptomatic cardiotoxicity is largely responsible for the observed VO2peak impairment. However, resting LVEF was within the normal range, suggesting that concomitant or independent injury to the other O2 transport components (ie, pulmonary, vascular, and skeletal muscle function) must therefore play a contributing role.
Cardiopulmonary dysfunction was also apparent at rest. Strikingly, 5% of patients presented with resting sinus tachycardia before adjuvant therapy, the incidence of which significantly increased to 37% in those undergoing anticancer therapy. The causes of sinus tachycardia are poorly understood, but may include the effects of treatment-induced anemia, autonomic neuropathy, and/or cardiac dysfunction.32 The clinical consequences of tachycardia in breast cancer are unknown, but resting heart rate is a strong independent predictor of cardiovascular disease mortality in healthy women.33 The resting blood pressure data also were indicative of vascular dysfunction. This was especially apparent in the after adjuvant therapy group, which presented with a mean blood pressure in the range of prehypertension34; the prognostic value of blood pressure is well established.35
A major research and clinical goal in oncology practice is accurate quantification of functional/physical performance status or physiologic age in patients with cancer.36,37 Our findings indicate that CPET is one tool that may provide complementary information to existing assessments because it provides a comprehensive, integrative assessment of the reserve capacity of the heart, as well as other O2 transport components (ie, VO2peak), which is not captured by current measures used in clinical practice. Furthermore, exercise capacity is an established, strong predictor of mortality in numerous clinical populations.22,38–40 For example, Gulati et al38 found a two-fold risk of death in women achieving less than 85% of age-predicted exercise capacity relative to those achieving ≥ 85% in 5,721 asymptomatic women. In our study, 78% of operable patients did not achieve 85% of age-predicted sedentary VO2peak. As a consequence, these patients have increased susceptibility to late-occurring cardiovascular disease41 and premature mortality. Our exploratory analyses provide the first data to suggest that the prognostic significance of VO2peak extends to women with metastatic breast cancer. Large-scale prospective studies are now warranted. The prognostic value of VO2peak in women with operable breast cancer remains to be determined.
Preventive and/or treatment strategies will be required to offset the direct and indirect effects of anticancer therapy. Aerobic (exercise) training is the most effective therapy to improve VO2peak in healthy individuals given that it improves the reserve capacity of all O2 transport organs, which together lead to favorable improvements in VO2peak.5 A meta-analysis by our group found that exercise training in patients with cancer caused a significant improvement in VO2peak (weighted mean difference = +2.90 mL · kg−1 · min−1; 95% CI, 1.16 to 4.64 mL · kg−1 · min−1), relative to sedentary control.42 Investigation of the most effective type and intensity of exercise training to augment VO2peak and other outcomes, as well as the physiologic mechanisms underlying the exercise training – VO2peak relationship in breast and other cancer populations, is the focus of ongoing trials.
This study has important limitations. Our findings are based on cross-sectional (involving different patients in the breast cancer subgroups) as opposed to prospective data. Also, there is treatment heterogeneity and likely comorbidity differences among patient cohorts. Patient selection bias may exist as a result of different patient inclusion and exclusion criteria adopted across pooled studies as well as exclusion of patients deemed physically unable to perform CPET. Relatedly, our comparison “healthy” sedentary data were obtained from population-based normative data with differences in prevalence of comorbidities and Vo2 assessment (peak v maximum Vo2); in addition, 22% of patients did not achieve the criteria for a true peak Vo2 (ie, respiratory exchange ratio ≥ 1.10). Together, these factors may have resulted in an overestimation of the observed impairment in cardiopulmonary function in patients with breast cancer. Finally, the survival analysis only included death from any cause. The specific cause of death is not known. With the exception of performance status, months since diagnosis and age analyses were also not adjusted for other established parameters of survival in the metastatic setting. Future studies are needed to fully define the point at which functional dependence occurs, as well as evaluate the concordance between VO2peak and other existing functional measurement tools.
In summary, patients with breast cancer have significant and marked impairment in cardiopulmonary function over the entire continuum of disease. As a result, approximately one third of patients with breast cancer have a VO2peak less than the functional independence threshold and reach a predicted age-related VO2peak, on average, 20 to 30 years earlier than healthy women without a history of breast cancer. Given the projected number of cancer survivors, future studies investigating the clinical utility of quantitative measures of functional capacity as well as the efficacy of exercise interventions to offset the devastating consequences of the “multiple hit” are a high priority.
Footnotes
Listen to the podcast by Dr Schwartz at www.jco.org/podcasts
Supported by National Institutes of Health Grants No. CA143254, CA142566, CA138634, CA133895, and CA125458 and funds from George and Susan Beischer (L.W.J.). K.S.C. is supported by the Canada Research Chairs Program.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lee W. Jones
Provision of study materials or patients: John R. Mackey
Collection and assembly of data: Lee W. Jones, Edith N. Pituskin, Mark Haykowsky
Data analysis and interpretation: Lee W. Jones, Kerry S. Courneya, John R. Mackey, Hyman B. Muss, Jessica M. Scott, Whitney E. Hornsby, April D. Coan, James E. Herndon II, Pamela S. Douglas, Mark Haykowsky
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 2.Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: Is our ear really to the ground? J Clin Oncol. 2008;26:1201–1203. doi: 10.1200/JCO.2007.14.8742. [DOI] [PubMed] [Google Scholar]
- 5.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society, American College of Chest Physicians: ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 7.Kavanagh T, Mertens DJ, Hamm LF, et al. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation. Circulation. 2002;106:666–671. doi: 10.1161/01.cir.0000024413.15949.ed. [DOI] [PubMed] [Google Scholar]
- 8.Kavanagh T, Mertens DJ, Hamm LF, et al. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J Am Coll Cardiol. 2003;42:2139–2143. doi: 10.1016/j.jacc.2003.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Kubozono T, Itoh H, Oikawa K, et al. Peak VO(2) is more potent than B-type natriuretic peptide as a prognostic parameter in cardiac patients. Circ J. 2008;72:575–581. doi: 10.1253/circj.72.575. [DOI] [PubMed] [Google Scholar]
- 10.Aaronson KD, Mancini DM. Is percentage of predicted maximal exercise oxygen consumption a better predictor of survival than peak exercise oxygen consumption for patients with severe heart failure? J Heart Lung Transplant. 1995;14:981–989. [PubMed] [Google Scholar]
- 11.Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: Systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 13.Haykowsky MJ, Mackey JR, Thompson RB, et al. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15:4963–4967. doi: 10.1158/1078-0432.CCR-09-0628. [DOI] [PubMed] [Google Scholar]
- 14.Courneya KS, Jones LW, Peddle CJ, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: A randomized controlled trial. Oncologist. 2008;13:1012–1020. doi: 10.1634/theoncologist.2008-0017. [DOI] [PubMed] [Google Scholar]
- 15.Courneya KS, Mackey JR, Bell GJ, et al. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 16.Jones LW, Haykowsky M, Pituskin EN, et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor–positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 17.Jones LW, Haykowsky M, Peddle CJ, et al. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007;16:1026–1031. doi: 10.1158/1055-9965.EPI-06-0870. [DOI] [PubMed] [Google Scholar]
- 18.Jones LW, Eves ND, Mackey JR, et al. Safety and feasibility of cardiopulmonary exercise testing in patients with advanced cancer. Lung Cancer. 2007;55:225–232. doi: 10.1016/j.lungcan.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald MD, Tanaka H, Tran ZV, et al. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: A meta-analysis. J Appl Physiol. 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 20.Hill AV, Long CN, Lupton H. Muscular exercise, lactic acid and the supply and utilisation of oxygen: Parts VII-VIII. Proc R Soc B. 1924;97:84–138. [Google Scholar]
- 21.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 22.Paterson DH, Cunningham DA, Koval JJ, et al. Aerobic fitness in a population of independently living men and women aged 55-86 years. Med Sci Sports Exerc. 1999;31:1813–1820. doi: 10.1097/00005768-199912000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney C, Schmitz KH, Lazovich D, et al. Functional limitations in elderly female cancer survivors. J Natl Cancer Inst. 2006;98:521–529. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 24.Zeng J, Zhang J, Hollis DR, et al. Impact of incidental cardiac irradiation on the development of shortness of breath and changes in pulmonary function tests in patients receiving radiation for lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:S157–S158. [Google Scholar]
- 25.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 26.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 27.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grotto HZ. Anaemia of cancer: An overview of mechanisms involved in its pathogenesis. Med Oncol. 2008;25:12–21. doi: 10.1007/s12032-007-9000-8. [DOI] [PubMed] [Google Scholar]
- 29.Amaral SL, Papanek PE, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am J Physiol. 2001;281:H1163–H1169. doi: 10.1152/ajpheart.2001.281.3.H1163. [DOI] [PubMed] [Google Scholar]
- 30.van Norren K, van Helvoort A, Argilés JM, et al. Direct effects of doxorubicin on skeletal muscle contribute to fatigue. Br J Cancer. 2009;100:311–314. doi: 10.1038/sj.bjc.6604858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassett DR, Jr, Howley ET. Maximal oxygen uptake: “classical” versus “contemporary” viewpoints. Med Sci Sports Exerc. 1997;29:591–603. doi: 10.1097/00005768-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Cooney MT, Vartiainen E, Laatikainen T, et al. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619.e3. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 35.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 36.Hurria A, Balducci L, Naeim A, et al. Mentoring junior faculty in geriatric oncology: Report from the Cancer and Aging Research Group. J Clin Oncol. 2008;26:3125–3127. doi: 10.1200/JCO.2008.16.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: Understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 38.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 39.Mora S, Redberg RF, Cui Y, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: A 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 40.Erikssen G, Liestøl K, Bjørnholt J, et al. Changes in physical fitness and changes in mortality. Lancet. 1998;352:759–762. doi: 10.1016/S0140-6736(98)02268-5. [DOI] [PubMed] [Google Scholar]
- 41.Mann DL, Bristow MR. Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 42.Jones LW, Liang Y, Pituskin EN, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: A meta-analysis. Oncologist. 2011;16:112–120. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]