Abstract
Study Objectives:
To assess the acute effects of SB-649868 in male subjects with Primary Insomnia with regard to (1) objective and subjective sleep parameters, (2) safety and tolerability, (3) next-day residual effects.
Design:
Multicenter, randomized, double-blind, placebo-controlled crossover study using a complete set of Williams orthogonal Latin Squares
Setting:
9 sleep centers in Germany
Patients:
52 male subjects with a diagnosis of primary insomnia (difficulty in sleep initiation and maintenance) confirmed by polysomnography
Interventions:
SB-649868 (10, 30, 60 mg) and placebo administered after dinner 90 minutes before bedtime
Measurements and Results:
Sleep effects assessed by polysomnography during 2 consecutive nights and by sleep questionnaires completed by subjects after each night at the sleep laboratory. Safety and tolerability were assessed by adverse events collection, electrocardiogram (ECG), vital signs, laboratory tests. Next-day residual effects were assessed by Digit Symbol Substitution Test, and modified Verbal Learning Memory Test administered at “lights on” after night 2. SB-649868 significantly reduced latency to persistent sleep, wake after sleep onset (WASO), and increased total sleep time (TST) compared to placebo. A dose-dependent effect was observed. A dose-dependent increase in absolute and percent REM sleep and reduction in REM sleep latency was observed mainly at the 60-mg dose. SB-649868 was well tolerated with inconsistent next day residual effects. SB-649868 sleep effects were correlated with SB-649868 circulating levels.
Conclusion:
The data demonstrate the sleep-promoting properties of the orexin antagonist SB-649868 in male patients with insomnia.
Clinical Trials Information:
This study was registered on ClinicalTrials.gov with the following identifier: NCT00426816. URL: http://www.clinicaltrial.gov/ct2/show/NCT00426816?term=649868&rank=5.
Citation:
Bettica P; Squassante L; Zamuner S; Nucci G; Danker-Hopfe H; Ratti E. The orexin antagonist SB-649868 promotes and maintains sleep in men with primary insomnia. SLEEP 2012;35(8):1097-1104.
Keywords: Orexin, hypocretin, SB-649868, insomnia, polysomnography
INTRODUCTION
Insomnia is the most prevalent sleep disorder; epidemiologic studies show that prevalence of primary insomnia ranges from 1% to 2% of the population, but increases up to 22% of the population if one considers insomnia comorbid to other medical conditions.1 Although, insomnia can be only transient, in most cases it tends to become chronic prompting patients to ask for treatment. Behavioral therapy and pharmacotherapy are both used to treat patients with chronic insomnia. Until recently the only approved pharmacological treatments for insomnia were acting on the GABA system by either binding to the benzodiazepine ligand site of the GABA receptor or by modulating the GABA receptor directly.2 More recently, melatonin slow release has been approved for the treatment of a subset of patients with insomnia and ramelteon (a melatonin agonist) has been approved for the treatment of patients with impaired sleep induction.3 Although these treatments are effective, there is still need for improved drugs that can cover a broader population of patients with insomnia, i.e., patients with impaired sleep induction, impaired sleep maintenance, or both, and which do not have any of the limitations current treatments may have, such as next day residual symptoms, tolerance, dependence, restoration of a physiological sleep profile, and muscle relaxation.
A large body of evidence supports the role of orexins (also known as hypocretins) in controlling arousal and sleep/wake states.4 Intracerebroventricular injection of orexin-A in rats was initially found to increase locus coeruleus (LC) firing as well as locomotor activity, and rearing and grooming behavior.5 Further studies using electroencephalography (EEG) and electromyography (EMG) recordings in rats demonstrated that the administration of orexin-A at the beginning of the normal sleep period increased the time spent awake and decreased both paradoxical (rapid eye movement, REM) and slow wave (deep) sleep.6 In addition to activating LC neurones, orexin-A was subsequently found to increase the neuronal firing of serotonergic raphe neurones7 and of the histaminergic cells of the tuberomammillary nucleus,8 both of which are postulated to play important roles in the maintenance of wakefulness.9
Additional evidence for the role of orexin-A in the maintenance of wakefulness is provided by studies in knockout (KO) mice. KO mice devoid of the orexin precursor prepro-orexin have exhibited a behavioral phenotype similar to human narcolepsy. Similar effects have been observed in orexin 1 (OX1) KO, orexin 2 (OX2) KO, and dual OX1/OX2 KO mice.10) Some types of human narcolepsy have been associated with a loss of orexin activity and reduced orexin levels in the cerebrospinal fluid, further indicating that reduced orexin function in humans can produce decreased wakefulness and an increase in propensity to sleep.11 Taken together, these lines of evidence support the investigation of compounds that target orexin receptors as novel therapeutic targets for sleep and other disorders.
SB-649868 is a potent, orally acting, selective OX1/OX2 receptor antagonist under investigation for the treatment of insomnia. Studies in rodent and primate models have demonstrated sleep-promoting effects and lack of motor impairment following administration of SB-649868.12 In healthy volunteers, SB-649868 was shown to be safe and well tolerated at doses up to 80 mg, with mechanism-related adverse events (e.g., somnolence and fatigue) observed in a majority of subjects after 60 and 80 mg single doses.13 Evening administration of doses up to 60 mg to healthy volunteers without sleep complaint and under normal sleeping conditions resulted in significant dose-dependent decrease in latency to persistent sleep, and wake after sleep onset, and increase in total sleep time as measured by polysomnography.13
In this report, we present the results of a recent Phase IIa study which was conducted to evaluate the effect of SB-649868 on sleep in a male population affected by primary insomnia. The primary objective of the study was to evaluate the acute effect of SB-649868 on sleep parameters as determined objectively by polysomnography (PSG). Other clinical endpoints of the study were daytime cognitive functioning on the morning following dosing, assessed by tests of alertness, memory, attention and fine motor control, and subjective sleep quality using self-reported post-sleep questionnaires. In addition, SB-649868 blood samples were collected to gather relevant pharmacokinetic (PK) characteristics of SB-649868 in subjects with primary insomnia, and to investigate the concentration-response relationship on key sleep parameters.
MATERIALS AND METHODS
This was a multicenter, randomized, double-blind, placebo-controlled, crossover study (GSK protocol 107714) using a complete set of Williams orthogonal Latin Squares. The study was approved by the German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) and by the competent ethics committees. After signing the informed consent, subjects with primary insomnia who met all the inclusion and exclusion criteria then participated in 4 separate 2-night polysomnography (PSG) sessions in which they were randomized to receive placebo or SB-649868 (10 mg, 30 mg, or 60 mg), one treatment for each session, in a balanced order. Each session was separated by a minimum of 1 week and occurred on the same day of the week (± 1 day).
Potential subjects who had signed the informed consent participated in a screening period consisting of a clinical screening visit and 2-night PSG recordings in the sleep laboratory. Subjects were eligible for inclusion in this study if they were males aged 18 through 64 years (inclusive), if they suffered from primary insomnia as defined by Diagnostic and Statistical Manual of Mental Disorders-Text Revision (DSM-IV-TR) criteria 307.42, if their apnea-hypopnea index and their periodic leg movement arousal index were < 10/h on screening PSG, and if on the 2 nights screening PSG their mean total sleep time (TST) ranged between 240 and 420, their mean latency to persistent sleep (LPS) was ≥ 30 min, but not < 20 min on either night, and their mean wake after sleep onset time (WASO) was ≥ 30 min, with neither night < 20 minutes.
Subjects' time in bed had to be between 6.5 and 9 h for ≥ 5 nights per week over the preceding 3 months, and their bedtime had to be between 21:00 and 24:00, with variations no more than 1 hour. Subjects were not eligible for the study if on nightshift or rotating-shift work within the last 2 work weeks or during the study period, or if they planned to travel across > 3 time zones during the study or in the 2 weeks prior to screening.
The subjects enrolled in the study had to be in good health as determined by medical and psychiatric history, physical examination, electrocardiogram (ECG), serum chemistry, hematology, and urinalysis results.
Subjects were excluded from this study if they had any clinically significant unstable medical or surgical condition (treated or untreated); any psychiatric disorder other than primary insomnia as defined by DSM-IV-TR; any history of a clinically significant hepatic, cardiac (e.g., myocardial infarction), renal, neurologic (e.g., seizures), cerebrovascular, metabolic, or pulmonary disease; and any clinically significant abnormalities in hematology, blood chemistry, ECG, urinalysis, physical exam, vital signs, or other protocol-specified screening test which were not resolved by the baseline visit. Known seropositivity for HIV, hepatitis B, or hepatitis C was also exclusionary.
Subjects were allowed into the study if they agreed to use protocol-specified methods of contraception or practice sexual abstinence, as appropriate; consumption < 300 mg per day on average of xanthine-containing beverages (e.g., coffee, cola, tea, chocolate) over the preceding 1 month (NOTE: 12 oz soda " 50 mg, 7 oz coffee or 2 oz espresso " 100 mg, 7 oz tea " 75 mg of caffeine); smoking < 1 pack of cigarettes per day on average; and typical consumption < 14 alcoholic units in any week or < 5 alcoholic units in any single day [NOTE: 1 unit = 8 oz beer, 3 oz wine, or 1 oz hard liquor].
Use of any psychotropic medications or other medications, including over-the-counter and herbal products, within 1 week or 5 half-lives (whichever was longer) prior to screening or need to use any of these medications at any time during the study were exclusionary, as was the use of any investigational drug within 30 days or 5 half-lives of the study compound prior to the Screening Visit. Subjects were excluded if positive at urine drug screen (amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, or opiates).
Subjects were required to report to the sleep laboratory in sufficient time to complete the pre-dose assessments and to maintain the appropriate sleep schedule. The itinerary for each evening of the treatment session was as follows:
20:30 Subjects received a standard dinner
21:00 Administration of placebo or SB-649868 dose
22:00 Subjects retired to bed, 12-lead ECG, PK sampling
22:30 PSG recording started, perfusor connected and tested, “lights-off”
During all 4 treatment sessions, subjects remained at the site from the moment they were admitted before the first night session until all activities after the second night session were completed and the subjects were considered fit to leave the site by the Investigator.
During the 1 week between sessions, no other drugs were allowed (particularly no hypnotics). No changes of lifestyle or extreme exercise were allowed.
Approximately 60 min after the double-blind study-drug administration, the following assessments were performed: 12-lead ECG and semi-supine vital signs (blood pressure, heart rate, respiratory rate, oral temperature). After they retired to bed nocturnal PSG and PK blood sample collection were performed. In the morning upon awakening (8 h after “lights-off”) the following procedures were performed by the subjects: post-sleep questionnaire (about 25 min, but not before 15 min after “lights-on,” lasting 5 min), psychometric testing (35 min [ ± 15 min] after “lights-on”)—Digit Symbol Substitution Test (DSST), modified Verbal Learning Memory Test (VLMT), VAS for sleepiness/alertness—question about the kind of treatment they received (unmasking/masking effects), physical examination, 12-lead ECG (approximately 10 h after double-blind dose), semi-supine vital signs, PK blood sample (10 h from time of dosing the night before), adverse events inquiry, Romberg test, heel-to-toe test, and clinical laboratory tests.
The following clinical laboratory tests were conducted during each visit at the sleep lab, before treatment and at discharge, and then at the follow-up visit: alkaline phosphatase, transaminases, albumin, bicarbonates, bilirubin total, BUN, calcium, creatinine, gamma glutamyltransferase, glucose, protein total, potassium, sodium, total cholesterol, total triglycerides, LDH, uric acid, complete hematology, and urinalysis.
Polysomnography
This multicenter clinical trial was performed at 9 sites in Germany. Sleep was assessed by PSG from which objective measures of sleep have been obtained by blinded experienced scorers at the central scoring site at the Competence Center of Sleep Medicine (Charit́e University Medicine, Berlin). Prior to each study site initiation, sites had to send a PSG recording with a set-up for the first screening night for certification and standardization. Recording of the first screening night comprised 4 electroencephalographic (EEG) channels (C3A2, C4/A1, O1/A2, and O2A1), 2 electroculographic (EOG) leads, 2 electromyographic (EMG) leads (mental and submental), 1 ECG channel, 2 tibialis EMG channels (left and right leg), an oral and/or nasal respiratory flow measurement channel as well as a chest and an abdominal motion measurement channel, and a channel to record oxygen saturation. This PSG was used to exclude subjects with sleep related breathing disorders or periodic leg movements in sleep (PLMS) or both. Subjects without such breathing disorders or PLMS returned for the second screening PSG night if the LPS was ≥ 20 min, WASO was ≥ 20 min, and TST was between 240 and 420 minutes. Recording of the second screening night and the treatment nights included all channels except the breathing and the 2 tibialis EMG channels. PSG was recorded for 8 h, starting around 22:30. All PSGs were scored visually at the central scoring lab based on 30-sec intervals (epochs) according to Rechtschaffen and Kales criteria.14
The primary efficacy endpoints were TST, defined as duration of REM plus NREM (stage 1, stage 2, stage 3, and stage 4) sleep from “lights off” to “lights on”, WASO defined as number of wake epochs from persistent sleep onset to “lights on” divided by 2, and LPS defined as time between “lights off” and the first epoch of 20 consecutive non-wake epochs including stage 1 (persistent sleep onset).
Secondary endpoints were time (min and % of TST) spent in stages 1, 2 and 3 or 4 (slow wave sleep, SWS) of NREM sleep and time (min and % of TST) spent in REM sleep. The duration in minutes was calculated as number of epochs of the respective stage from “lights off” to “lights on” divided by 2. Sleep efficiency (SEI defined as (TST/TIB) *100, with TIB: Time in Bed as duration of the recording from “lights off” to “lights on”) and latency to REM sleep (REML defined as latency from persistent sleep to the first epoch of REM) were also considered as secondary endpoints.
Pharmacokinetics
Serial blood samples for pharmacokinetic analyses of plasma SB-649868 were collected at pre-dose, 1 h, 1.5 h, 3 h, 4 h, 6 h, and 10 h post dose on the first night. PK samples at 1.5, 3, 4, 6, and 10 h post dose were collected using a perfusor which was placed outside the sleep lab to not interfere with the subject's sleep. A cannula 1-1.5 m long connected the perfusor to the subject's arm. PK samples were also collected at pre-dose, 1, 1.5, and 10 h post dose on the second night. Blood samples were analyzed for SB-649868 using a validated analytical method based on protein precipitation, followed by HPLC/MS/MS analysis.15) The pharmacokinetics of SB-649868 were assessed by determining the following pharmacokinetic parameters: AUC(0–t), Cmax and tmax. Plasma concentration-time data were analyzed using standard non-compartmental methods (WinNonlin Professional, Version 4.0.1, Pharsight Corp, Mountain View, CA).
Pharmacokinetics/Pharmacodynamics
A nonlinear mixed effect modelling approach using individual key PSG parameters as LPS, TST, and WASO was applied using NONMEM software, version VI (Globomax Corp.).16 The analysis was conducted on the untransformed score, and the placebo data was included in the model. The concentration response was described using an Emax model in which the placebo effect was used to establish the maximal possible sleep improvements. Plasma concentration before the “lights off” were used for PK/PD analysis of LPS endpoint, while predicted average plasma concentrations during the “lights off” period were used for both WASO and TST PK/PD analyses.
Cognitive Tests
DSST is part of the revised Wechsler Adult Intelligence Scale17 and assesses information processing, concentration, and psychomotor performance. The DSST has a long reputation of usage in detecting residual effects of hypnotic drugs.18,19
VLMT20 is a modified German version of Rey's Auditory Verbal Learning Test21 used in memory research as well as in clinical studies.
Statistics
The study was properly powered according to statistical justification. A sample size of 48 patients completing the study should have provided more than 99% power to detect a difference between SB-649868 and placebo ≥ 22 minutes in TST, assuming a within-subject SD of 22.44 minutes. Power was also expected to be 98% for LPS and 83% for WASO to detect a difference ≥ 11 min for each of these PSG parameters, assuming a within-subject SD of 12.9 min and of 18.2 min, respectively.
Primary statistical analysis was performed on the average of the 2 nights primary PSG endpoints (TST, WASO, LPS) collected at each treatment period. A mixed effect model was applied, with period and treatment as fixed effects and subject as random effect. Estimates for mean treatment differences of each active dose from placebo were derived. Similar statistical models were applied for the other PSG endpoints.
At each treatment period, mean values over the 2 consecutive nights were derived and analysed for post-sleep questionnaire, DSST, and VLMT. Estimates for mean treatment differences of each SB-649868 doses from placebo were derived according to the same mixed model. Derived pharmacokinetic parameters were summarized by SB-649868 dose by means of descriptive summary statistics.
RESULTS
Fifty-two (52) subjects were randomized into the study, and 49 completed. Three subjects withdrew from the study (only 1 subject due to a mild adverse event, headache). Table 1 summarizes the demographics of the study population.
Table 1.
Study population demographics

As reported in Table 2, the administration of SB-649868 was associated with a significantly longer TST than placebo (P < 0.001 at all the doses) and significantly shorter LPS (P < 0.001 at all the doses) and WASO (P ≤ 0.001 for 30 and 60 mg). The estimated differences in (mean of 2 nights) TST versus placebo were +22.4, +49.7 and +69.8 min for the 10, 30, and 60 mg doses, respectively. The estimated differences in (mean of 2 nights) LPS versus placebo were −26.1, −33.0 and −43.7 min, and in (mean of 2 nights) WASO versus placebo were −18.4 and −29.0 min for the 30 and 60 mg doses, respectively. When the PSG changes were analyzed separately for each of the 2 nights of administration, the results were confirmed.
Table 2.
PSG Parameters: summary statistics and estimates of SB-649868 treatment differences from placebo (mean of the two nights)

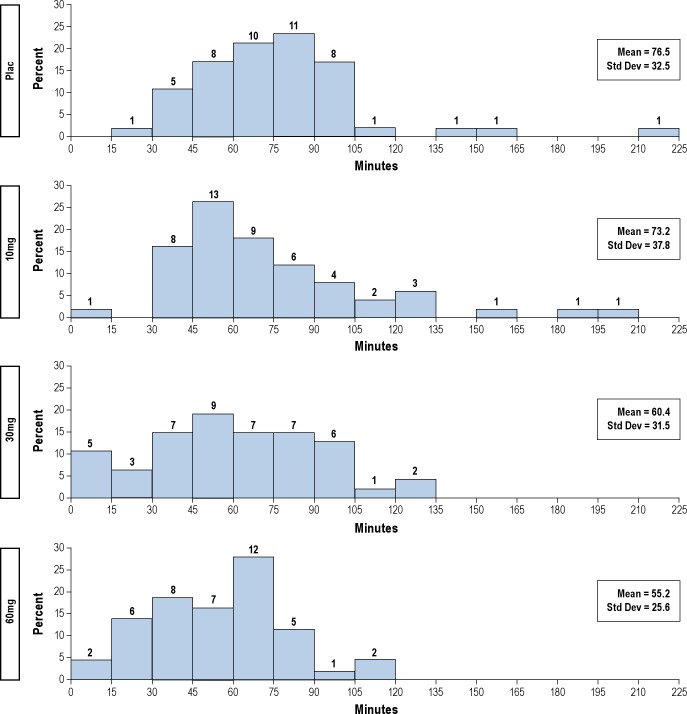
The administration of SB-649868 was associated with a dose-dependent increase of time (minutes) spent in both stage 2 and REM, with no significant differences for the other stages of sleep. When the sleep architecture was analyzed as percentage of TST, NREM stage 1 and SWS were significantly reduced, while REM was dose-dependently increased by the administration of SB-649868 (see Table 2). SB-649868 dose-dependently reduced latency to REM sleep. As shown in Figure 1, none of the patients treated with placebo had REM sleep latencies < 15 min, while 1, 5, and 2 subjects had REM latencies < 15 min after SB-649868 10, 30, and 60 mg, respectively.
Figure 1.
REM sleep latency from persistent sleep to first epoch of REM (on the top of each bar, the label shows the number of observations associated).
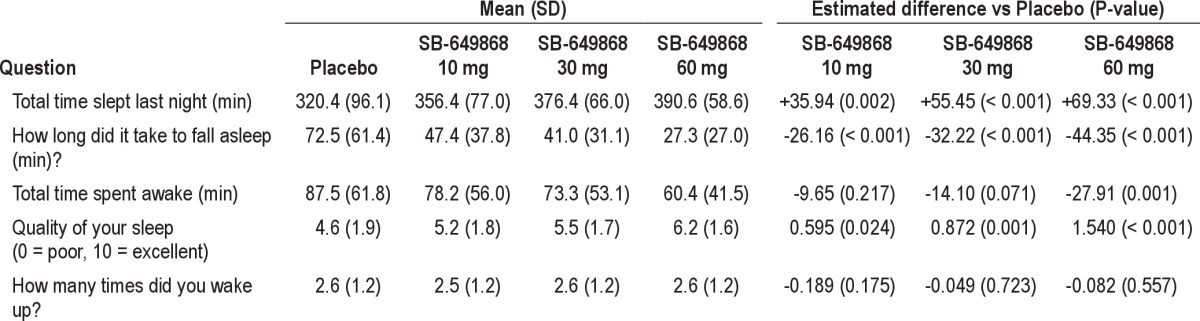
Results of subjective sleep assessments were consistent with those shown with the objective PSG measures. Moreover, SB-649868 statistically significantly improved sleep quality compared to placebo at every dose (see Table 3).
Table 3.
Post-sleep questionnaire: summary statistics and estimates of SB-649868 treatment differences from placebo (mean of the two nights)
SB-649868 10 mg significantly improved DSST total score, while no effect was seen for the other doses (see Table 4). The number of correctly recalled words was significantly reduced by SB-649868. When immediate and delayed recall were assessed, only the immediate correct recall was significantly affected by the SB-649868 30 mg dose (see Table 4).
Table 4.
Cognitive tests: summary statistics and estimates of SB-649868 treatment differences from placebo (mean of the two nights)

PK and PK/PD Results
Summary statistics of SB-649868 PK parameters are presented in Table 5. PK results in insomnia patients are consistent with results in healthy volunteers.13 Inter-subject variability for SB-649868 PK parameters was generally low to moderate, ranging between 30% and 56% across the different treatments and different derived parameters.
Table 5.
Pharmacokinetic parameters following single doses (10, 30, and 60 mg) of SB-649868

In insomniacs, PK-PD analysis demonstrated a consistent and highly statistically significant concentration-response relationship for LPS, TST, and WASO. Results from PK-PD analyses together with the Emax model equations are reported in Table 6. Maximal effects for the 3 Emax models were established using the placebo response. For the TST model, the theoretical maximal SB-649868 effect was estimated to be equal to the difference between the placebo response and the maximal time available for sleep during the study nights (i.e., 480 min). Model equation describing the WASO effect accounted for a minimum threshold effect (WASO min) that resulted to be approximately 5 min in our subject population. For all 3 models, the distribution of placebo response was found to be different between the 2 consecutive nights (P < 0.0001).
Table 6.
Population PK-PD parameters estimated (mean and SD) for TST, LPS, and WASO

Safety Results
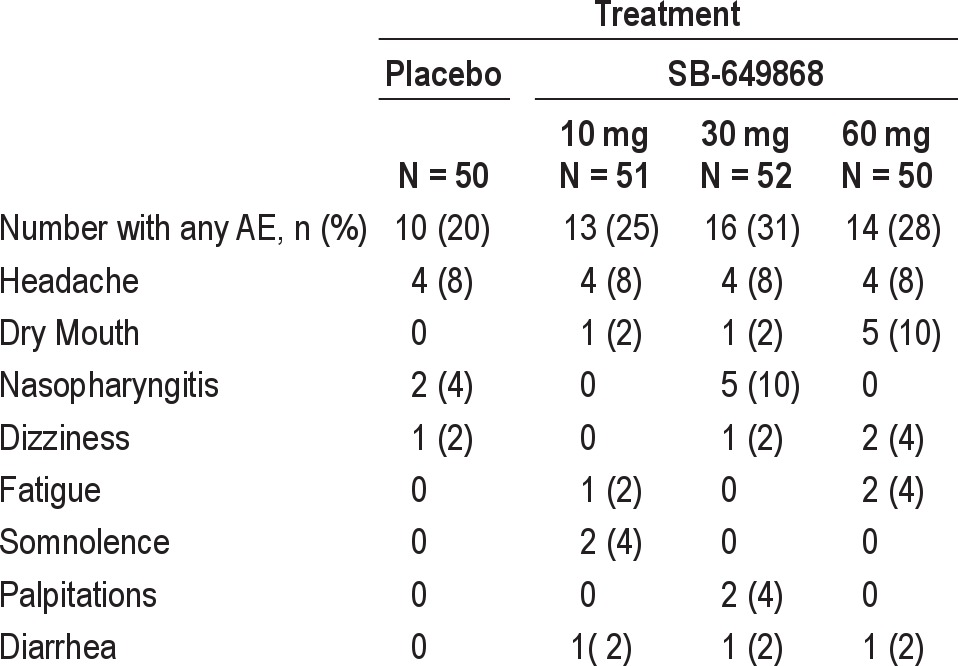
A total of 66 AEs were reported by 31 subjects (59.6%) after dosing. The most common treatment emergent AEs, i.e., those reported by more than one subject in any treatment group are summarized by dose in Table 7. Overall, headache was the most commonly reported AE and the most common AE reported in the placebo and SB-649868 10 mg groups. Nasopharyngitis was the most common AE in the 30 mg group, and dry mouth was the most common AE in the SB649868 60 mg group. There appeared to be a dose-dependent increase in the incidence of dry mouth. No significant changes were observed in vital signs, ECG, or laboratory tests.
Table 7.
Number (%) of subjects with most common (i.e., in more than 1 subject) treatment emergent adverse events
DISCUSSION
In the present study we have shown that SB-649868, an OX1/OX2 dual antagonist in development for the treatment of insomnia, significantly improves sleep induction and sleep maintenance in male patients with primary insomnia. The study also shows that the drug is well tolerated and that drug circulating levels correlated significantly with the hypnotic effect.
Treatment with SB-649868 led to an increase in TST ranging from 22 up to almost 70 minutes, depending on the dose used. In a meta-analysis to assess the hypnotic effect of benzodiazepines, non-benzodiazepine GABA modulators, and antidepressants, Buscemi and colleagues22 showed that the average change in PSG-assessed TST induced by these 3 classes was 32.7, 11.4, and 79.6 minutes, respectively. Although this meta-analysis combined randomized clinical trials of different treatment duration, while this study assessed just the acute effect of treatment, it is fair to say that the effects observed with SB-649868 in this study are at least comparable to those reported for other pharmacological treatments of insomnia. The effect on TST was a combination of SB-649868 effects on sleep induction (significant at every dose) and sleep maintenance (significant at 30 and 60 mg). Also, in the case of sleep induction and sleep maintenance, the effects observed with SB-649868 were at least comparable to those observed with other pharmacological treatments of insomnia.21
The improvements in sleep induction and sleep maintenance observed at PSG were in line with those reported by the patients in their sleep questionnaires. Moreover, SB-649868 significantly and dose-dependently improved sleep quality. Although data are not fully consistent, different studies have suggested a modest correlation between subjective and objective effects of pharmacological treatments for insomnia, with an overestimation of the effects on sleep induction and an underestimation of those on sleep maintenance.23 In this study we show a very good correspondence between objective and subjective assessments; these data could suggest that the beneficial effects of SB-649868 are well perceived by the patient, further supporting the clinical relevance of these effects.
Three different doses of SB-649868 were tested in this study. A clear dose and concentration-dependent effect was observed, with all doses significantly improving sleep induction (LPS) while only the intermediate (30 mg) and high (60 mg) dose significantly improved sleep maintenance (WASO). In addition, the developed PK-PD models implied that the magnitude of SB-649868 response was also linked to the placebo response: more severely affected subjects were more likely to have bigger improvements in key PGS parameters. Results of the PK/PD analysis provided additional insight about the concentrations required to induce sleep and those required to maintain sleep. In accordance to the results from the statistical analysis, higher concentrations were needed to maintain sleep than to induce it. The stronger effect on LPS may be an artifact of the timing of administration in this study (see below for discussion); however, since the PK/PD analysis also showed that EC50 for LPS was approximately 2-3 fold lower than for WASO, this result may be real and thus deserves further exploration.
Small but significant reductions in percentage TST spent in stage 1 sleep and in slow wave sleep were observed, while SB-649868 dose-dependently increased percentage TST spent in REM sleep. In absolute terms, however, SB-649868 dose-dependently increased the minutes spent in stage 2 and in REM sleep and reduced REM latency, while the other sleep stages were not affected. Our comprehension of the role of sleep stages is still incomplete. SWS is believed to be mainly under a homeostatic control and is thought to contribute to the recovery processes that occur during sleep.24 REM sleep is believed to be important for memory consolidation.25 In particular, it is still unclear what is the absolute and/or percentage time in SWS or REM required to maintain the beneficial effects of sleep. However, these considerations are based mainly on studies of sleep deprivation in healthy individuals.26,27 The relevance of these results to long term changes of some of the sleep stages in insomnia patients still remains unclear, also considering that the effects of SB-649868 on SWS may be simply an artifact.
A relatively small number of subjects reported adverse events both after placebo and after SB-649868. Most of the adverse events occurred in too few subjects to draw any conclusion. Headache was the most commonly reported AE followed by dry mouth and nasopharyngitis. A clear dose-dependence was observed for dry mouth. Such an AE is not uncommon after treatment with strong hypnotics. The risk of narcolepsy/cataplexy is always a concern when using pharmacological treatments which inhibit the action of orexins, based on preclinical and human data suggesting a deficiency of the orexin system in narcolepsy.11 Hallucinations, sleep paralysis, and cataplexy—symptoms which are common in narcolepsy/cataplexy (Narcolepsy fact sheet, National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders)—have not been reported. However, this risk cannot be completely excluded, since SOREM (sleep onset REM, i.e., REM episodes within 15 min from persistent sleep28 episodes were reported only after the administration of SB-649868. Future studies will be needed to assess the risk of SB-649868 to induce narcoleptic symptoms. No effects of SB-649868 were detected in a thorough battery of laboratory tests assessed at different time-points in this study or on multiple assessments of vital signs and ECG.
The assessment of next day residual effects gave mixed results. The only clear effect observed was a reduction in the number of correct words recalled at the end of the test (delayed recall). The effects on the other parameters were either absent or inconsistent (i.e., observed only at one dose). Next day residual effects have been tested after the administration of SB-649868 in healthy volunteers in normal sleeping conditions (doses 10, 30, and 60 mg)13) and undergoing a model of situational insomnia (doses 10 and 30 mg).29 In both studies next day residual effects were not observed. Future studies are needed to assess appropriately the risk of next day residual effects after treatment with SB-649868.
Although the current study provides good evidence about a clinically relevant hypnotic effect, some limitations were present. Only men were enrolled in the study since no data on reproductive toxicology of SB-649868 were available at the time of study start. Gender differences have been shown previously for hypnotics.30 The time of administration of the drug in this study was 90 minutes before bedtime. This timing, which is relatively unusual for an insomnia treatment, was selected to make sure that significant concentrations of SB-649868 were present once subjects retired to bed. The formulation of SB-649868 used in this study was very preliminary and did not address the slow rate of absorption and relatively high concentration variability observed previously.13 The administration after food was also aimed at improving the rate of compound absorption. This timing of administration, however, may have affected some results either favorably (LPS and next-day residual effects) or unfavorably (SOREM). Cataplexy-like symptoms were not assessed in this study. Finally, this was the first study conducted in patients; therefore, efficacy was tested only in acute conditions.
In conclusion, this study has shown that SB-649868 is well tolerated and induces clinically significant dose-dependent improvements in both sleep induction and sleep maintenance in subjects with primary insomnia.
DISCLOSURE STATEMENT
This manuscript describes investigational use of the experimental compound SB-649868. This study was sponsored by GlaxoSmithKline. Drs. Bettica, Squassante, Nucci, Zamuner, and Ratti were full-time employees of GlaxoSmithKline at the time of study. The other author has indicated no financial conflicts of interest.
REFERENCES
- 1.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–94. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson SA, Rajaratnam SM, Dawson D. Melatonin agonists and insomnia. Expert Rev Neurother. 2010;10:305–18. doi: 10.1586/ern.10.1. [DOI] [PubMed] [Google Scholar]
- 4.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 5.Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piper DC, Upton N, Smith MI, et al. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci. 2000;12:726–30. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown RE, Sergeeva O, Eriksson KS, et al. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–9. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson KS, Sergeeva O, Brown RE, et al. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saper CB, Fuller PM, Pedersen NP, et al. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;20(98):437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Zeitzer JM, Mignot E. The hypocretins and narcolepsy. In: Lecea L, Sutcliffe JG, editors. Hypocretins: integrators of physiological functions. New York: Springer; 2005. pp. 233–52. [Google Scholar]
- 12.Gerrard PA, Porter RA, Holland V, et al. Preclinical pharmacology of SB-649868: a novel orexin OX1/OX2 receptor antagonist possessing potent hypnotic activity in rodents and primates. Sleep. 2009;32:A42. [Google Scholar]
- 13.Bettica P, Nucci G, Pyke C, et al. Phase I studies on the safety, tolerability, pharmacokinetics and pharmacodynamics of SB-649868—A novel dual orexin receptor antagonist. J Psychopharmacology. 2011 Jul 5; doi: 10.1177/0269881111408954. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A manual for standardized terminology, techniques and scoring system for sleep stages on human subjects. Washington DC: National Institute of Health; 1968. Publication 204. [Google Scholar]
- 15.Renzulli C, Nash M, Wright M, et al. Disposition and metabolism of [14C]SB-649868 an orexin 1 and 2 receptor antagonist in humans. Drug Metab Dispos. 2011;39:215–27. doi: 10.1124/dmd.110.035386. [DOI] [PubMed] [Google Scholar]
- 16.Beal SL, Sheiner LB. NONMEM user's guides. San Francisco: NONMEM Project Group, ; 1998. [Google Scholar]
- 17.Wechsler D. Wechsler Adult Intelligence Scale-Revised. Manual. New York: Psychological Corporation, ; 1981. [Google Scholar]
- 18.Jansen AA, de Gier JJ, Slangen JL. Diazepam-induced changes in signal detection performance. A comparison with the effects on the Critical Flicker-Fusion Frequency and the Digit Symbol Substitution Test. Neuropsychobiology. 1986;16:193–7. doi: 10.1159/000118325. [DOI] [PubMed] [Google Scholar]
- 19.Danjou P, Paty I, Fruncillo R, et al. A comparison of the residual effects of zaleplon and zolpidem following administration 5 to 2 h before awakening. Br J Clin Pharmacol. 1999;48:367–74. doi: 10.1046/j.1365-2125.1999.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmstaedter C, Durwen HF. The Verbal Learning and Retention Test. A useful and differentiated tool in evaluating verbal memory performance. Schweiz Arch Neurol Psychiatr. 1990;141:21–30. [PubMed] [Google Scholar]
- 21.Rey A. L'examen psychologique dans les cas d'enćephalopathie traumatique. Paris: Presses Universitaires de France, ; 1964. [Google Scholar]
- 22.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–33. [PMC free article] [PubMed] [Google Scholar]
- 24.Dijk DJ. Slow-wave sleep deficiency and enhancement: implications for insomnia and its management. World J Biol Psychiatry. 2010;11(Suppl 1):22–8. doi: 10.3109/15622971003637645. [DOI] [PubMed] [Google Scholar]
- 25.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 26.Banks S, Van Dongen HP, Maislin G, et al. Neurobehavioral dynamics following chronic sleep restriction: dose-response effects of one night for recovery. Sleep. 2010;33:1013–26. doi: 10.1093/sleep/33.8.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornung OP, Regen F, Schredl M, et al. Manipulating REM sleep in older adults by selective REM sleep deprivation and physiological as well as pharmacological REM sleep augmentation methods. Exp Neurol. 2006;197:486–94. doi: 10.1016/j.expneurol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–21. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 29.Bettica P, Squassante L, Groeger JA, et al. Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology. doi: 10.1038/npp.2011.310. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth T, Lines C, Vandormael K, Ceesay P, et al. Effect of gaboxadol on patient-reported measures of sleep and waking function in patients with Primary Insomnia: results from two randomized, controlled, 3-month studies. J Clin Sleep Med. 2010;6:30–9. [PMC free article] [PubMed] [Google Scholar]