Abstract
The development of induced pluripotent stem cell (iPSC) technology has generated enthusiasm about the therapeutic potential of these cells for treating a variety of diseases. However, the evidence that they actually will be clinically useful is limited. Here, we discuss the potential therapeutic applications of iPSCs for treating cancer and other diseases and highlight the current barriers restricting their use.
Induced pluripotent stem cells (iPSCs) are a new type of stem cell that is generated by reprogramming the genome of an adult somatic cell, such as a skin fibroblast, to a pluripotent state. Such iPSCs share many similarities with embryonic stem cells (ESCs). Reprogramming of adult somatic cells to iPSCs requires certain pluripotency factors, including the transcription factors Oct4, Sox2, and Klf4 (1). These iPSCs are able to renew themselves indefinitely and to differentiate into many different cell types, including pancreatic-β cells, liver hepatocytes, cardiomyocytes, hematopoietic cells, and dopaminergic neurons. Consequently, there has been much enthusiasm about using iPSCs to generate tissues to treat a variety of diseases, including diabetes, liver cirrhosis, leukemia, and Parkinson’s disease, but ultimately how useful these cells will be for regenerative medicine is still not clear (2).
The first clinical trials to use human ESCs, which are closely related to human iPSCs, have just begun in patients with retinal disease and severe spinal cord injury (2). The goal of these early trials is to establish safety, hopefully with a hint of efficacy. If ESCs are established as a safe cellular therapy, then this will provide a foundation for clinical trials using human iPSCs. Unlike human ESCs, which must be prepared from human embryos, iPSCs can be reprogrammed from many (perhaps most) types of adult cells (3), thus avoiding the real and perceived ethical and pragmatic issues that arise with human ESCs. In addition, because iPSCs can be derived directly from the patient, iPSC-derived tissues would be genetically identical to tissues of the patient enabling autologous transplantation. In contrast, it would not be feasible to make ESCs for every patient, and so the use of ESCs in regenerative medicine would be limited to allogeneic transplantation.
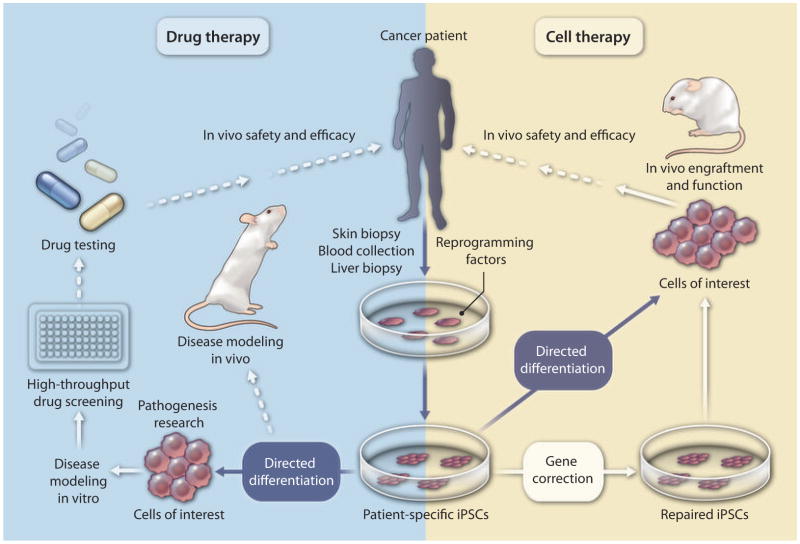
Given that iPSCs do not have the ethical or immunogenic limitations associated with ESCs, they represent a stem cell technology that is more likely to be translatable to the clinic. One of the advantages of iPSCs is that they can be generated from diverse tissues of the human body and methods to improve human iPSC technology now allow the generation of iPSCs from various adult human tissues, including skin, hair, blood, and liver (3). Building on the knowledge obtained from human ESC studies, direct differentiation methods have been implemented to obtain highly specialized cell types from human iPSCs (3). The enthusiasm of scientists for using human iPSCs is due in part to the fact that nuclear reprogramming of adult human cells generates iPSCs endowed with the unlimited potential to reconstruct genetically identical tissues. This biomedical tool offers unprecedented opportunities to develop scalable, yet personalized cell-based therapy for patients with a variety of different diseases. Not only could a patient’s iPSCs be used to generate cells for transplantation to repair damaged tissue (for example, transplanting iPSC-derived liver cells to repair or regenerate injured liver), but also the differentiated progeny of such cells could be used to screen candidate drugs to treat the disease (Fig. 1). In addition, by focusing on diseases of unknown or unclear etiology, iPSC technology offers a robust platform to efficiently dissect molecular and cellular pathophysiology for the purposes of developing diagnostic, therapeutic, and preventive applications (Fig. 1).
Fig. 1. Roadblocks to translating human iPSC technology to the clinic.
Human iPSC technology potentially can be used for screening new cancer drugs (blue box) and ultimately for providing cells for transplant to treat a variety of diseases, including cancer (yellow box). Genetic mutations can be corrected in patient-derived iPSCs by gene targeting approaches. The main hurdles to using patient-specific iPSCs for disease modeling, drug screening, and transplantation purposes are (i) a lack of effective differentiation protocols, (ii) little or no engraftment capability for the majority of human iPSC-derived specialized cells, (iii) difficulties in modeling multifactorial diseases, (iv) the need for GMP-compliant conditions at each step, and (v) safety concerns regarding the potential tumorigenicity of iPSCs associated with their pluripotent state or with insertional- or culture-driven mutagenesis. Dotted arrow, not yet tested; solid arrow, only a few studies available; blue arrow, feasible but requires further study.
POTENTIAL APPLICATION OF iPSCS FOR CANCER TREATMENT
Human iPSCs have been touted as the route to produce a variety of tissues that could be used in regenerative medicine. For example, iPSCs generated from the fibroblasts of type 1 diabetes patients have been induced to differentiate into insulin-producing pancreatic-β cells in vitro (4). In a mouse model of sickle cell anemia, transplantation of hematopoietic progenitor cells derived from autologous mouse iPSCs that had been genetically corrected resulted in rescue of the disease phenotype (5).
But how can the regenerative potential of iPSCs be commandeered to help treat cancer patients? There are two possible scenarios where iPSCs might be of value. First, one could envisage using iPSC-derived tissue to replace or repair tissues of cancer patients that have been injured by radiation, chemotherapy, or the surgical treatment necessary to eliminate the tumors. Because most cancers involve acquired genetic mutations in a specific tissue, iPSCs derived from other healthy tissues of the same patient theoretically could be used to regenerate those tissues damaged by the tumors themselves or subsequent treatments. However, human iPSC-mediated regenerative therapy requires that the iPSC-derived tissue shows robust engraftment in vivo. Unfortunately, only a few human ESC- or iPSC-derived cell types such as ESC-derived dopaminergic neurons or ESC- and iPSC-derived hepatocytes have been shown to engraft successfully in animal models of Parkinson’s disease and liver cirrhosis, respectively (6, 7). So far, many other cell types—such as hematopoietic cells derived from ESCs or iPSCs—that would have been predicted to engraft efficiently in vivo have proved surprisingly recalcitrant to engraftment. Recently, we have demonstrated that hepatic cells derived from iPSCs generated from healthy human donors or from patients with inherited liver disease, liver cirrhosis, or liver cancer were able to engraft the livers of mice treated with a chemical to induce liver injury (7, 8). Up to 30% of the mouse liver was composed of human hepatocytes after transplantation, and the engraftment levels were comparable with those observed after transplant of primary human hepatocytes (7, 8). Although this study is preliminary, it suggests that it may be possible to generate human hepatocytes from iPSCs derived from the nonliver tissue of patients with liver cirrhosis that could then be transplanted into these patients to promote liver regeneration and repair. Such a strategy might also have the potential to prevent patients with liver cirrhosis from progressing to hepatocellular carcinoma.
A second area in which patient-specific iPSCs may offer a distinct advantage for cancer treatment is immune therapy (9). It has been shown that human iPSCs derived from T lymphocytes retain the pre-rearranged T cell receptor (TCR) gene (10–13), suggesting that these iPSCs could be induced to differentiate into functionally active T cells. It will be interesting to see whether a large quantity of functional T lymphocytes carrying specificity against certain tumor antigens could be generated in vitro by reprogramming selected T cell clones into iPSCs and then subsequently differentiating them back into T lymphocytes that could then be infused into the patient. One of the concerns related to this approach, however, is safety. A mouse study has shown that mice derived from a mature T cell reprogrammed by somatic cell nuclear transfer (a procedure in use before the advent of iPSC technology) developed spontaneous T cell lymphomas at high frequency (14). It is currently unclear if this observed phenomenon is intrinsic to T cells bearing pre-rearranged TCRs. Future studies are needed to demonstrate the safety of human iPSCs derived from T cells.
One area where human iPSCs could be very useful for cancer treatment is for screening new drugs. Human iPSCs can be obtained from the cancer cells of patients, and these iPSCs and their progeny would provide cellular targets containing all of the mutations of the patient’s tumor on the genetic background of the patient. The cell types obtained by differentiating patient iPSCs derived from cancer tissues may be more biologically relevant to human tumors than the traditional cancer cell lines, mouse tumors, or mouse xenograft models that are currently used in drug screening. In addition, hepatocytes generated from human iPSCs of different genetic backgrounds will also aid efforts to test the toxicity of candidate cancer drugs—hepatotoxicity is the most common reason that many promising cancer drugs never make it into the clinic (15).
CURRENT HURDLES TO iPSC-BASED CANCER THERAPY
There are many roadblocks that may hinder or delay clinical applications of human iPSCs for treating a variety of diseases, including cancer. Such roadblocks include (i) safety issues related to the generation of iPSCs, (ii) a lack of effective protocols for differentiating human iPSCs into different functional cell types, and (iii) a lack of GMP-compliant protocols for deriving, expanding, and differentiating human iPSCs.
A principal safety concern is insertional mutagenesis associated with the traditional derivation of iPSCs from adult human cells by use of a transcription factor transgene cocktail delivered by retrovirus or lentivirus vectors. This roadblock has been partly addressed by using virus-free and integration-free methods such as episomal vectors or mRNA- or protein-based strategies to reprogram adult human cells; these strategies work with varying efficiencies to generate iPSCs (16–21). Human iPSCs derived by these protocols are free of exogenous DNA sequences, thereby eliminating the potential for insertional mutagenesis and undesired cell behaviors caused by reactivation of reprogramming transgenes. However, just like other processes involving cell division and expansion, genomic mutations are inevitable during reprogramming and the subsequent expansion steps, as has been demonstrated in a recent whole-exome sequencing study (22). This study showed that human iPSC lines, regardless of the reprogramming methods used, contain approximately six protein-coding point mutations per exome. As whole-genome sequencing technology continues to develop and the costs for such studies continue to drop, it will become both feasible and necessary to examine individual human iPSC lines for their genomic integrity to ensure their safety for clinical applications.
Many cell types have been generated from human iPSCs; however, the functionality of most of the human iPSC-derivatives remains unknown and requires extensive testing. Although differentiation protocols have been established for obtaining hematopoietic progenitor-like cells as well as more specialized blood cell types, it has proved a daunting task to generate transplantable hematopoietic stem cells from human ESCs or iPSCs that can home to bone marrow after transplant and engraft successfully (23, 24). In contrast, human ESC- and iPSC-derived hepatic progenitors and mature hepatocytes have been shown to engraft mouse liver tissue after transplant and to be functional (7, 8, 25–28). The functionality of these iPSC-derived liver cells still needs to be improved for them to be comparable with their primary human hepatocyte counterparts. To achieve this goal will require more advanced knowledge about normal liver development. Studies using mouse iPSCs and more recently human iPSCs have suggested that there is epigenetic memory retained in the iPSCs reflecting their derivation from adult somatic (parental) cells and that such molecular memory may influence their potential to differentiate into certain tissues, including hematopoietic cells and pancreatic cells (7, 29–31). Understanding the mechanisms underlying the epigenetic memory (7, 29–31) retained by iPSCs will also benefit efforts to derive functional cell types that are safer and more suitable for therapy.
Currently, most of the reprogramming and differentiation experiments have been conducted as proof-of-principle studies. Translating this research into clinical therapies will require significant efforts to develop GMP-compliant conditions. Clinical grade iPSCs and differentiation reagents need to be developed. Given that it takes several months to generate, expand, and select human iPSCs even before the differentiation process begins, for clinical purposes it may be more practical to establish human iPSC banks that contain a wide range of iPSC lines derived from diverse human lymphocyte antigen (HLA) haplotypes. These banked iPSCs could then be used to produce HLA-compatible tissues and organs for allogeneic rather than autologous transplantation.
TREATING HEMATOLOGICAL MALIGNANCIES WITH ADULT STEM CELLS
Allogeneic blood or bone marrow transplantation (BMT) is the standard-of-care treatment for many hematological malignancies, such as leukemias, that cannot be cured with conventional-dose therapies. Histocompatibility barriers have historically limited the applicability of BMT to those patients who have HLA-matched donors. Transplants of HLA-mismatched bone marrow produce mortality rates in excess of 50%, mostly as a result of graft-versus-host disease (GVHD) (32, 33). Accordingly, upwards of 50% of patients, and the majority of some ethnic groups such as African-Americans (34), in need of allogeneic BMT lack appropriate donor options. The ability to generate hematopoietic stem cells from patient-derived iPSCs could theoretically provide a graft for all in need of BMT. However, the potent immunological allogeneic graft-versus-tumor effect, which is generally the most important antitumor activity of allogeneic BMT, would be lacking if the patient’s own iPSC-derived hematopoietic stem cells were used unless similar anticancer activity could be engineered into these cells. HLA-matched allogeneic iPSCs would be another alternative. On the other hand, recent data indicate that HLA barriers may no longer be a major impediment to the more widespread use of allogeneic BMT. Recent data show that allogeneic BMT from mismatched (haploidentical)–related BMT donors can be just as effective as that from matched siblings, with similar rates of GVHD and survival (35, 36). Importantly, such an approach maintains the allogeneic graft-versus-tumor effect.
WHAT DOES THE FUTURE HOLD?
It is currently difficult to predict whether human iPSCs have real potential in regenerative medicine because in vivo studies reporting engraftment of cells derived from iPSCs are sparse. The two main uses of human iPSC technology would be for drug discovery and cell transplantation (Fig. 1). Using iPSCs for drug development would require establishing patient iPSC-based disease models for testing potential drugs. Pathogenesis research using patient-relevant models of complex diseases such as cancer would help in the discovery of better cellular and molecular targets for drug development. Regenerative therapy using iPSCs will require correction of the genetic defect (when necessary) and in vivo functional testing of these cells. Perhaps a more immediate use of human iPSC-derived cells will be for in vitro screening of candidate antitumor drugs given that improving and evaluating in vivo function for most iPSC-derived cell types is still in the future. Patient iPSC-based drug screening is likely to be particularly beneficial for cancer patients with late-stage inoperable tumors because conventional cancer cell lines and animal models have not been effective for developing drugs that are powerful enough to combat the intra- and intertumor heterogeneity of advanced cancers (37). Although there are many technical hurdles to overcome before establishing iPSC-based tailored therapy for cancer patients, the potential of pluripotent stem cell therapy is great. Together with continued improvements in current cancer therapies, iPSC technology provides an additional therapy to add to the antitumor armamentarium, but much more work needs to be done to demonstrate their true value in the clinic.
Acknowledgments
Funding: Supported by grants CA15396, R21AA020020, and RO1 DK 070971 and by MSCRF grants 2010-MSCRFII-0101-00 and 2011-MSCRE-00-51-00.
REFERENCES AND NOTES
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun YS, Chaudhari P, Jang YY. Applications of patient-specific induced pluripotent stem cells; Focused on disease modeling, drug screening and therapeutic potentials for liver disease. Int J Biol Sci. 2010;6:796–805. doi: 10.7150/ijbs.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 6.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci Transl Med. 2011;3:82ra39. doi: 10.1126/scitranslmed.3002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SM, Kim Y, Liu H, Chaudhari P, Ye Z, Jang YY. Liver engraftment potential of hepatic cells derived from patient-specific induced pluripotent stem cells. Cell Cycle. 2011;10:2423–2427. doi: 10.4161/cc.10.15.16869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown ME, Rondon E, Rajesh D, Mack A, Lewis R, Feng X, Zitur LJ, Learish RD, Nuwaysir EF. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS ONE. 2010;5:e11373. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, Urbach A, Heffner GC, Grskovic M, Vigneault F, Lensch MW, Park IH, Agarwal S, Church GM, Collins JJ, Irion S, Daley GQ. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, Okada Y, Seimiya H, Fusaki N, Hasegawa M, Fukuda K. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serwold T, Hochedlinger K, Swindle J, Hedgpeth J, Jaenisch R, Weissman IL. T-cell receptor-driven lymphomagenesis in mice derived from a reprogrammed T cell. Proc Natl Acad Sci USA. 2010;107:18939–18943. doi: 10.1073/pnas.1013230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, Giacomini KM, Krauss RM. Identifying genetic risk factors for serious adverse drug reactions: Current progress and challenges. Nat Rev Drug Discov. 2007;6:904–916. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SM, Liu H, Chaudhari P, Kim Y, Cheng L, Feng J, Sharkis S, Ye Z, Jang YY. Reprogramming of EBV-immortalized B-lymphocyte cell lines into induced pluripotent stem cells. Blood. 2011;118:1801–1805. doi: 10.1182/blood-2011-03-340620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou BK, Mali P, Huang X, Ye Z, Dowey SN, Resar LM, Zou C, Zhang YA, Tong J, Cheng L. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu K, Yu J, Suknuntha K, Tian S, Montgomery K, Choi KD, Stewart R, Thomson JA, Slukvin II. Efficient generation of transgene-free induced pluripotent stem cells from normal and neoplastic bone marrow and cord blood mononuclear cells. Blood. 2011;117:e109–e119. doi: 10.1182/blood-2010-07-298331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Z, Cheng L. Potential of human induced pluripotent stem cells derived from blood and other postnatal cell types. Regen Med. 2010;5:521–530. doi: 10.2217/rme.10.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H, Yin X, Wu C, Che J, Lu S, Ding M, Deng H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233–1242. doi: 10.1038/cr.2009.107. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Ye Z, Kim Y, Sharkis S, Jang YY. Generation of endoderm-derived human induced pluripotent stem cells from primary hepatocytes. Hepatology. 2010;51:1810–1819. doi: 10.1002/hep.23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J, Ahrlund-Richter L, Skepper J, Semple R, Weber A, Lomas DA, Vallier L. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–3136. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K, Wang G, Daley GQ, Lee JH, Church GM, Forbes SJ, Iredale JP, Wilmut I. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-Nur O, Russ HA, Efrat S, Benvenisty N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, Bradley BA, Casper JT, Flomenberg N, Gajewski JL, Gluckman E, Henslee-Downey PJ, Hows JM, Jacobsen N, Kolb HJ, Lowenberg B, Masaoka T, Rowlings PA, Sondel PM, van Bekkum DW, van Rood JJ, Vowels MR, Zhang MJ, Horowitz MM. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 33.Shaw PJ, Kan F, Woo Ahn K, Spellman SR, Aljurf M, Ayas M, Burke M, Cairo MS, Chen AR, Davies SM, Frangoul H, Gajewski J, Gale RP, Godder K, Hale GA, Heemskerk MB, Horan J, Kamani N, Kasow KA, Chan KW, Lee SJ, Leung WH, Lewis VA, Miklos D, Oudshoorn M, Petersdorf EW, Ringdén O, Sanders J, Schultz KR, Seber A, Setterholm M, Wall DA, Yu L, Pulsipher MA. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors. Blood. 2010;116:4007–4015. doi: 10.1182/blood-2010-01-261958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dew A, Collins D, Artz A, Rich E, Stock W, Swanson K, van Besien K. Paucity of HLA-identical unrelated donors for African-Americans with hematologic malignancies: The need for new donor options. Biol Blood Marrow Transplant. 2008;14:938–941. doi: 10.1016/j.bbmt.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolaños-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, Devine SM, Wingard JR, Aljitawi OS, Cutler CS, Jagasia MH, Ballen KK, Eapen M, O’Donnell PV Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yap TA, Gerlinger M, Futreal PA, Pusztai L, Swanton C. Intratumor heterogeneity: Seeing the wood for the trees. Sci Transl Med. 2012;4:127ps10. doi: 10.1126/scitranslmed.3003854. [DOI] [PubMed] [Google Scholar]