Abstract
Objectives:
Sleep is regulated by circadian and homeostatic processes and is highly organized temporally. Our study was designed to determine whether this organization is preserved in patients receiving mechanical ventilation (MV) and intravenous sedation.
Design:
Observational study.
Setting:
Academic medical intensive care unit.
Patients:
Critically ill patients receiving MV and intravenous sedation.
Methods:
Continuous polysomnography (PSG) was initiated an average of 2.0 (1.0, 3.0) days after ICU admission and continued ≥ 36 h or until the patient was extubated. Sleep staging and power spectral analysis were performed using standard approaches. We also calculated the electroencephalography spectral edge frequency 95% (SEF95), a parameter that is normally higher during wakefulness than during sleep. Circadian rhythmicity was assessed in 16 subjects through the measurement of aMT6s in urine samples collected hourly for 24-48 hours. Light intensity at the head of the bed was measured continuously.
Measurements and Results:
We analyzed 819.7 h of PSG recordings from 21 subjects. REM sleep was identified in only 2/21 subjects. Slow wave activity lacked the normal diurnal and ultradian periodicity and homeostatic decline found in healthy adults. In nearly all patients, SEF95 was consistently low without evidence of diurnal rhythmicity (median 6.3 [5.3, 7.8] Hz, n = 18). A circadian rhythm of aMT6s excretion was present in most (13/16, 81.3%) patients, but only 4 subjects had normal timing. Comparison of the SEF95 during the melatonin-based biological night and day revealed no difference between the 2 periods (P = 0.64).
Conclusions:
The circadian rhythms and PSG of patients receiving mechanical ventilation and intravenous sedation exhibit pronounced temporal disorganization. The finding that most subjects exhibited preserved, but phase delayed, excretion of aMT6s suggests that the circadian pacemaker of such patients may be free-running.
Clinical Trial Information:
Clinicaltrials.gov NCT01276652.
Citation:
Gehlbach BK; Chapotot F; Leproult R; Whitmore H; Poston J; Pohlman M; Miller A; Pohlman AS; Nedeltcheva A; Jacobsen JH; Hall JB; Van Cauter E. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. SLEEP 2012;35(8):1105-1114.
Keywords: Critical illness, intensive care, polysomnography, ventilator, sleep, melatonin, circadian rhythm, sedation, slow wave activity
INTRODUCTION
There is increasing evidence that sleep plays a crucial role, not only for cognitive function, but also for physical well-being, and that sleep disorders may exacerbate an existing illness.1–5 Unfortunately, the sleep of critically ill patients undergoing mechanical ventilation remains poorly characterized, despite the occurrence of nearly 800,000 hospital admissions requiring mechanical ventilation annually in the United States.6 Most studies in this area have been performed in patients receiving little to no intravenous sedation despite the frequent use of sedatives and narcotics in this population.7 There is a high potential for sleep disruption in the intensive care unit (ICU) environment from underlying illness, medications, inappropriate exposure to noise and light, and the ventilator itself.8–13 Progress in this area has been hampered by the logistical and methodological challenges of performing and interpreting continuous polysomnography (PSG) in this population. The electroencephalogram (EEG) in such patients may exhibit a variety of patterns not found during normal sleep due to the effects of acute illness and sedatives and analgesics,8,14,15 limiting the application of standardized sleep staging in this setting.16 While computer-derived quantitative analyses, such as power spectral analysis, may possess certain advantages over visual scoring,16 these tools have not yet been used to characterize the EEG of ICU patients over periods of time longer than 24 hours. Previous studies of sleep in the ICU have also not addressed potential disruptions of circadian rhythmicity, which have been shown to be variably altered in critically ill patients.17–22
Sleep timing, duration and architecture are determined by the interaction of a homeostatic mechanism relating sleep pressure to the duration of prior wakefulness and by an endogenous circadian rhythm. Slow wave activity (SWA; EEG power in the 0.75-4 Hz, or delta, frequency band) is the best accepted marker of the homeostatic process and levels of SWA normally decline across successive NREM-REM cycles across the sleep period. Whether the ultradian rhythmicity (with a 90-100 min period) and homeostatic decline of SWA is partly preserved in ICU patients is not known. It is also not known whether the sleep EEG of such patients reflects the influence of the circadian pacemaker. Certainly sedation and sleep share certain similarities, and studies in laboratory rodents suggest that some sedatives and anesthetics appear to reduce homeostatic sleep pressure,23,24 but there are major differences as well. Conceivably, disorganization of the circadian rhythm and the homeostatic control of sleep in critically ill patients may be associated with a severely sleep deprived state, inhibiting the numerous and varied restorative cellular and endocrine processes that derive from normal sleep.
Our study was therefore designed to simultaneously assess the sleep EEG and circadian rhythmicity to determine whether some of the characteristics of normal sleep would be detectable in acutely ill patients receiving mechanical ventilation and intravenous sedation. Circadian rhythmicity was assessed through a detailed analysis of the temporal profile of aMT6s excretion in urine samples collected hourly,25 while PSG was performed concurrently. Standard sleep scoring, power spectral analysis, and the computation of spectral edge frequency 95% (SEF95), a parameter that is normally higher during wakefulness than during sleep,26,27 were performed on each set of recordings. We further compared SEF95 between usual daytime and nighttime hours to determine if the sleep EEG shifted to lower frequencies during the night, as would be expected. To explore the possibility that the persistence of a diurnal rhythm in SEF95 was masked by shifts of circadian rhythmicity, we also used individual aMT6s profiles to determine the biological night (e.g., the period of elevated melatonin levels) for each individual and compared the SEF95 between the biological night and the biological day.
METHODS
Subjects
The study was designed to simultaneously assess sleep and circadian rhythmicity in critically ill patients. We also explored the feasibility of a non-pharmacological intervention to promote sleep and reinforce circadian rhythms, and explored different study designs for its application and for the analysis of its effect. The intervention combined an effort to consolidate nursing procedures during the daytime and reduce patient exposure to light and sound at night. This intervention was applied in 11 of 25 enrolled patients during a portion of the study and was well tolerated.
Adult (≥ 18 years of age) patients receiving mechanical ventilation in the University of Chicago medical intensive care unit (ICU) were enrolled. Patients were ineligible if they were expected to be extubated within 24 h; if they exhibited clinically apparent central nervous system disease such as cerebrovascular accident, dementia or other neurodegenerative disease, meningitis or encephalitis, intracranial neoplasm, or metabolic or hypoxic encephalopathy; if they had a history of untreated obstructive sleep apnea; or if they were admitted with a confirmed or suspected drug overdose. Patients receiving neuromuscular blocking agents were also excluded. Severity of illness was calculated using the Acute Physiology and Chronic Health Evaluation (APACHE) II Score,28 and all patients were followed to hospital discharge. Sepsis was defined using standard criteria.29 The study was approved by the institutional review board at the University of Chicago, and written informed consent was obtained from participants or their authorized representatives.
Ventilator and Sedative Management
Subjects were ventilated in the assist control mode of mechanical ventilation from 21:00 to 06:00 to minimize the potential for nocturnal sleep disruption due to ventilator mode.10 Continuous sedative and analgesic medications were administered by the treating clinicians according to the medical ICU sedation protocol, a goal-directed protocol that targets the Richmond Agitation Sedation Scale30 and includes a daily sedative interruption.31 Each patient was assessed daily while on-study for the presence of delirium using the Confusion Assessment Method for the ICU.32
Measurements and Analysis
Light exposure
The Actiwatch-Light device (Minimitter Company, Inc) was secured at the head of the bed at the level of the patient’s eyes in the usual direction of gaze in order to measure light intensity in the vicinity of the eyes at a pre-programmed interval of one minute. This device measures ambient light exposure in a range from 0.1 to 150,000 lux.
Assessment of circadian rhythm
Melatonin secretion and the excretion pattern of its metabolite, urinary aMT6s, serve as an accurate and practical “hand of the clock.”25 The levels of aMT6s were measured on urine samples collected at hourly intervals from bladder catheters that were already in place in order to assess circadian phase and amplitude. Specimen collection began the morning after subject enrollment and concluded 24 h later, except in 10 subjects who underwent an additional 24 h of specimen collection in order to investigate the reproducibility of the profile. Samples were not collected from patients who were receiving or considered likely to receive dialysis during the course of the study. Samples were initially frozen at −70°C and subsequently analyzed in batch at a commercial laboratory (Pharmasan Labs, Inc., Osceola, Wisconsin) using a highly sensitive, competitive ELISA kit (IBL Laboratories, Hamburg, Germany). The lower limit of detection of the assay is 1.0 ng/mL. The intra-assay coefficient of variation is 5.2% to 12.2% in the range of 5.8–204 ng/mL. The inter-assay coefficient of variation is 5.1% to 14.9% in the range of 12.4–220 ng/mL.
Analysis of 6-sulfatoxymelatonin profiles
A small number (< 3% of all samples) of values were interpolated or excluded due to missing samples or outlying positions. Several different analyses were performed:
a) Total aMT6s excretion. The total amount of aMT6s excreted by each subject during the usual sleep time hours (23:00 to 07:00) and during the usual wake time hours (07:00 to 23:00) was calculated.
b) Analysis of individual temporal profiles of hourly aMT6s excretion. A 3-point moving average was performed on each subject’s 24-h temporal profile in order to smoothe short-term fluctuations in aMT6s excretion. The acrophase and nadir of each 24-h profile were defined as the time of occurrence of the maxima and minima, respectively. The amplitude of the cycle was defined as 50% of the difference between the values of the acrophase and nadir. The onset of nocturnal melatonin secretion for each subject was estimated as the time when the excretion of aMT6s exceeded the mean of the 3 previous samples by 2 standard deviations.33 The normal timing of peak melatonin excretion is between midnight and 05:00 in adults over 40 years of age. Patients in whom the melatonin onset occurred after midnight or the acrophase occurred after 05:00 were considered to be phase delayed, while patients whose acrophase occurred prior to midnight were considered to be phase advanced. These analyses were based on the first day of data collection, except in one subject in whom day 1 data were deemed unreliable.
c) Analysis of the temporal profile of mean hourly aMT6s excretion for the group. Interindividual differences in the quantity of melatonin secreted during a 24-h period may vary 20-fold34; therefore, temporal variability in the rate of excretion of aMT6s was reported as a percentage of the 24-h mean. This analysis was repeated for the 10 subjects with 48 h of aMT6s data to confirm the reproducibility of the profile.
Polysomnography
Continuous polysomnographic recording was initiated the night of enrollment (Day 0) and continued ≥ 36 h unless the patient was extubated. One exception was a patient who was extubated and then reintubated 15 min later in whom the recording was continued. Day 1 of recording refers to a 24-h period beginning at 09:00 the day after enrollment. The DigiTrace recording system (DigiTrace Care Services, Boston, MA) was used for the first 4 subjects. Subsequently, the Nihon Kohden Polysleep 912-1 portable recording system was used. We recorded ventilator pressure, flow, and volume waveforms; EEG (C3-A1/A2, C4-A1/A2, O1-A1/A2, O2-A1/A2); electrooculography; and electromyography. Most of the subjects were attended by trained research assistants; in the remainder, the hookup was inspected by a registered sleep technologist at least twice daily and periodically by one of the investigators (BKG).
Manual scoring of the EEG
Two investigators (HW and BKG), attempted, in consultation with an expert neurophysiologist and sleep medicine specialist (JHJ), to score all PSG recordings in 30-sec epochs using conventional criteria.35 This extensive review demonstrated a number of pathologic EEG findings precluding accurate classification of this material using conventional criteria (data not shown), findings consistent with the experience of previous investigators.14–16 REM sleep, while scarce, was more readily identifiable when present, in keeping with the findings of Ambrogio et al.16 Accordingly, we focused our efforts on quantitative EEG analysis.
Quantitative EEG procedure
All recordings obtained with the Nihon Kohden system were digitized and submitted to spectral analysis on a central EEG lead using a commercially available electrophysiological recording analyzer software package (PRANA, PhiTools, Strasbourg, France) designed and co-developed by one of the authors (FC). Visual examination of raw recordings indicated the presence of artifacts due to movement, suctioning and other patient care activities, the mechanical ventilator, grimacing, and other unidentified causes. Therefore, an iterative process was used for artifact detection and rejection. First, an automated artifact detection procedure was performed by the software package, and the results inspected. Rejected material was confirmed, after which additional ocular, muscular, and movement artifacts were identified and rejected manually. Power spectra were obtained by a fast Fourier transform algorithm using 4-sec overlapping windows. A Hanning window was used to minimize leakage from adjacent frequencies. Elementary spectra of each 30-sec epoch were then averaged to obtain one mean spectral estimate for every scoring epoch. Power in each EEG frequency band was obtained by summing the spectral power of the corresponding 0.25 Hz frequency bins, the lower frequency bin being included and the upper one excluded. Frequency bins were defined as follows: delta (0.75–4.5 Hz), theta (4.5–8.5 Hz), alpha (8.5–12.5 Hz), sigma (12.5–15.5 Hz), beta (15.5–22.5), and gamma (22.5–45.5 Hz). We then used individual spectral power profiles as an aide to identifying and eliminating residual artifacts prior to performing analyses of absolute delta power or the SEF95. After these extensive artifact-removal procedures, the median amount of recorded material available for quantitative analysis averaged 70% across subjects.
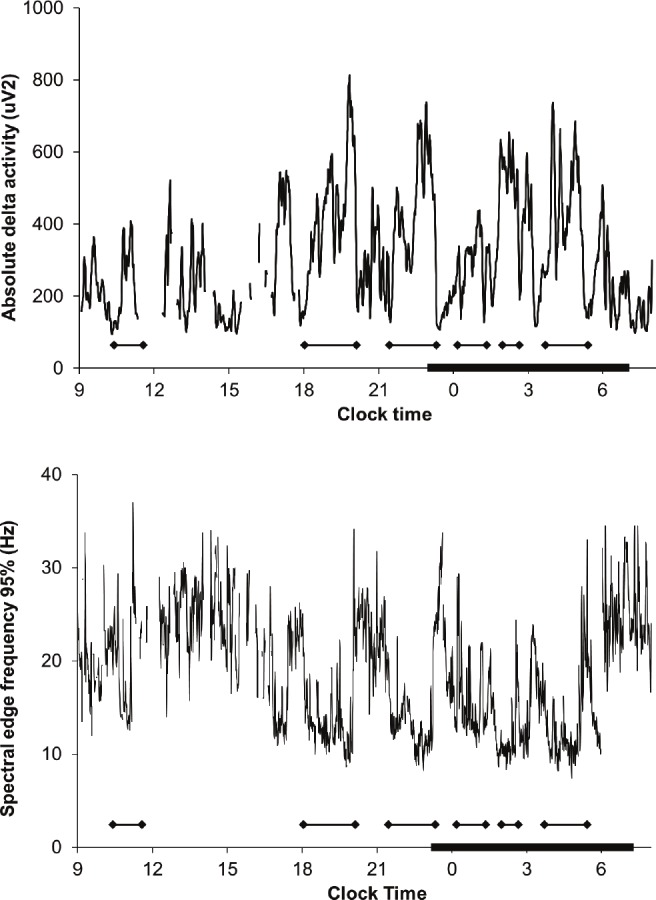
In addition to conventional EEG power spectral analysis, we also calculated the SEF95 for each 30-sec epoch in an effort to minimize the contribution of residual high-frequency power due to artifacts. The SEF95 is defined as the frequency below which 95% of the spectral power resides. Under normal conditions, the SEF95 is higher during wakefulness (> 20 Hz) than during sleep (approximately 10 Hz or less during slow wave sleep), and the SEF95 of REM sleep is similar to that of wakefulness.26,27 The profiles of SEF95 data were smoothed using a 3-point moving average. Figure 1 shows typical temporal profiles of absolute delta activity and SEF95 from a healthy subject. For each subject, we determined the average SEF95 for the entire duration of recording as well as for usual wake time hours (07:00-23:00) and usual sleep time hours (23:00-07:00).
Figure 1.
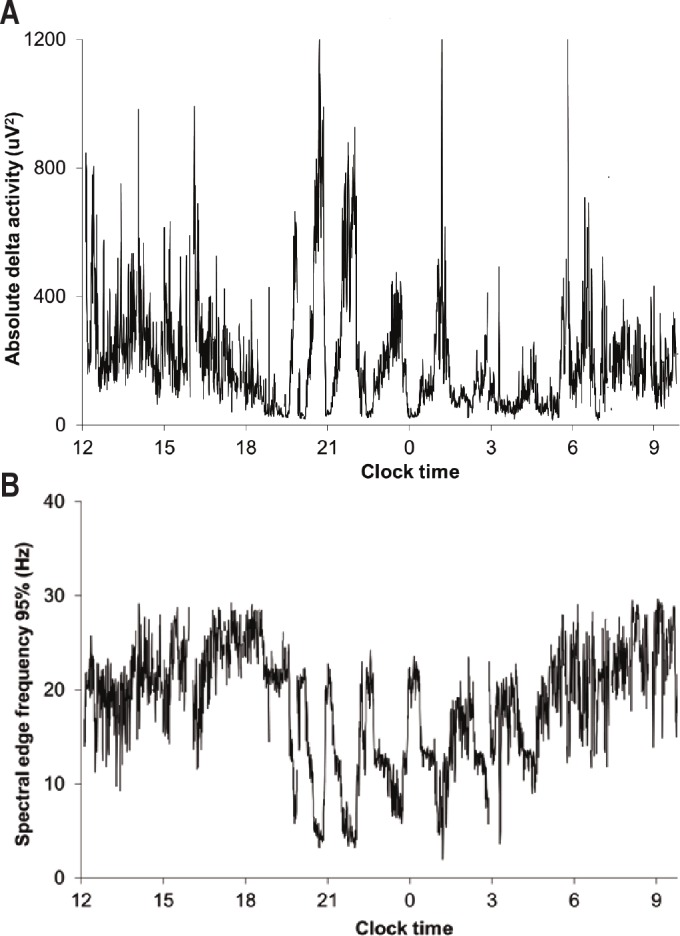
Twenty-four hour profiles of absolute delta activity (top panel) and spectral edge frequency 95% (SEF95, bottom panel) in a representative normal subject. SEF95 is higher during the daytime than at night and exhibits cyclic alterations that are inversely related to slow wave activity. Compared to absolute delta power, SEF95 is more robust to artifact (reflected in sharp spikes in delta power) in this unattended study performed in the field. The subject’s relatively early bedtime is typical of his society in this warm climate.
To examine the EEG for evidence of normal sleep-wake regulation we used several approaches. (1) We constructed for the cohort the 24-h temporal profiles of absolute delta activity and SEF95 from Day 1 of recording. Each parameter was expressed as a percentage of the 24-h mean for each time series and the pattern examined for evidence of a circadian rhythm of EEG activity. (2) We compared the average daytime SEF95 to the average nighttime SEF95 to determine if the SEF95 was lower during the usual nighttime than during the usual daytime as would be expected in healthy adults. (3) We analyzed whether the SEF95 activity was lower during the biological night when compared with the biological day. In healthy individuals, the onset of melatonin secretion is considered to be phase locked to the opening of the “gates of sleep” shortly thereafter, and is thus normally followed by a decrease in EEG frequency and in SEF95. To determine whether, for each patient, the EEG frequency was lower during the biological night than during the day we performed an analysis on 9 subjects who exhibited circadian rhythmicity to aMT6s excretion and also had concurrent EEG data. For the purposes of this analysis we defined the biological “night” as a 5-h period beginning 2 h after melatonin onset and ending 7 h after melatonin onset, a period during which sleep propensity is normally high.36 A 5-h period beginning 6 h prior to melatonin onset and ending 1 h prior to melatonin onset was selected to represent the biological “day.” The median SEF95 was determined for each subject for the biological day and night periods, after which the median SEF95 values for the biological day and night were compared. We also performed sensitivity analyses in which the definitions of biological day and night were altered, as reported in the Results below.
Statistical Analysis
Summary data are reported as mean ± standard deviation or median (interquartile range) as appropriate. Differences in the proportion of patients with normal circadian timing between patients with and without sepsis were tested using the Fisher exact test. Linear regression was used to examine the relationship between APACHE II scores and circadian amplitude of aMT6s excretion. Differences in total 24-h aMT6s excretion between our subjects and healthy controls34 were tested using a t-test. Differences between usual wake time hours (07:00-23:00) and usual sleep time hours (23:00-07:00) in light intensity, absolute delta power, and SEF95 were tested using the Wilcoxon signed rank test or paired t-test as appropriate. Differences in light intensity and aMT6s excretion between subjects who received the intervention on Day 1 and those who did not were examined using a t-test or Mann-Whitney U rank sum test as appropriate. Differences in SEF95 between the melatonin-derived biological day and night were tested using the Wilcoxon signed rank test. Statistical tests were 2-sided, and a P value < 0.05 was judged as statistically significant. Calculations were performed using SigmaStat for Windows Version 2.03 (SPSS Inc.).
RESULTS
A total of 25 patients were enrolled. Two patients developed exclusion criteria almost immediately after consent was obtained and were withdrawn from the study. One patient experienced difficulties synchronizing respiration to the ventilator and became agitated, after which consent was withdrawn by the family, prior to the performance of any study-related procedures. This manuscript reports the results of our analyses on 22 subjects.
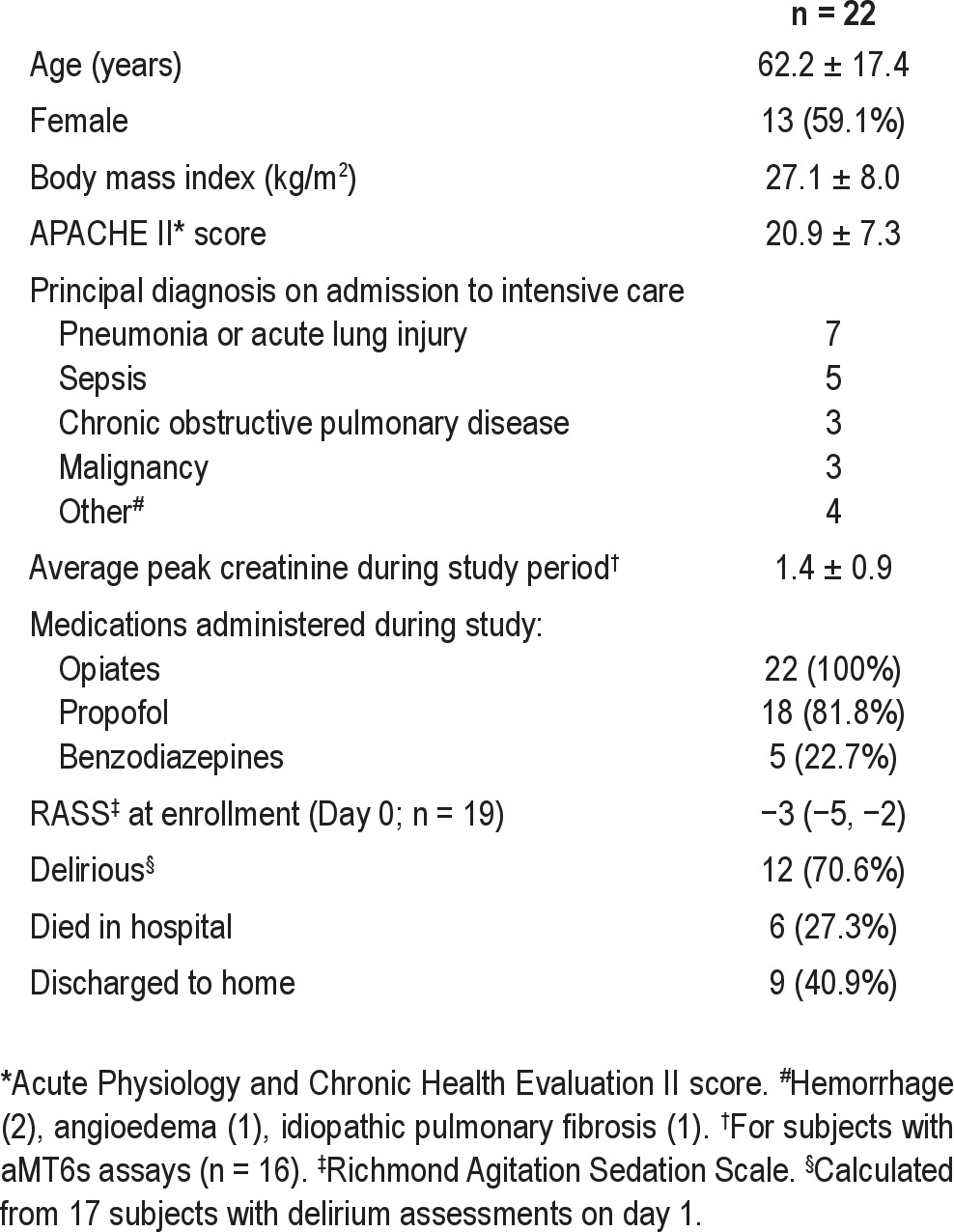
Group characteristics and details of sedative administration and monitoring are shown in Table 1. Patients were moderately to deeply sedated at the time of enrollment, with a median RASS score of −3 (−5, −2), and all patients received intravenous opiates while on-study. Approximately half (12/22, 54.5%) the subjects met standard criteria for sepsis including, but not limited to, patients for whom sepsis was the principal diagnosis on ICU admission. One patient (who did not have aMT6s assays) had graft versus host disease of the liver but did not have clinically evident ascites or hepatic encephalopathy. There were no other subjects with liver disease. Subjects were enrolled a median of 2.0 (1.0, 3.0) days after ICU admission, and 2.0 (1.1, 5.0) days after hospital admission.
Table 1.
Clinical characteristics and sedative data
Ambient light intensity was measured in 18 subjects, 15 of whom also had urinary aMT6s assays performed. While average light intensity during usual sleep time hours (23:00 to 07:00) was conducive to sleep, averaging 3.7 (1.0, 11.3) lux; average light intensity during normal wake time hours (07:00 to 23:00) was also low, averaging 79.7 (27.9, 195.3) lux (P < 0.001 for comparison of the 2 periods). Light intensity on Day 1 of aMT6s excretion in the 15 subjects with aMT6s assays averaged 59.8 (23.9, 168.8) lux during the daytime and 6.7 (1.8, 15.2) lux during the nighttime (P < 0.001) and the difference in light intensity between the usual wake and sleep time hours did not differ between subjects who received the intervention and subjects who did not (P = 0.43).
Analysis of Circadian Rhythm
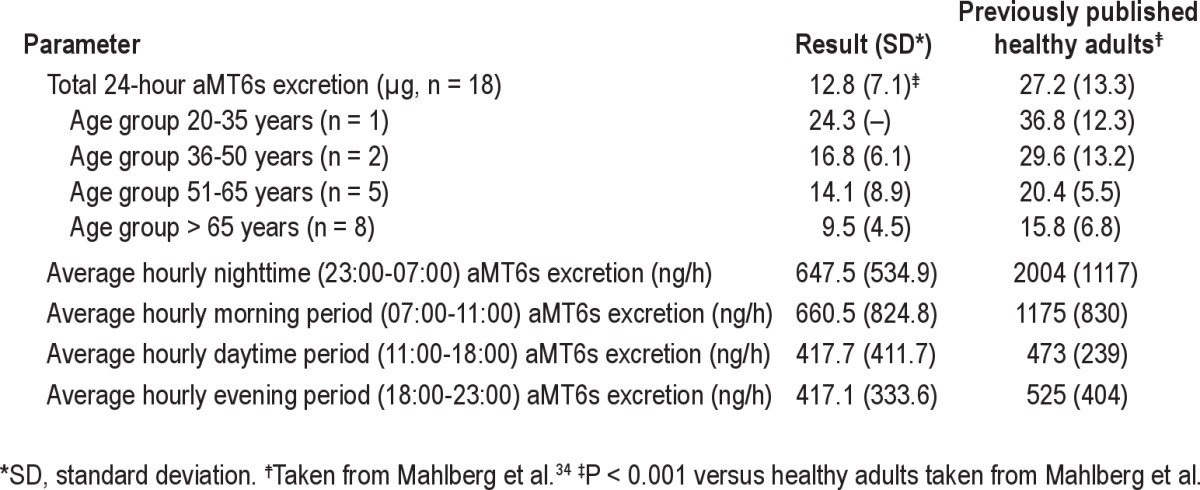
Urinary aMT6s assays were performed in 16 subjects, 5 of whom received the intervention on Day 1. Continuous infusions of one or more vasoactive drugs (norepinephrine, phenylephrine, vasopressin, dopamine, or dobutamine) were administered to 9/16 subjects with aMT6s assays during at least a portion of the collection period; one of these subjects also received enteral metoprolol. No patients received α-blockers, aspirin, nonsteroidal antiinflammatory drugs, selective serotonin reuptake inhibitors, or tricyclic antidepressants during this period. Table 2 shows the average daily excretion of aMT6s for the first day of collection. Average 24-h excretion of aMT6s was reduced (12.8 ± 7.1 μg versus 27.2 ± 13.3 μg; P < 0.001) when compared with previously published healthy controls as reported by Mahlberg et al. in a study that used a similar ELISA assay from the same manufacturer.34 Total excretion tended to decrease with age as expected, although group sizes were too small to permit formal statistical comparisons.
Table 2.
Urinary 6-sulfatoxymelatonin (aMT6s) excretion
The 24-h temporal profile of aMT6s excretion for the group exhibited a phase delay (Figure 2A) with the median melatonin onset occurring at midnight (22:00, 01:00) and the median acrophase occurring at 05:00 (04:00, 07:00). Figure 2B shows the temporal profile for the 10 subjects who underwent 48 h of aMT6s collection and shows that the timing of the acrophase of aMT6s excretion was similar between days 1 and 2. Most (13/16, 81.3%) subjects exhibited a circadian rhythm to aMT6s excretion. Individual aMT6s excretion profiles showed significant variability in the timing of melatonin excretion, with 4 subjects exhibiting normal timing, 7 subjects exhibiting a phase delay, 2 subjects exhibiting a phase advance, and 2 subjects with no discernible rhythm of aMT6s excretion (Figure 2C). In one subject, the presence of a circadian rhythm and the timing of melatonin onset could not be determined due to missing samples. There was no difference between patients who received the intervention and those who did not in the amplitude of aMT6s excretion (475.5 ± 432.8 ng versus 451.9 ± 359.5 ng, P = 0.91) or in the timing of melatonin onset and acrophase (data not shown). There was no difference between patients with and without sepsis in the proportion of patients with normal circadian timing (2/8 patients with sepsis compared with 2/7 subjects without sepsis, P = 1.0). There was no correlation between APACHE II scores and aMT6s amplitude (R2 = 0.08).
Figure 2.
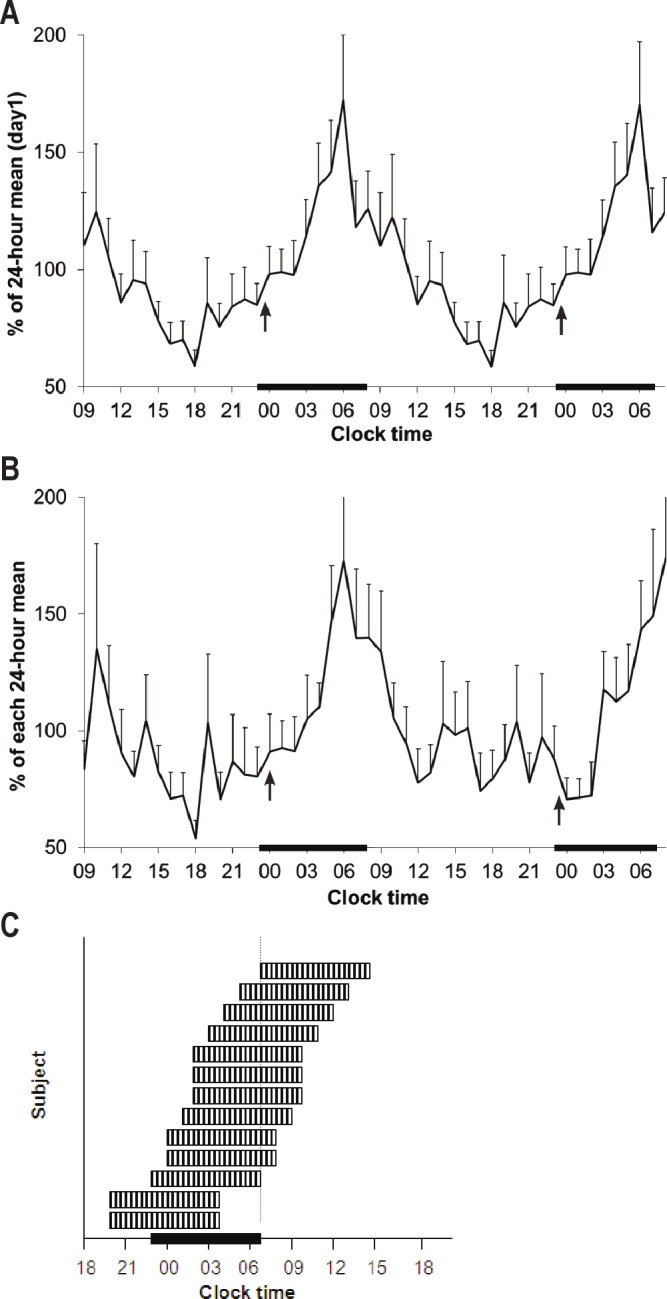
Temporal profiles of 6-sulfatoxymelatonin (aMT6s) excretion. Error bars denote standard error of the mean for each value. The arrows indicate median melatonin onset. The black bars denote usual sleep time (23:00-07:00). (A) aMT6s excretion on Day 1 (n = 16). The data are double plotted for easier visualization. (B) aMT6s excretion on Days 1 and 2 in the subset of subjects who had 48 hours of urinary collection (n = 10). The reproducibility of the profile is evident. (C, inset) Graphical depiction of individual rest periods as determined by melatonin assays. Each hashed band represents a putative 8-h rest period (e.g., recommended sleep time) for each subject as determined by analysis of individual aMT6s assays. The rest period is considered to begin 2 h after melatonin onset. The vertical dotted line represents change of shift for the nurses, and the beginning of the day shift.
Polysomnographic Analysis
Polysomnography was performed in 22 patients. One recording was lost due to technical failure. In the remaining 21 subjects, total recording time varied between 645 and 3589 minutes, for a total of 49,179 minutes (819.7 h or 34.2 days) of recording time. REM sleep could be identified in only 2 subjects, one of whom was 22 years old (total REM time = 55 min of 45.9 total h of recording) and the other of whom had all sedative and narcotic infusions discontinued shortly after enrollment (total REM time = 208 min of 43.6 total h of recording). Other sleep stages could not be reliably identified.
Quantitative EEG analysis was performed in 18 subjects whose recordings were obtained with the Nihon Kohden system. One of these subjects had no EEG data from Day 1 due to technical failure. As suggested by the relatively flat profile of average delta power on Day 1 (Figure 3, top), there was no difference in absolute delta power between the usual wake time (7:00-23:00) and usual sleep time (23:00-7:00) periods (302.2 ± 184.0 μV2 versus 275.4 ± 187.5 μV2, P = 0.34). Additionally, absolute delta power was not low during the daytime and did not decline in amplitude over the course of the night, as would be expected in healthy adults with the dissipation of the homeostatic drive to sleep.
Figure 3.

Twenty-four hour profiles of absolute delta activity (top panel) and spectral edge frequency 95% (SEF95, bottom panel) on day 1 (n = 17). The dark symbols represent average values for each parameter. The shaded areas represent the standard error of the mean. The black bars denote usual sleep time (23:00-7:00).
The temporal profile of SEF95 for the group on Day 1 also showed little temporal variability (Figure 3, bottom). The average SEF95 for the group was low (6.3 [5.3, 7.8] Hz) and did not differ between the usual wake time (7:00-23:00) and usual sleep time (23:00-7:00) periods (7.7 ± 3.9 Hz versus 7.7 ± 3.6 Hz, respectively; P = 0.86). Similarly, median SEF95 did not differ between the melatonin-derived biological day and the biological night (5.8 Hz day versus 5.8 Hz night, P = 0.64) in the 9 subjects in whom this analysis was performed. There was no difference between the biological day and the biological night in the average number of epochs available for analysis for each subject (463.2 epochs/subject during the day versus 388.3 epochs/subject during the night, P = 0.24). Sensitivity analyses were performed in which the definitions of biological day and night were altered. Nearly identical results were obtained when the biological night was defined as occurring from 1 h hour after melatonin onset to 6 h after melatonin onset (median SEF95 also 5.8 Hz). Similar results were also obtained when the biological day for each subject was defined as the period from 12:00 to 17:00, a period during which daytime light and care activities would be expected to contribute to wakefulness (median SEF95 6.0 Hz during this period).
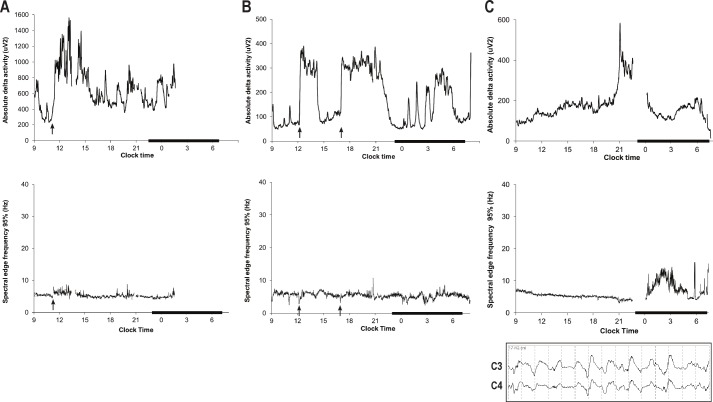
Individual temporal profiles of SWA and SEF95 were consistently abnormal (see Figure 4 for examples) and reflected the effects of sedatives and/or various toxic-metabolic encephalopathies.37–39 Individual profiles did not show the expected cyclicity of SWA reflecting the circadian and temporal organization of normal sleep. Similarly, individual profiles of SEF95 generally lacked the diurnal and ultradian rhythmicity characteristic of normal sleep and showed no consistent effect of the sleep promotion intervention, although our analysis was limited by the small number of subjects enrolled. The one exception was a 22-year-old woman with a history of previous episodes of respiratory failure whose raw EEG appeared relatively normal (Figure 5).
Figure 4.
Individual temporal profiles of slow wave activity and spectral edge frequency 95% on day 1. A 5-minute moving average was used to plot the slow wave activity profiles. The black bars denote usual sleep time (23:00-7:00). SEF95, spectral edge frequency 95%; EEG, electroencephalogram. (A) Lightening of sedation. 70-year-old woman with interstitial lung disease receiving propofol and fentanyl. Discontinuation of propofol alone (arrow) results in an increase in delta power and a simultaneous shift to higher frequencies as indicated by the small increase in SEF95. Given that propofol itself induces slow wave activity,40 the response in this case reflects significant cortical suppression prior to propofol discontinuation caused by the joint administration of a sedative and narcotic (fentanyl). The patient was interacting with visitors at noon. (B) Arousal. 64-year-old man receiving propofol and fentanyl continuously and unarousable except for cough and gag. The EEG was highly suppressed when delta power was low (< 100 μV2) and showed a monotonous (encephalopathic) delta pattern when delta power was high. Increases in delta power generally followed stimulation and were associated with an increase in heart rate and a transient decrease in SEF95, consistent with the “paradoxical arousal” or “synchronization” response to noxious stimuli occasionally seen in patients receiving anesthetics.38,39 Subsequent increases in SEF95 coincided with increased fast-frequency activity and persistently elevated heart rate, compatible with a classical arousal response. The two major episodes during the day (arrows) followed linen changes. (C) Periodic patterns. 70-year-old man with refractory septic shock and multiple organ failure. Propofol and fentanyl were discontinued at 11:00, after which he remained unresponsive. The raw EEG demonstrated a low-frequency pattern with intermittent 1- to 3-second periods of suppression, consistent with a marked encephalopathy. The increase in delta power at 21:00 is attributable to the appearance of rhythmic delta activity, as shown in the 15-sec EEG tracing. The early morning increase in SEF95 was not associated with an increase in responsiveness and is attributable to progressive attenuation of the EEG and a relatively greater contribution of higher frequencies to the overall EEG. He arrested at 07:34.
Figure 5.
Relatively normal profile. 22-year-old woman with pneumonia and a history of prior lung transplantation. Propofol was administered continuously except between 11:30 and 14:00. The patient interacted with staff and family when awake and received the sleep and circadian rhythm intervention on this day. Cyclic decreases in SEF95 coincide with cyclic increases in delta activity and with periods of behaviorally scored sleep (bold arrows) as recorded by the bedside attendant. The raw EEG exhibited few pathologic features other than frequent stage transitions.
DISCUSSION
To our knowledge, our investigation is the first to simultaneously study the sleep EEG and circadian rhythms of critically ill patients receiving mechanical ventilation and intravenous sedation. Our study demonstrated that: (1) The circadian rhythm of aMT6s excretion was usually preserved. (2) Circadian timing was frequently disturbed, most commonly in the form of a phase delay. (3) Normal features of sleep could not be identified by expert visual sleep stage scoring; REM sleep could be identified in only 2 of 21 patients. (4) The normal features of the EEG typical of the alternation of wake and sleep states were absent, with SWA showing neither circadian nor ultradian organization and the SEF95 also showing little temporal variation, either between usual daytime and nighttime hours or between the melatonin-derived biological daytime hours and nighttime hours. Taken together, these results demonstrate a profound degree of temporal disorganization in the sleep EEG and circadian rhythms of our subjects.
Abnormalities in the circadian rhythms of critically ill patients have been previously described, although most studies suffer from various methodological limitations, including infrequent sampling of melatonin or a reliance on the analysis of temperature curves. The overall preservation of the circadian rhythm of aMT6s excretion in our study differs from the results of Mundigler et al.17 and Paul and colleagues,22 who reported abolition of the 24-hour pattern of melatonin secretion in most of their subjects. Our frequent sampling interval, the use of a three-point moving average to minimize short-term fluctuations in aMT6s excretion, and the representation of the profile of melatonin excretion as a percentage of 24-hour mean to normalize the well-documented inter-individual differences in melatonin secretion34 allowed for an unequivocal demonstration of the persistence of circadian rhythmicity in our cohort as shown clearly in Figure 1A and Figure 1B.
Most of our subjects were not wearing eyeshades during the period of aMT6s collection. This is similar to the approach used by Paul et al.22 but differs from that of Mundigler and colleagues.17 While light may acutely suppress melatonin secretion,41 simultaneous visual inspection of the light and aMT6s profiles revealed no instances in which aMT6s excretion was acutely suppressed (data not shown). This may be due not only to low ambient light levels, but to the fact that our subjects’ eyes were likely closed a great deal of the time, as suggested by the enrollment RASS score. While we cannot entirely exclude an effect of light exposure on nocturnal aMT6s excretion, the possibility that early evening light exposure may have been partly responsible for the frequent occurrence of a phase delay in our subjects42 appears unlikely because of the very low levels of retinal light exposure. We cannot exclude the possibility that abnormalities of circadian timing were present even prior to ICU admission and reflected the influence of home sleep schedules and/or illness. In adults in the older age range typical of the patients included in the present study, an advance, rather than a delay, of circadian phase has been the most consistent finding. Our finding of a phase delay in the ICU is therefore more likely to reflect the absence of effective zeitgebers in the ICU and in the hospital generally, as discussed below. Whatever the cause, disorders of circadian timing may contribute to sleep disruption in this setting.
Our analysis of the PSG is the most detailed to date in this patient population, incorporating visual analysis, standard power spectral analysis, and the analysis of temporal trends in SEF95. These analyses reveal the presence in our subjects of a highly abnormal state bearing little resemblance to normal sleep or wake. REM sleep was identifiable but scarce, and our subjects’ EEG recordings showed a number of pathologic phenomena consistent with varying degrees of encephalopathy. Individual SWA profiles lacked the normal cyclicity and homeostatic decline with increasing sleep duration. Finally, the SEF95, which typically declines in sleep as the raw EEG shifts to lower-frequency activity,26,27 exhibited no evidence of the diurnal variation present in a normal sleep/wake cycle. The SEF95 also was not higher during the melatonin-derived biological day than during the biological night. In sum, despite the use of multiple analytical approaches, and the simultaneous monitoring of circadian rhythmicity, we were unable to identify in our ICU patients any of the elements of normal sleep-wake regulation that are otherwise apparent in PSG recordings of not only healthy subjects but also of patients suffering from a wide variety of pathological conditions. This suggests that critically ill sedated patients receiving mechanical ventilation and intravenous sedation exist not only in an altered state of consciousness but may be continuously sleep deprived, a condition that could exacerbate their condition and compromise their recovery.
It is true that sleep, anesthesia, and sedation exhibit certain phenotypic similarities and involve overlapping neurophysiological processes,43 suggesting that mechanically ventilated patients receiving sedation do not experience complete sleep deprivation. Such episodic “sleep-like states” are unlikely to be fully restorative, however, even if disruptions from patient care activities and from weak day-night schedules could be completely eliminated. For instance, the slow waves of propofol differ from those of natural sleep in several important respects,40 and sedatives or anesthetics may increase slow wave activity without satisfying all homeostatic needs.44 In addition, while the function of REM sleep is controversial, the severe REM sleep deprivation found in our study and in others15,45 may itself have adverse short- and long-term effects. Recently, REM sleep deprivation has been shown to inhibit hippocampal neurogenesis in rats,46 providing one potential mechanism among many whereby the sleep disruption of critical illness may contribute to the adverse long-term neuropsychological sequelae of critical illness47 by reducing brain plasticity. Collectively, the results of our study powerfully illustrate the monotonous experience of most patients receiving mechanical ventilatory support and intravenous sedation where the activity of the brain is concerned. In fact, the dispersion of “sleep-like states” and/or sedation over a 24-hour cycle may contribute to the reduced amplitude of aMT6s excretion seen in our study, given that melatonin amplitude is reduced by recent episodes of sleep.48
Certain characteristics of the ICU environment and its heterogeneous patient population present unique challenges to the study of sleep. Our study was not designed to investigate the many potential interactions between patient characteristics and treatments and the observed results. For instance, sedative practices have continued to evolve since the inception of this study with the introduction of dexmedetomidine into the ICU and with the advent of protocols that attempt to minimize or altogether eliminate sedatives when possible.49 Whether similar abnormalities of circadian rhythmicity or sleep-wake regulation would be found in such settings is not known. It is also possible that different results would be found in patients treated in ICUs characterized by greater daytime illumination than ours. An additional methodological challenge in this population is the presence of many potential sources of EEG artifact, to which conventional power spectral analysis is very sensitive.50 This may have interfered with our ability to detect normal sleep processes. We addressed this challenge through a multifaceted approach. (1) We employed trained research assistants at the bedside to troubleshoot EEG acquisition whenever possible. (2) We used a parameter—the SEF95—that by definition is more resistant to the effects of high-frequency artifact than conventional power spectral analysis. This parameter also does not rely on the electromyogram signal, which in our study proved highly sensitive to artifactual contamination, similar to the experience of Olofsson and colleagues18 in a study that employed the bispectral index monitor. Finally (3), we used an iterative process for artifact identification and rejection that was aided by the examination of individual spectral power profiles. Despite this comprehensive and intensive approach, we cannot entirely exclude the possibility that residual artifacts—including low-frequency respiratory artifacts that would depress the SEF95—were present in our analyzed material.
Uncoupling of the circadian timing system from the sleep-wake cycle is seen in the majority of non-sighted individuals, and can be provoked experimentally under conditions of prolonged continuous dim light exposure (as in forced desynchrony protocols).51,52 Indeed, the finding that our cohort was generally phase delayed suggests that the circadian rhythms of our patients may have been free-running. Our study was not designed to determine the differential effects of sedation, the ICU environment, and acute illness on circadian rhythmicity. We speculate, however, that the disordered circadian rhythmicity found in our subjects is to a large extent iatrogenic, reflecting the influence of weak light-dark cycles, the dispersion of sleep-like activity over 24 hours from continuous intravenous sedation, and the presence of 24-hour care and feeding schedules. Although not measured in our study, sedative-induced eye closure may have further minimized daytime retinal light exposure and inhibited entrainment.
It is interesting to speculate whether similar abnormalities of circadian timing and sleep-wake regulation would occur if healthy subjects were subjected to prolonged sedation; animal models may be useful in answering this question. We also are interested in the potential effects of daily sedative interruption—a practice employed in our ICU but not performed at a fixed time of day—on circadian rhythmicity. Specifically, we wonder if efforts to correct the delay in circadian timing would be enhanced by interrupting sedation at the same time every morning while simultaneously enhancing light exposure. Given recent interest in the circadian modulation of the immune response in sepsis,53 as well as in the direct effects of melatonin on immune function and regulation,54 our findings should be explored further. Conceivably, protocols that provide patients with a restorative process that is more closely related to normal sleep—an evolutionarily conserved process marked by a high degree of temporal organization at multiple levels55—may lead to better outcomes.
SUMMARY
Critically ill patients receiving mechanical ventilation and intravenous sedation exhibit severe disorganization of circadian rhythmicity and sleep-wake regulation. REM sleep is scarce, and individual temporal profiles of SWA and SEF95 do not exhibit the expected diurnal and ultradian organization of normal sleep. Although the circadian rhythm of aMT6s excretion is generally preserved, alterations in timing are frequent, consistent with the absence of effective zeitgebers for such patients in a typical ICU environment. The virtual absence of normal sleep architecture in such patients is likely to be associated with severe sleep deprivation and may impair recovery from critical illness.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Chapotot is a co-founder of PhiTools, the company that developed and markets the PRANA software used to analyze the polysomnograms in this study. Mr. Whitmore has received project funding from Respironics. Ms. Pohlman has received remuneration from speaking engagements from B. Braun Inc. and Carefusion. Dr. Van Cauter has received research grants from Philips/Respironics and from Amylin/Eli Lilly, and has participated in speaking engagements sponsored by Johnson and Johnson, Sanofi-Aventis. She has also served as a consultant to Lamson and Dugan, attorneys. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the nurses and staff of the intensive care units at the University of Chicago for their assistance with this study. We also thank Matt Luchette and William Schweickert for their assistance. All work was performed at the University of Chicago. Dr. Gehlbach receives support from grant number K23HL088020 from the National Heart, Lung, and Blood Institute. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institute of Health. This study was also supported by the Brain Research Foundation and by the Institute for Translational Medicine (CTSA grant number UL1 RR024999), the Diabetes Training and Research Center (P60 DK020595), and the Department of Medicine at the University of Chicago.
ABBREVIATIONS
- aMT6s
6-sulfatoxymelatonin
- APACHE
Acute Physiology and Chronic Health Evaluation
- EEG
electroencephalography
- ICU
intensive care unit
- RASS
Richmond Agitation Sedation Scale
- SEF95
spectral edge frequency 95%
- PSG
polysomnography
- REM
rapid eye movement
Footnotes
A commentary on this article appears in this issue on page 1029.
REFERENCES
- 1.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bank S, Dinges DF. Behavioral and physiological consequences of sleep restriction. [PMC free article] [PubMed] [Google Scholar]
- 3.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–61. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–53. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 7.Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Crit Care Med. 2007;35:393–401. doi: 10.1097/01.CCM.0000254339.18639.1D. [DOI] [PubMed] [Google Scholar]
- 8.Weinhouse GL, Watson PL. Sedation and sleep disturbances in the ICU. Crit Care Clin. 2009;25:539–49. doi: 10.1016/j.ccc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–7. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 10.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–9. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 11.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: Pressure support versus proportional assist ventilation. Crit Care Med. 2007;35:1048–54. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 12.Toublanc B, Rose D, Gláerant JC, et al. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33:1148–54. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 13.Cabello B, Thille AW, Drouot X, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36:1749–55. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 14.Weinhouse GL. Crit Care Clin. 2008. Pharmacology I: Effects on sleep of commonly used ICU medications; pp. 477–91. [DOI] [PubMed] [Google Scholar]
- 15.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–18. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 16.Ambrogio C, Koebnick J, Quan SF, Ranieri VM, Parthasarathy S. Assessment of sleep in ventilator-supported critically ill patients. Sleep. 2008;31:1559–68. doi: 10.1093/sleep/31.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mundigler G, Delle-Karth G, Koreny M, et al. Impaired circadian rhythm melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30:536–40. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–84. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 19.Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med. 2007;33:1954–8. doi: 10.1007/s00134-007-0769-x. [DOI] [PubMed] [Google Scholar]
- 20.Frisk U, Olsson J, Nylen P, Hahn R. Low melatonin excretion during mechanical ventilation in the intensive care unit. Clin Sci. 2004;107:47–53. doi: 10.1042/CS20030374. [DOI] [PubMed] [Google Scholar]
- 21.Shilo L, Dagan Y, Smorijk Y, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317:278–81. doi: 10.1097/00000441-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Paul T, Lemmer B. Disturbance of circadian rhythms in analgosedated intensive care unit patients with and without craniocerebral injury. Chronobiol Int. 2007;24:45–61. doi: 10.1080/07420520601142569. [DOI] [PubMed] [Google Scholar]
- 23.Tung A, Lynch JP, Mendelson WB. Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg. 2001;92:1232–6. doi: 10.1097/00000539-200105000-00028. [DOI] [PubMed] [Google Scholar]
- 24.Nelson AB, Faraguna U, Tononi G, Cirelli C. Effects of anesthesia on the response to sleep deprivation. Sleep. 2010;33:1659–67. doi: 10.1093/sleep/33.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bojkowski CJ, Arendt J, Shih MC, Markey SP. Melatonin secretion in humans assessed by measuring its metabolite, 6-sulfatoxymelatonin. Clin Chem. 1987;33:1343–8. [PubMed] [Google Scholar]
- 26.Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94:125–9. doi: 10.1097/00000539-200201000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Rey M, Bastuji H, Garcia-Larrea L, Guillemant P, Mauiguiêre F, Magnin M. Human thalamic and cortical activities assessed by dimension of activation and spectral edge frequency during sleep wake cycles. Sleep. 2007;30:907–12. doi: 10.1093/sleep/30.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 29.American College of Chest Physicians. Society of Critical Care Medicine consensus conference: Definitions of sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 30.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 31.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 32.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 33.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–9. [PMC free article] [PubMed] [Google Scholar]
- 34.Mahlberg R, Tilmann A, Salewski L, Kunz D. Normative data on the daily profile of urinary 6-sulfatoxymelatonin in healthy subjects between the ages of 20 and 84. Psychoneuroloendocrinology. 2006;31:634–41. doi: 10.1016/j.psyneuen.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. [Google Scholar]
- 36.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol Regulatory Integrative Comp Physiol. 1999;277:1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21:307–18. [PubMed] [Google Scholar]
- 38.Thomsen CE, Prior PF. Quantitative EEG in assessment of anaesthetic depth: comparative study of methodology. Br J Anaesth. 1966;77:172–8. doi: 10.1093/bja/77.2.172. [DOI] [PubMed] [Google Scholar]
- 39.Otto KA. EEG power spectrum analysis for monitoring depth of anaesthesia during experimental surgery. Lab Anim. 2008;42:45–61. doi: 10.1258/la.2007.006025. [DOI] [PubMed] [Google Scholar]
- 40.Murphy M, Bruno MA, Riedner BA, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283–91. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gooley JJ, Chamberlain K, Smith KA, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrin Metab. 2011;96:E463–72. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown EN, Lydic R, Schiff ND. Mechanisms of disease: general anesthesia, sleep, and coma. N Engl J Med. 2010:2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mashour GA, Lipinski WJ, Matlen LB, et al. Isoflurane anesthesia does not satisfy the homeostatic need for rapid eye movement sleep. Anesth Analg. 2010;110:1283–9. doi: 10.1213/ANE.0b013e3181d3e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardin KA, Seyal M, Stewart T, Bonekat HW. Sleep in critically ill chemically paralyzed patients requiring mechanical ventilation. Chest. 2006;129:1468–77. doi: 10.1378/chest.129.6.1468. [DOI] [PubMed] [Google Scholar]
- 46.Guzman-Marin R, Suntsova N, Bashir T, Nienhuis R, Szymusiak R, McGinty D. Rapid eye movement sleep deprivation contributes to reduction of neurogenesis in the hippocampal dentate gyrus of the adult rat. Sleep. 2008;31:167–75. doi: 10.1093/sleep/31.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clin. 2009;25:615–28. doi: 10.1016/j.ccc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Zeitzer JM, Duffy JF, Lockley SW, Dijk D-J, Czeisler CA. Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. Sleep. 2007;30:1437–43. doi: 10.1093/sleep/30.11.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strøm T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomized trial. Lancet. 2010;375:475–80. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 50.Campbell IG. EEG recording and analysis for sleep research. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns1002s49. Chapter 10: Unit 10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eastman C, Rechtschaffen A. Circadian temperature and wake rhythms of rats exposed to prolonged continuous illumination. Physiol Behav. 1983;31:417–27. doi: 10.1016/0031-9384(83)90061-6. [DOI] [PubMed] [Google Scholar]
- 52.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 53.Srinivasan V, Pandi-Perumal SR, Spence DW, Kato H, Cardinali DP. Melatonin in septic shock: some recent concepts. J Crit Care. 2010;656:e1–e6. doi: 10.1016/j.jcrc.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 54.Carrillo-Vico A, Lardon PJ, Naji L, et al. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J Pineal Res. 2005;39:400–8. doi: 10.1111/j.1600-079X.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 55.Mignot E. Why we sleep: the temporal organization of recovery. PLoS Biology. 2008;6:e106. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]