Abstract
Study Objectives:
Although acute sleep loss during 24- to 30-h extended duration work shifts (EDWS) has been shown to impair the performance of resident physicians, little is known about the effects of cumulative sleep deficiency on performance during residency training. Chronic sleep restriction induces a gradual degradation of neurobehavioral performance and exacerbates the effects of acute sleep loss in the laboratory, yet the extent to which this occurs under real-world conditions is unknown. In this study, the authors quantify the time course of neurobehavioral deterioration due to repeated exposure to EDWS during a 3-week residency rotation.
Design:
A prospective, repeated-measures, within-subject design.
Setting:
Medical and cardiac intensive care units, Brigham and Women's Hospital, Boston, MA.
Participants:
Thirty-four postgraduate year one resident physicians (23 males; age 28.0 ± 1.83 (standard deviation) years)
Measurements and Results:
Residents working a 3-week Q3 schedule (24- to 30-h work shift starts every 3rd day), consisting of alternating 24- to 30-h (EDWS) and approximately 8-h shifts, underwent psychomotor vigilance testing before, during, and after each work shift. Mean response time, number of lapses, and slowest 10% of responses were calculated for each test. Residents also maintained daily sleep/wake/work logs. EDWS resulted in cumulative sleep deficiency over the 21-day rotation (6.3 h sleep obtained per day; average 2.3 h sleep obtained per extended shift). Response times deteriorated over a single 24- to 30-h shift (P < 0.0005), and also cumulatively with each successive EDWS: Performance on the fifth and sixth shift was significantly worse than on the first shift (P < 0.01). Controlling for time of day, there was a significant acute (time on shift) and chronic (successive EDWS) interaction on psychomotor vigilance testing response times (P < 0.05).
Conclusions:
Chronic sleep deficiency caused progressive degradation in residents' neurobehavioral performance and exacerbated the effects of acute sleep loss inherent in the 24- to 30-h EDWS that are commonly used in resident schedules.
Citation:
Anderson C; Sullivan JP; Flynn-Evans EE; Cade BE; Czeisler CA; Lockley SW. Deterioration of neurobehavioral performance in resident physicians during repeated exposure to extended duration work shifts. SLEEP 2012;35(8):1137-1146.
Keywords: Extended duration work shift, sleep deficiency, psychomotor vigilance test, chronic, acute, resident, graduate medical education
INTRODUCTION
Considerable attention has been focused on the effect of extended duration work shifts (EDWS, > 24 h) on the performance of resident physicians.1–5 The Accreditation Council for Graduate Medical Education has recently limited the maximum duration of a work shift to 16 h for the approximately 24,000 postgraduate year (PGY)-1 residents (i.e., residents in their first year after medical school), while continuing to allow the approximately 84,000 intermediate-level and senior resident physicians (PGY-2 and above) to work 28-h shifts twice per week.6 Working continuous EDWS has been shown to reduce overall total sleep time, increase the rate of attentional failures overnight,7 increase the risk of errors on clinical tasks,8 result in higher rates of medical errors,2,9 and increase the risk of crashes as a result of driving while drowsy.10 It has been demonstrated in the laboratory that 1-2 weeks of chronic sleep deficiency can be as detrimental to waking performance as 1-2 days of acute sleep deprivation.11–13
Moreover, acute and chronic sleep deprivation interact such that performance during a night of lost sleep is up to 10-fold worse when individuals have been chronically sleep deprived beforehand in comparison with acute sleep loss alone.13 Previous studies that examined the cumulative effects of chronic sleep loss11,12 did not do so on a background of acute sleep deprivation, as experienced in trainee physicians. Furthermore, because the study by Cohen et al.13 was conducted in the controlled laboratory environment, the extent to which acute and chronic sleep deprivation interact in a real-world occupational setting is unknown.
Given that medical and surgical resident physicians are routinely required to undergo both acute sleep deprivation and chronic sleep deficiency when working repeated 24- to 30-h EDWS, we sought to evaluate the effect of such schedules on performance in resident physicians. Because much of our understanding on the effect of acute and chronic sleep loss is based on highly controlled laboratory studies, the study also aimed to assess whether their effect on performance could be detected in an operational environment.
METHODOLOGY
Design Overview
Residents were studied during a 3-week rotation in the medical intensive care unit or the coronary care unit while following a Q3 shift schedule (as described in the next paragraphs). Residents completed a daily sleep- and work-hour log, and completed a 10-min psychomotor vigilance test (PVT) before, during, and at the end of each scheduled shift.
Participants
PGY-1 residents in the internal medicine residency training program at Brigham and Women's Hospital (2002-2004) were invited to participate in the study. Thirty-four PGY-1 resident physicians (23 males; age 28.0 ± 1.83 standard deviation (SD) years; range 24-32 years) whose schedules were compatible with an available study slot were placed in the study on a “first-come, first-serve” basis (Figure 1). The study was approved by the Human Research Committee of Partners Healthcare, consistent with principles of the Declaration of Helsinki. All participants provided written informed consent and were reimbursed for their participation.
Figure 1.
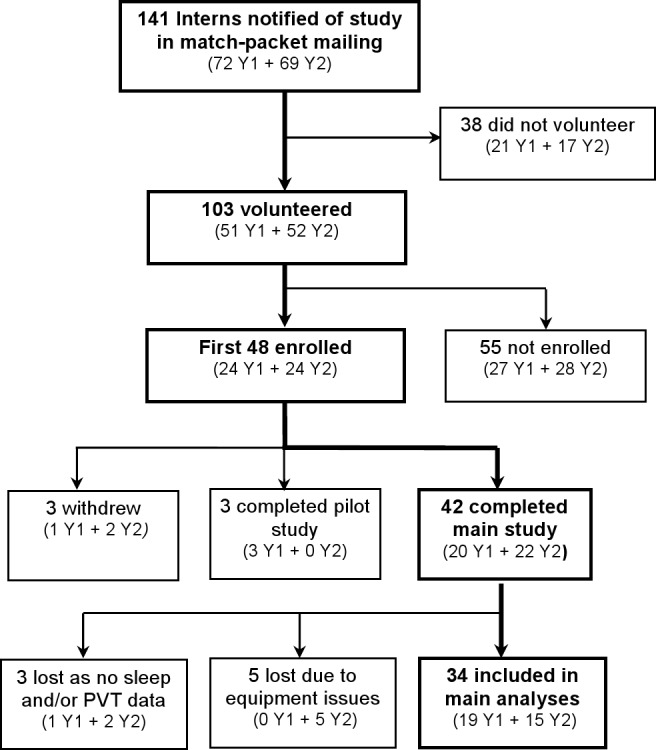
A flow chart showing subject progression through the study stages (for years 1 and 2 of the study).
Shift Schedules
The hospital schedule required residents to work EDWS of 24-30 scheduled consecutive hours every other shift (starting every 3rd calendar day “Q3”) for 3 weeks.2,7 While on the traditional schedule, a team of 3 interns provided continuous coverage on a repeated 3-day schedule, consisting of a day shift (approximately 07:00 to approximately 15:00) on Day 1 followed by an EDWS from 07:00 on Day 2 to approximately 12:00 on Day 3. Interns had the day off when a day shift occurred Saturday-Monday. Interns had the opportunity for sleep, at home, for 2 full nights in between each EDWS.
For two weeks before the study, residents worked on a noncall clinic rotation without extended shifts. The study rotation therefore began with residents relatively “well slept” (total sleep time) 54.3 h/week).2,7 No neurobehavioral data were collected during this time.
Data from the first 6 successive EDWS were analyzed to examine change in neurobehavioral performance during the rotation. The seventh extended shift was not available in 17 of 34 participants due to scheduling considerations, and was therefore excluded from the analyses. As the timing of EDWS were staggered systematically within the 3-person resident team, EDWS-X does not depict the same study day for each participant but does depict the same order of EDWSs. For example, Intern 1 started an EDWS on days 1, 4, 7, 10, 13, and 16 whereas Intern 2 started EDWS on days 2, 4, 8, 11, 14, and 17 and so on. The term first EDWS always refers to the first EDWS for each intern; second EDWS always refers to the second extended duration shift, etc.
Measurements and Outcomes
Sleep Measurements
Residents completed a daily sleep/work log and continuous wrist actigraphy (Actiwatch-L; Mini-Mitter, Co., Inc., Bend, OR) throughout the study. Sleep logs were used to collect data on sleep timing and duration, sleep quality, awakenings, and work schedules and were available for 676 of 714 nights. The sleep logs were previously verified from polysomnography (PSG) from the first 20 residents, recorded for 3-4 days/week during shift rotations (Vitaport-2/3, TEMEC Instruments, The Netherlands), showed 95.6% agreement epoch-to-epoch (r = 0.94, P < 0.001). These data are described in another study.7 When sleep logs were unavailable for (38 of 714 nights; 5.32%), actigraphy data were substituted to determine sleep onset and offset time, total sleep time (including naps) per day (00:00-23:59), and sleep parameters in relation to each PVT.
Neurobehavioral Performance
Neurobehavioral performance was assessed using a standard measure of sustained visual attention: 10-minute Psychomotor Vigilance Test (PVT). Random inter-stimulus intervals ranged from 1-9 sec and a typical task administration involved approximately 100 stimuli presentations. The PVT has been demonstrated to have little to no practice effects12 and is sensitive to both time awake and circadian phase in the laboratory.14–16 Residents completed the PVT on 3-6 occasions throughout each EDWS, typically at the beginning and end of the shift, and approximately every 6 h during the shift. Each PVT was assigned: (1) duration since last sleep episode ≥ 30 min; (2) duration at work; and (3) total sleep, including naps, in the previous 24 h.
To address the effect of time on shift, PVT data were averaged within-subject, and across all 6 EDWS, for each of the 4 time bins: DAY 1: 06:00 – 14:59; EVENING: 15:00-22:59; NIGHT: 23:00 – 05:59; DAY 2: 06:00 – 14:59. One participant did not have PVT data in all 4 time bins, for all shifts, and was excluded from this analysis.
To address the change in neurobehavioral performance with each successive EDWS, we calculated average PVT performance, within-subject, across each shift. Any EDWS without PVT data in both the first and 2nd half of the shift was excluded. This occurred in 13 cases, in 3 participants (6.4%; 13 of a possible 204 cases [34 subjects*6 EDWS]). These participants were excluded from the analyses. Multiple tests for an individual participant were averaged within either shift-half.
To assess the interaction between the effects of time on shift (acute effect) and successive extended duration shift (chronic effect), we evaluated performance at the start and end of each shift (to keep time of day constant) for each extended shift. Of a possible 408 data points (6 shifts × 2 time points × 34 subjects), 49 were missing (12%). Missing data points were evenly distributed across shifts and time on shift (sample size ranged from 29-32 for each data point). All participants completed the PVT during each EDWS but not in every time bin. A linear mixed model approach was used to accommodate missing data points and to account for inter-individual variability.
Mean reaction time (RT), the mean of the 10% slowest responses and number of lapses (responses ≥ 500 ms) were used as measures of neurobehavioral performance. To normalize the data distribution, mean RT was expressed as the log of mean RT and lapses were normalized ((√n)+(√(n+1)). Mean, standard error, and/or confidence intervals are reported.
Medication and Caffeine Intake
Participants provided information on daily caffeine intake and prescribed/over-the-counter medications in a daily log. Caffeine content was defined as 202 mg/16 oz cup of coffee (17 mg/oz), 30 mg/12 oz cup of tea, and 40 mg/12 oz can of soda.17,18 There was no change in estimated weekly caffeine intake (P = 0.95) and therefore caffeine was not used as a covariable.
Three participants reported taking medication that could affect sleep or alertness for the entire study (i.e., antidepressant, diuretic, thyroid medication) and 2 occasionally (i.e. allergy medication, muscle relaxants), totaling 14 days (1.96%) between them. These days were included in the analysis and checked to ensure these were not distributed preferentially in any shift.
Statistical Analysis
Paired sample t tests were used to assess changes in sleep/work parameters at the start and end of each EDWS. Repeated measures 1-way analysis of variance was used to examine changes in performance due to successive EDWS (chronic effect) or time on duty (acute effect). Paired comparisons with Holm-Bonferroni corrections were performed as post hoc tests and missing data were excluded on a case-by-case basis. For any violation of sphericity, the Huynh-Feldt statistic and associated epsilon (ϵ) value are reported. To assess any interaction between acute and chronic effects, a linear mixed model was used for fixed (time on shift; successive EDWS) and random (subject) effects using a scaled identity covariance structure. To consider stability in interindividual variability, we calculated intraclass coefficients (ICC) and these were assessed using the Wald test.19 Here we followed published criteria for categorizing levels of stability: “slight” (0.0-0.02), “fair” (0.2-0.4), and “moderate or beyond” (> 0.4).20
To examine whole RT distribution more extensively for cognitive and perceptual processing speed21 we calculated the 5th-95th percentiles, in 5% increments, for each PVT at the start and end of EDWS 1 and EDWS 6. This method enables an examination of the slowest responses (in addition to nonlapse response times), fastest responses, and variability of responses simultaneously. For instance, although some individuals may have larger mean RTs, this finding may be attributed only to long response times for a small number of responses. Response time distributions are not normal distributions, but rather have a long tail caused by longer RTs. The data were therefore fitted with a Weibull distribution curve, as previously described by Santhi et al.22 Using this method, we were able to evaluate all percentiles to describe and display succinctly the observed data in its entirety.
The 4-parameter Weibull curve fit to the data provides 4 outcome parameters describing the distribution of the data: (1) Shift - leading edge of the RT distribution reflecting the fastest possible response; (2) Scale - the spread of distribution, i.e. variability; (3) Shape - a measure of the skew of the distribution23; and (4) Midpoint(X0) - the 50th percentile (median). We examined these parameters using a 1-way repeated measures analysis of variance and subsequent paired comparisons. As performing paired comparisons at every percentile point to see where the distribution varies would increase the likelihood of a type 1 error, the Weibull analysis permits statistical assessment of the overall distribution of the responses and provides an estimation of the curve of the RT distribution by group (acute*chronic). This method also protects to some extent against “subject bias”; the overall distribution of data is very difficult to contrive, whereas simple measures such as number of lapses and slowest RTs can be manipulated much more easily by the participant (several 10-s reaction times will have a dramatic effect on the average RT, for example). After the acute and chronic interaction demonstrated by Cohen et al.,13 planned comparisons on the Scale parameter were performed to compare the spread of distribution at the end of the sixth shift (acute*chronic) compared with the end of the first shift (acute only). All data were analyzed using SPSS 18.0 software, SPSS Inc., Chicago, IL.
RESULTS
Sleep and Wake
Acute Sleep Loss
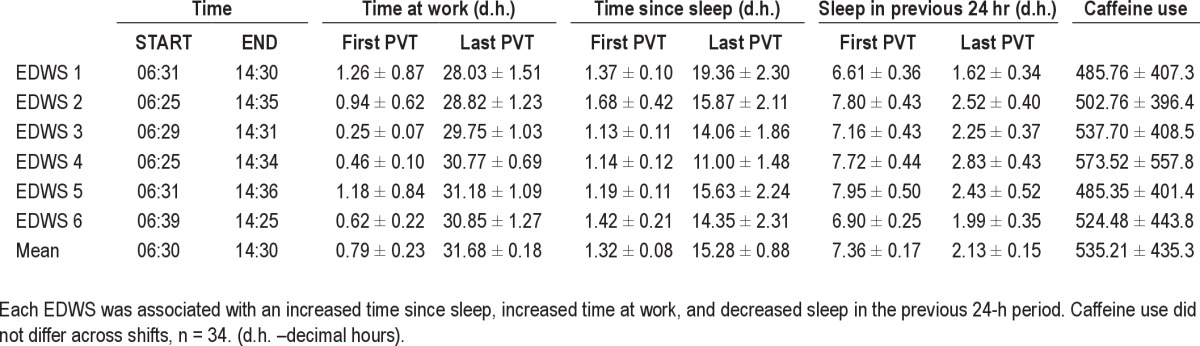
Each EDWS averaged 31.92 h ± 0.35 h in duration (95% confidence interval (CI): 31.26 – 32.59 h) and 30.4% (62 of 204) of these were completed without any sleep, such that, approximately 75% of participants (25 of 34) completed at least 1 EDWS without sleep. An average of 2.3 h of sleep was obtained per shift. As each EDWS was preceded by a day off or a swing shift, sleep in the previous 24 h at the beginning of the EDWS was of longer duration (8.44 ± 0.21 h) than at the end of shift, demonstrating acute sleep loss over the shift (Table 1). Compared to PVTs at the start of the shift, PVTs at the end of the shift were associated with greater time awake (t(1,33) = 10.89, P < 0.0005) and less sleep in the previous 24 h (t(1,33) = 14.28, P < 0.0005). Sleep duration in the 24 h before the PVT at the end of each EDWS differed (F(5,190) = 3.135, P < 0.01), such that EDWS 4 (P = 0.05) and EDWS 5 (P = 0.02) were preceded by more sleep than EDWS 1, demonstrating that any change in performance during the rotation was not simply due to changes in sleep duration before the test.
Table 1.
Average sleep/wake parameters (± standard deviation) at the time of psychomotor vigilance test (PVT) at the start (approximately 06:30 Day 1) and end (approximately 14:30 Day 2) of the extended duration work shift (EDWS)
Chronic Sleep Deficiency
Cumulative total sleep time at the end of each week was 43.4 h ± 0.92 h (95% CI: 42.5-44.3 h), 88.0 h ± 1.68 h (95% CI: 86.5–89.8 h) and 130.4 h ± 2.07 h (95% CI: 130.2–134.3 h), respectively, with a nightly average less than that obtained during two weeks of ambulatory clinic (7.75 h/night; Figure 2). EDWS resulted in cumulative sleep deficiency over the 21-day rotation, with an average sleep duration of 6.3 h ± 3.15 h per 24 h over the entire 21-day study.
Figure 2.
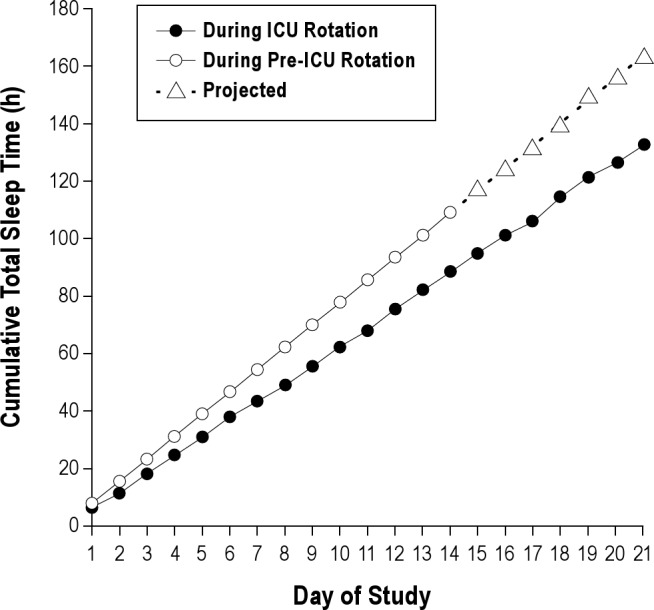
Cumulative total sleep time during the residents' intensive care unit (ICU) schedule including nocturnal sleep and diurnal naps in comparison with cumulative sleep over 14 days prior to the ICU rotation (including 7 days of projected cumulative sleep; dotted lines, open circle). Compared with daily sleep duration (7.75 h; 95% confidence interval 6.5-9.0 h) obtained when not on alternating extended duration shifts, when on a schedule including alternating 30-h shifts residents obtained 10.9 h less sleep by day 7, 20.4 h less sleep by day 14, and a projected 30.6 h less sleep by day 21, n = 34.
Effect of Time on Shift
There was a significant deterioration of mean RT with increasing duration of time on duty (F(3,96) = 16.918, P < 0.0005, η2 = 0.35), such that mean RT had slowed by more than a third at the end of the shift as compared with the start (Figure 3A). The number of lapses more than doubled during the shift (F(3,96) = 18.004, P < 0.0005, η2 = 0.36; Figure 3B) as did the RT for the slowest 10% of responses (485 to 1,083 ms; F(2.50,80.09) = 9.483, P < 0.0005, ϵ = 0.83, η2 = 0.23; Figure 3C). For mean RT, average reaction times were 284.6 ± 9.10 ms (95% CI 266.8-302.4 ms) at the beginning of the shift versus 379.4 ± 23.9 ms (95% CI 332.6–426.2 ms) at the end of the shift. For lapses, there were 2.7 ± 0.59 lapses (95% CI 1.52–3.8) at the beginning of shift which increased to 6.7 ± 0.82 (95% CI 5.2–8.5 lapses) at the end of the shift. For slowest 10% of RTs this increased from 485 ± 41.2 ms (95% CI 404.2–565.8 ms) at the beginning of the shift to 1083.1 ± 146.0 (95% CI 796.9–1299.2 ms) at the end of the shift. Post hoc tests showed that performance during NIGHT and DAY 2 was worse than DAY 1 (P < 0.0005) and EVENING trials (P < 0.001) (Figure 3C). Almost all interns (32 of 34, 94.1%) exhibited increased lapses from the start to the end of the shift.
Figure 3.
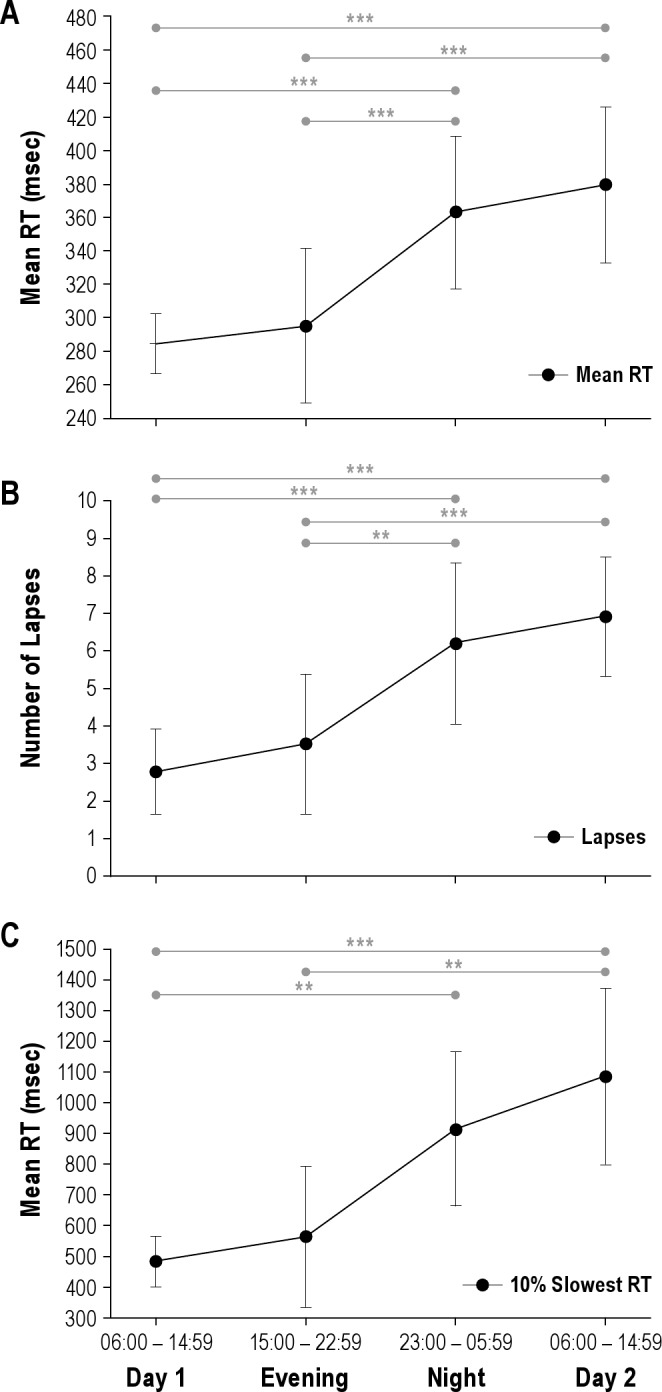
Mean change in mean PVT reaction time (RT) (A), lapses (B), and slowest 10% RTs (C) over the duration of an extended shift. Post hoc differences are shown (***P < 0.0005; **P < 0.001; *P < 0.005).Mean ± standard error of the mean reported, n=33.
Effect of Successive Shifts
There was a significant slowing of PVT mean RT with increasing number of successive EDWS (F(3.75, 89.99) = 4.714, P = 0.002, ϵ = 0.75, η2 = 0.16) such that mean RT slowed by 20% (from 320.0 ms to 385.4 ms, Figure 4A). Similarly, the rate of lapses (F(5,120) = 7.037, P < 0.0005, η2 = 0.23) and the RTs for the slowest 10% of responses (F(4.66, 111.94) = 2.250, P = 0.05, ϵ = 0.93, η2 = 0.08) also increased with successive shifts, whereby lapses increased 2.4-fold from 2.8 to 6.7 (Figure 4B), and the slowest 10% was slower by 361.7 ms, changing from 744.5 ms on EDWS 1 to 1106.2 ms on EDWS 6 (Figure 4C). Post hoc tests are shown in Figure 4B for lapses and Figure 4C for slowest 10% of responses. The observed effect of worsening performance from the first to the sixth extended duration shift was apparent in most of the interns (26 of 34, 76.5%).
Figure 4.
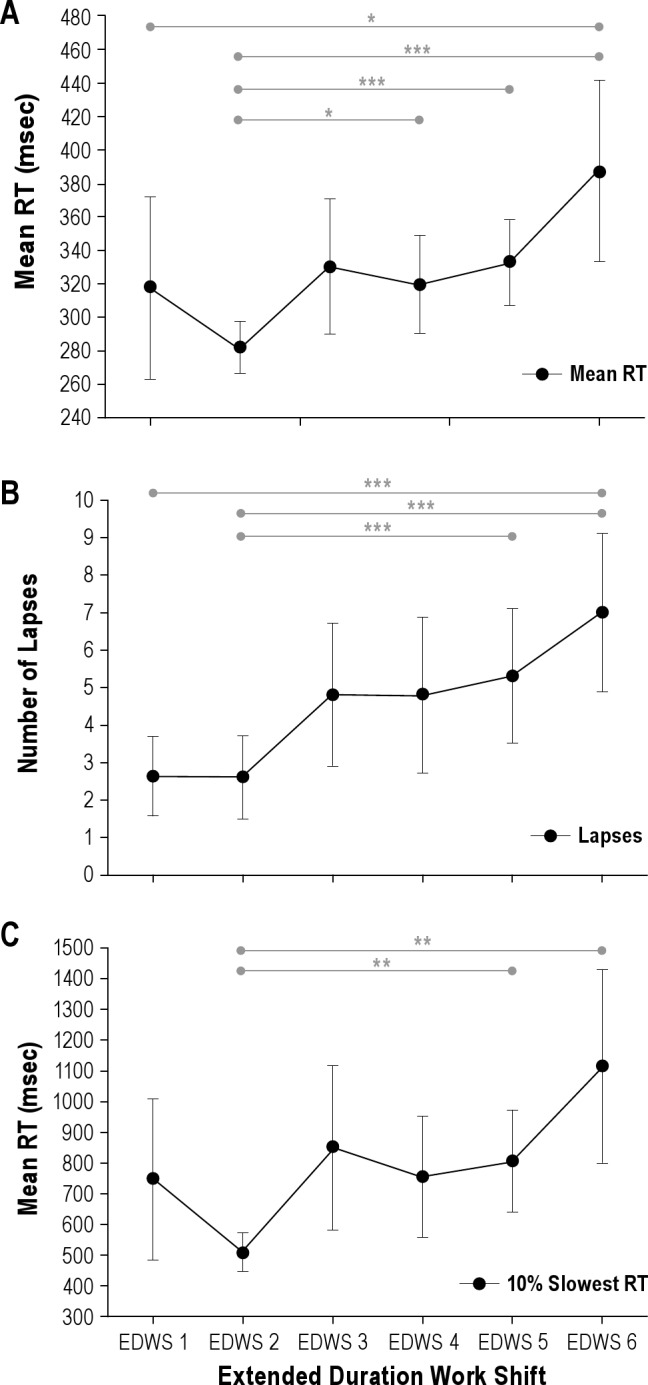
Degradation of neurobehavioral performance over 6 successive extended duration shifts on a 21-day rotation. Change in mean PVT reaction time (RT) (A), number of lapses (B), and slowest 10% RTs (C) from EDWS1 to EDWS6. Post hoc differences are shown (***P < 0.0005; **P < 0.001; *P < 0.005).Mean ± standard error of the mean reported, n = 31.
Interaction of Time on Shift and Number of Successive EDWS
For mean RT, there was a main effect of TIME (F(1,326.49) = 56.04, P < 0.0005), EDWS (F(5,321.35) = 4.692, P < 0.0005), and a significant EDWS × TIME interaction (F(11,315.27) = 8.599, P < 0.0005) (Figure 5A). Lapses exhibited a main effect of TIME (F(1,325.99) = 67.321, P < 0.0005) and EDWS (F(5,321.30) = 5.147, P < 0.0005), and significant TIME × EDWS interaction (F(11,315.16) = 10.301, P < 0.0005) (Figure 5B). For slowest 10% RTs, there was a significant main effect of TIME (F(1,329.30) = 25.58, P < 0.0005) and EDWS (F(5,322.895) = 2.764, P = 0.018), and a significant EDWS × TIME interaction (F(11,316.87) = 4.364, P < 0.0005) (Figure 5C). Moderately stable interindividual differences for lapses were seen in response to both time over the shift (ICC–0.37, Z = 3.482, P < 0.0005) and successive EDWS (ICC 0.35, Z = 3.436, P < 0.001) but were less stable for mean RT (ICC 0.26-0.29, Z > 3.10, P < 0.002) and the slowest 10% of responses (ICC 0.10-0.12, Z > 2.10, P < 0.03).
Figure 5.
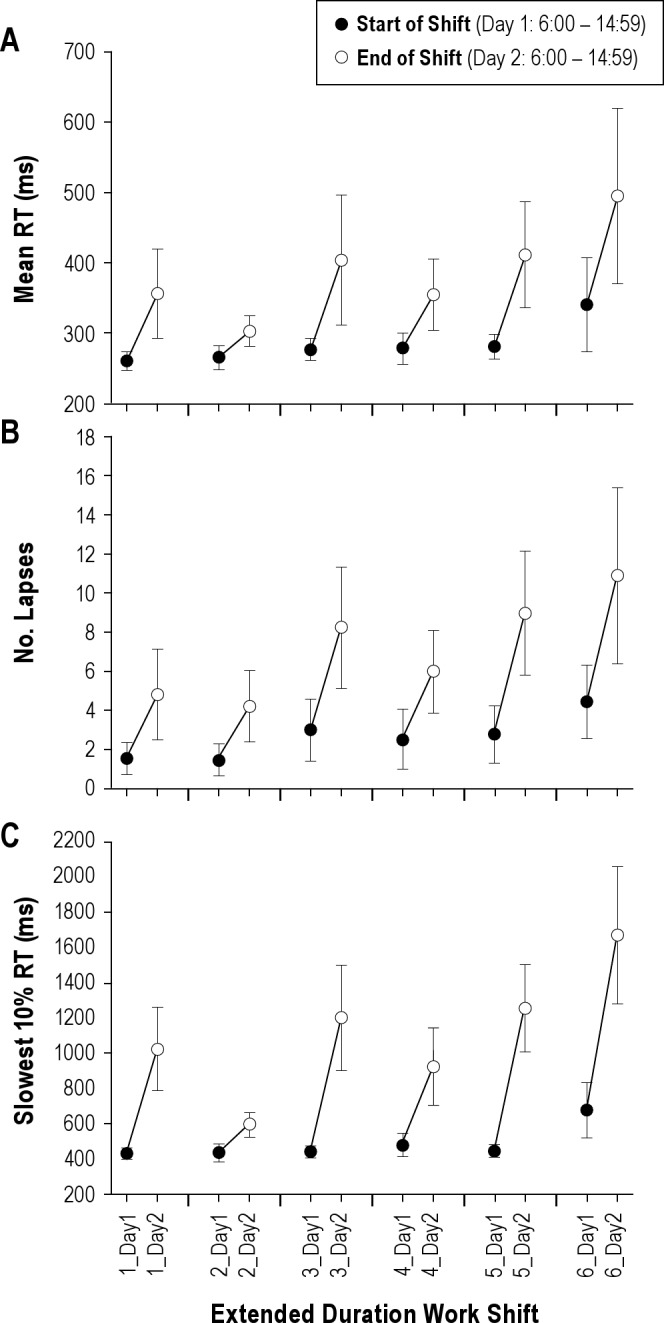
Interaction of chronic and acute effects: change in performance from the start of the extended duration work shift (Day 1 – 06:00-14:59) to the end of the extended duration work shift (06:00-14:59) mediated by the effects of each successive extended duration shift. Performance at the end of the shift shows greater chronic effects, whereas performance at the start of shifts remains relatively protected. Mean ± standard error of the mean reported,n = 34.
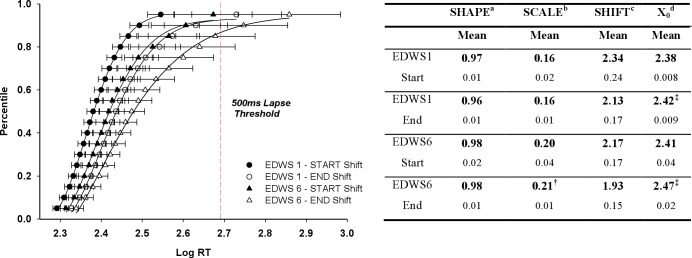
To examine the acute and chronic effects beyond simple mean RT, we evaluated the change in RT distribution at the start and end of the first and last EDWS (Figure 6). For the Shape (skew of the distribution) and Shift parameter (reflects fastest possible RT) there was no change across the 4 shifts (P = 0.076; P = 0.482, respectively). Although there was no main effect of EDWS on the Scale parameter (P = 0.159), which depicts the spread of distribution and longer RTs, planned pairwise comparisons did show a larger Scale parameter at the end of the sixth EDWS when compared with the end of the first EDWS (P = 0.003); Figure 6. The midpoint (X0) parameter is the median RT, and reflects cognitive slowing. This parameter significantly differed between shifts (F(3,20) = 16.551, P < 0.0005, η2 = 0.71), such that there was a significant slowing of responses at the end of the first and sixth EDWS when compared with the start of the first EDWS (P < 0.0005), and when comparing the end of the sixth EDWS with the end of the first EDWS (P = 0.004) and start of the sixth EDWS (P = 0.03). There were no significant differences for Shape, Scale, Shift, or midpoint parameters between the end of the first EDWS (acute effect) and the beginning of the sixth EDWS (chronic effect).
Figure 6.
Psychomotor vigilance test (PVT) cumulative reaction time (RT) distribution (left panel) with means and 95% confidence intervals and Weibull function parameters fitted to cumulative RT distribution (right panel). These data represent the average RT percentiles (mean ± 95% confidence intervals) and the fitted cumulative distribution function (4-parameter Weibull) from PVTs completed at the start and end of the first and sixth extended duration work shift (EDWS). The top and bottom axis represents log-transformed RTs and equivalent mean RTs, respectively. The y-axis represents the percentile value. A rightward shift corresponds to an overall cognitive slowing, and a stretching of the tail indicates increased variability and long RTs. Data distribution at the end of the first EDWS (EDWS 1 – End (open circle) was strikingly similar to the start of the sixth extended duration work shift (EDWS 6 – Start (filled triangle). Data distribution at the end of the EDWS suggests increased cognitive slowing and increased variability due to long RTs. The end of the sixth EDWS was associated with significantly greater variability than the start and end of the first shift, and enhanced cognitive slowing (offset midpoint), (n = 34). †P < 0.03, ‡P < 0.0005. aMeasure of skew (1.0 = exponential). bSpread of distribution reflecting variability. cLeading edge of RT (fastest possible response). dMidpoint (50th percentile) - cognitive slowing.
DISCUSSION
Our study shows that medical residents working 24- to 30-h EDWS every other shift exhibit impaired neurobehavioral performance over the course of each individual EDWS due to acute sleep loss; a cumulative deterioration of neurobehavioral performance with each successive EDWS due to chronic sleep deficiency; and a nonlinear interaction between acute and chronic sleep loss that multiplies the detrimental effects on performance. Our data show that repeated exposure to chronic sleep deficiency inherent in resident schedules exacerbates the degradation of performance due to acute sleep deprivation experienced during an EDWS.
The 24- to 30-h EDWS induced prolonged acute sleep deprivation, such that residents only obtained an average of 2.3 h sleep per shift, and no sleep at all for approximately 30% of these shifts. As predicted from previous laboratory studies,12,24 such acute sleep deprivation caused neurobehavioral performance to deteriorate, slowing average reaction time by 33% and more than doubling the average response times for the slowest 10% of responses to more than 1 sec, and increasing the rate of vigilance lapses 2.5-fold.
The effect of chronic sleep deficiency on the time course of neurobehavioral degradation is less well studied than acute sleep loss alone, and only one previous laboratory study has examined the interaction between chronic and acute sleep loss. In the current study, residents obtained only 6.3 h of sleep per day on average during the 21-day intensive care unit rotation and with a sporadic pattern. This failure to obtain adequate sleep each day caused a cumulative deterioration in performance across successive shifts, slowing average reaction time by 20% and the slowest 10% of responses by 49%, and caused more than a doubling in vigilance lapses by the sixth EDWS when compared with the first. Sleep obtained during the EDWS (2.3 h/shift) was extremely short but was followed by long recovery sleep (approximately 10 h) on scheduled days off or the intervening day (swing) shift (approximately 8.5 h). Consistent with laboratory data,13 these two full nights of recovery sleep in between each EDWS were insufficient to restore performance: On the sixth EDWS, residents' performance at the start of the EDWS was already impaired and then deteriorated much more rapidly when challenged by acute sleep deprivation than during the first EDWS (Figure 5). The rate of lapses in attention was 7 times higher at the end of the sixth EDWS compared with the beginning of the first extended shift and the slowest 10% of responses were more than 3 times slower. Cognitive slowing as examined using RT distribution showed that performance at the end of the first EDWS (acute effect) was strikingly similar to performance levels at the start of the sixth EDWS (chronic effect). In summary, although the acute and chronic effects alone were of similar magnitude, together they multiplied the performance impairment.
These findings echo a recent controlled laboratory study by Cohen et al.13 where a 10-fold increase in lapses was observed due to the interaction of acute and chronic sleep deprivation. Unlike our study, however, participants in the laboratory had tightly controlled sleep-wake schedules, controlled access to nutrition, and were living in very dim light. We were able to detect a decline in neurobehavioral performance in working residents despite the fact that they had unrestricted opportunity for recovery sleep between shifts, often napped during work shifts,25 and had free access to countermeasures including caffeine (an average of 535 mg/EDWS),26 physical activity,27 upright posture,28 and light.26 Although our data show moderately stable interindividual differences in PVT performance over time, this was less stable than previous laboratory studies,19 which may be due to other factors present in the field such as changeable levels of sleep, caffeine, work demands, etc. Given the instability of these factors in the field, it is remarkable that we observe these interindividual consistencies in PVT performance over time (whether over the course of one extended work shift or for responses to each subsequent extended work shift). This progressive decline in performance in individuals whose sleep opportunities and countermeasures access are not restricted has not previously been demonstrated in either the laboratory or in a field setting.
Our study used a proxy measure of neurobehavioral function, the PVT, rather than a direct measure of medical performance. The PVT is a well-validated test of sustained attention widely used to study the effects of sleep loss, including impairments in neurobehavioral function due to acute sleep deprivation (number of hours awake),12,15,29 chronic sleep deficiency,11–13 circadian phase (time of day),24,30 and alcohol and drug effects under both laboratory and field conditions, and may be considered a good indicator of medical performance. For example, Arnedt et al.3 demonstrated performance on the PVT after four weeks of “Heavy Call” (Q4 or Q5; EDWS starting every 4th or 5th day, respectively) deteriorated in a manner similar to that observed in an intern after four weeks of “Light Call” plus a blood alcohol concentration of 0.05%. We have also demonstrated that medical performance deteriorates over an EDWS in a manner similar to the PVT data using a high-fidelity medical performance simulator in a study of a subset of PGY-1 residents.4 Moreover, a comprehensive meta-analysis by the ACGME8 showed that sleep loss associated with 24- to 30-h EDWS reduced cognitive performance in the laboratory by almost 1 standard deviation (SD), and physicians' clinical performance by more than 1.5 SD, demonstrating that sleep loss degrades performance of physicians during clinical tasks at a comparable rate to nonclinical laboratory tests like the PVT.
In the current study, we only examined the effects of chronic sleep deficiency across a “Q3” shift and do not have data from other “call” shifts (e.g., “Q4”or “Q5”). The rate of accumulation of performance impairment due to chronic sleep deficiency is inversely correlated with the daily sleep opportunity that, at least over 14 days, shows an essentially linear trend.11,12 Consistent with this interpretation, we have shown previously that the number of EDWS worked per month is associated with an increased risk of motor vehicle accidents10 and percutaneous injury31 in residents. We would anticipate, therefore, that residents working a less frequent call schedule would still accumulate a chronic decline in performance but at a slower rate than the current study describes. In a recent systematic review, Levine et al.5 describe how reducing the number of extended calls shifts from a Q3 schedule to Q4 or less results in better resident quality of life, sleep and/or fatigue while increasing patient safety, which is likely due to a slower rate of impairment due to the combined effects of acute and chronic sleep deprivation and increased recovery time between extended duration shifts. The adverse acute effects on performance of working a 24- to 30-h shift apply the first and every time a 24- to 30-h EDWS is scheduled regardless of the frequency of the overnight call.10 Performance was always worse on Day 2 in comparison with the same time on Day 1 for every 1 of the 6 successive EDWS that we studied (Figure 3).
These data provide valuable insights into the time course of cumulative performance deterioration due to repeated exposure to EDWS and further highlight the need to reform resident schedules to prevent acute and chronic sleep deprivation and to allow for sufficient recovery time between shifts. Our study shows that even a single EDWS induces a measurable degree of cognitive impairment and that chronic repetition of these shifts exacerbates this impairment. In addition, these findings provide the first demonstration that laboratory data on the acute and chronic effects of sleep loss11–13 translates to real working environments for individuals who are subjected to acute and chronic sleep as an inherent part of their work shift schedules.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr Lockley has received two investigator-initiated research grants from the ResMed Foundation and an unrestricted equipment gift from ResMed Inc, in support of the studies described in this article; received consulting fees from Apollo Lighting, Naturebright, Sound Oasis, and Wyle Integrated Science and Engineering, and federally funded projects at Brigham and Women's Hospital, Thomas Jefferson University, and Warwick Medical School; lecture fees from Takeda Pharmaceuticals North America, I Slept Great/Euforma, LLC, and Emergency Social Services Association Conference, UK; unrestricted equipment gifts from Philips Lighting and Bionetics Corporation; an unrestricted monetary gift to support research from Swinburne University of Technology, Australia; a fellowship gift from Optalert, Pty Ltd, Melbourne, Australia; advance author payment and royalties from Oxford University Press, and honoraria from Servier Inc for writing an article for Dialogues in Clinical Neuroscience and from AMO Inc, for writing an educational monograph, neither of which refer to the companies' products; honoraria or travel and accommodation support for invited seminars, conference presentations or teaching from the Second International Symposium on the Design of Artificial Environments, Eighth International Conference on Managing Fatigue, American Academy of Sleep Medicine, American Society for Photobiology, Apollo Lighting, Bar Harbor Chamber of Commerce, Bassett Research Institute, Canadian Sleep Society, Committee of Interns and Residents, Coney Island Hospital, FASEB, Harvard University, Illinois Coalition for Responsible Outdoor Lighting, International Graduate School of Neuroscience, Japan National Institute of Occupational Safety and Health, Lightfair, National Research Council Canada, New York Academy of Sciences, North East Sleep Society, Ontario Association of Fire Chiefs, Philips Lighting, Thomas Jefferson University, University of Montreal, University of Tsukuba, University of Vermont College of Medicine, Utica College, Vanda Pharmaceuticals, Velux, Warwick Medical School, Woolcock Institute of Medical Research, and Wyle Integrated Science and Engineering (NASA); investigator-initiated research grants from Respironics Inc, Philips Lighting, Apollo Lighting, and Alcon Inc; and a service agreement and sponsor-initiated research contract from Vanda Pharmaceuticals. Dr Lockley also holds a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women's Hospital per hospital policy and has received revenue from a patent on the use of short-wavelength light, which is assigned to the University of Surrey. Dr Lockley has also served as a paid expert witness on behalf of two public bodies on arbitration panels related to sleep, circadian rhythms, and work hours. Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Actelion, Ltd.; Bombardier, Inc.; Boston Celtics; Cephalon, Inc.; Delta Airlines: Eli Lilly and Co.; Garda Siochana Inspectorate; Gerson Lehrman Group; Global Ground Support; Johnson – Johnson; Koninklijke Philips Electronics, N.Y.; Minnesota Timberwolves; Norfolk Southern; Novartis; Portland Trail Blazers; Respironics, lnc.; Sepracor, Inc.; Sleep Multimedia, Inc.; Somnus Therapeutics, Inc.; Yanda Pharmaceuticals, Inc.; and Zeo Inc. Dr. Czeisler owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Yanda Pharmaceuticals, Inc., and Zeo Inc., and received royalties from the Massachusetts Medical Society/New England Journal of Medicine; McGraw Hill, the New York Times, Penguin Press and Philips Respironics, Inc. Dr. Czeisler has received lecture fees from Accreditation Council of Graduate Medical Education; Alliance for Epilepsy Research; American Academy of Sleep Medicine; Cephalon, Inc., Duke University School of Medicine; Harvard School of Public Health; Mount Sinai School of Medicine; National Academy of Sciences; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKINIH); National Sleep Foundation; New England College of Occupational and Environmental Medicine (NECOEM); North East Sleep Society; Office of Rare Diseases Research (NIH); Rockpointe; Sleep Research Society; Society for Obstetric Anesthesia and Perinatology (SOAP); St. Luke's Roosevelt Hospital; University of Chicago; University of Colorado; the University of Virginia Medical Center; the University of Washington Medical Center; the University of Wisconsin Medical School. Dr. Czeisler has also received research prizes with monetary awards from the American Academy of Sleep Medicine; clinical trial research contracts from Cephalon, Inc.; an investigator-initiated research grant from Cephalon, Inc.; and his research laboratory at the Brigham and Women's Hopi tall has received unrestricted research and education funds and/or support for research expenses from Cephalon, Inc., Koninklijke Philips Electronics, N.Y., ResMed, ResMed Foundation; Committee for Interns and Residents, the CIR Policy and Education Initiative and the Brigham and Women's Hospital. The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which Dr. Czeisler directs, has received unrestricted research and educational gifts and endowment funds from: Boehringer Ingelheim Pharmaceuticals, Inc., Cephalon, Inc., George H. Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Hypnion, Jazz Pharmaceuticals, Jordan's Furniture, Merck – Co., Inc., Peter C. Farrell, Ph.D., Pfizer, ResMed, Respironics, Inc., Sanofi-Aventis, Inc., Sealy, Inc., Sepracor, Inc., Simmons, Sleep Health Centers LLC, Spring Aire, Takeda Pharmaceuticals and Tempur-Pedic. The HMS/DSM has received gifts from many outside organizations and individuals including: Brigham and Women's Hospital (Development Office), Catalyst Group, Cephalon, Inc., Committee for Interns and Residents, Eisai, Inc., Farrell Family Foundation, Jordan's Furniture, Lilly USA, LLC, Neurocare Center for Sleep, Philips-Respironics, Inc., Praxair US Homecare, Sanofi-Aventis, Inc., Select Comfort Corporation, Sepracor, Inc., Sleep HealthCenters LLC, Somaxon Pharmaceuticals, Synchrony Healthcare Communications, Yanda Pharmaceuticals, Inc., Wake Up Narcolepsy, Inc., Watermark MedicaL and Zeo, Inc. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc. and Sepracor, Inc. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author Contributions
Each author made contributions to the conception, design, analysis, or interpretation of the data; provided a critical revision of the intellectual content; and had final approval of the version to be published. The corresponding author (Dr. Anderson) had full access to all the data in the study and had the final responsibility for submission of the manuscript.
Funding Support
This study was supported by grants from the National Institute of Occupational Safety and Health within the U.S. Centers for Disease Control and Prevention (RO1 OH07567), which provided a Certificate of Confidentiality for data protection, the Agency for Healthcare Research and Quality (RO1 HS12032) affording data confidentiality protection by federal statute (Public Health Service Act; 42 U.S.C.), the Department of Medicine, Brigham and Women's Hospital, and the Division of Sleep Medicine, Harvard Medical School. The study was performed at Brigham and Women's Hospital Harvard Clinical and Translational Science Center, supported by the National Center for Research Resources (M01 RR02635 and 1 UL1 RR025758). Dr. Flynn-Evans was the recipient of a predoctoral fellowship in the program of training in Sleep, Circadian and Respiratory Neurobiology at Brigham and Women's Hospital (NHLBI; T32 HL079010). Drs. Lockley and Czeisler are supported in part by the National Space Biomedical Research Institute, through the National Aeronautics and Space Administration (NCC 9–58).
Role of the Sponsor
The National Institute of Occupational Safety and Health and the Agency for Healthcare Research and Quality had no role in the design and conduct of the study, the collection, preparation, or interpretation of the data, or the preparation or approval of the manuscript.
Additional Contributions
The authors would especially like to thank the study volunteers and the staff of the Coronary Care Unit and Medical Intensive Care Unit whose cooperation was vitally important. The authors are also indebted to the dedication and diligence of the Division of Sleep Medicine (DSM) technicians, and would like to thank Orfeu M. Buxton, PhD and John W. Cronin, M.D., for their invaluable contribution in conducting the study, and Joseph M. Ronda, MS, for assisting with PVT implementation. They extend their gratitude to the large number of people who helped with the design, planning, scheduling, and running of the study including members of the Harvard Work Hours, Health and Safety Group, the Department of Medicine at Brigham and Women's Hospital, and the technical and administrative staff at Brigham and Women's Hospital who supported the original protocol from which these data were derived, as noted previously.2,7,10
REFERENCES
- 1.Ulmer C, Wolman D, Johns M Committee on Optimizing Graduate Medical Trainee (Resident) Hours and Work Schedules to Improve Patient Safety for the Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, D.C: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 2.Landrigan CP, Rothschild JM, Cronin JW, et al. Effect of reducing interns' work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351:1838–48. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 3.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294:1025–33. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- 4.Gordon JA, Alexander EK, Lockley SW, et al. Does simulator-based clinical performance correlate with actual hospital behavior? The effect of extended work hours on patient care provided by medical interns. Acad Med. 2010;85:1583–8. doi: 10.1097/ACM.0b013e3181f073f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine AC, Adusumilli J, Landrigan CP. Effects of reducing or eliminating resident work shifts over 16 hours: a systematic review. Sleep. 2010;33:1043–53. doi: 10.1093/sleep/33.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasca TJ, Day SH, Amis ES., Jr The new recommendations on duty hours from the ACGME Task Force. N Engl J Med. 2010;363:e3. doi: 10.1056/NEJMsb1005800. [DOI] [PubMed] [Google Scholar]
- 7.Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns' weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351:1829–37. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 8.Philibert I. Sleep loss and performance in residents and nonphysicians: a meta-analytic examination. Seep. 2005;28:1392–402. doi: 10.1093/sleep/28.11.1392. [DOI] [PubMed] [Google Scholar]
- 9.Baldwin DC, Jr, Daugherty SR, Tsai R, Scotti MJ., Jr A national survey of residents' self-reported work hours: thinking beyond specialty. Acad Med. 2003;78:1154–63. doi: 10.1097/00001888-200311000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Barger LK, Cade BE, Ayas NT, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125–34. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 11.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 13.Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythmns, sleep, and neurobehavioural function in humans living on a 20-h day. Am J Physiol. 1999;46:R1152–R63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 15.Dinges DF, Kribbs NB. Performing while sleepy: Effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley and Sons, Ltd.; 1991. pp. 97–128. [Google Scholar]
- 16.Wright KP, JR., Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol. 2002;283:R1370–R7. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- 17.Barone JJ, Roberts HR. Caffeine consumption. Food and Chemical Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 18.McCusker RR, Goldberger BA, Cone EJ. Caffeine content of specialty coffees. J Anal Toxicol. 2003;27:520–2. doi: 10.1093/jat/27.7.520. [DOI] [PubMed] [Google Scholar]
- 19.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–33. [PubMed] [Google Scholar]
- 20.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 21.Rouder JN, Sun D, Speckman PL, Lu J, Zhou D. A Hierachical Bayesian Statistical Framework for Response Time Distributions. Psychometrika. 2003;68:589–606. [Google Scholar]
- 22.Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS One. 2007;2:e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myerson J, Robertson S, Hale S. Aging and intraindividual variability in performance: analyses of response time distributions. J Exp Anal Behav. 2007;88:319–37. doi: 10.1901/jeab.2007.88-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–R9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 25.Ficca G, Axelsson J, Mollicone DJ, Muto V, Vitiello MV. Naps, cognition and performance. Sleep Med Rev. 2010;14:249–58. doi: 10.1016/j.smrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:463–4. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Neri DF, Oyung RL, Colletti LM, Mallis MM, Tam PY, Dinges DF. Controlled breaks as a fatigue countermeasure on the flight deck. Aviat Space Environ Med. 2002;73:654–64. [PubMed] [Google Scholar]
- 28.Caldwell JA, Prazinko BF, Hall KK. The effects of body posture on resting electroencephalographic activity in sleep-deprived subjects. Clin Neurophysiol. 2000;111:464–70. doi: 10.1016/s1388-2457(99)00289-8. [DOI] [PubMed] [Google Scholar]
- 29.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 30.Lee JH, Wang W, Silva EJ, et al. Neurobehavioral performance in young adults living on a 28-h day for 6 weeks. Sleep. 2009;32:905–13. doi: 10.1093/sleep/32.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayas NT, Barger LK, Cade BE, et al. Extended work duration and the risk of self-reported percutaneous injuries in interns. JAMA. 2006;296:1055–62. doi: 10.1001/jama.296.9.1055. [DOI] [PubMed] [Google Scholar]