Background: A search for cell surface proteins amenable to antibody drug conjugate (ADC) therapy was performed.
Results: Expression of PMEL17 was highly restricted to melanoma cells, and an ADC directed against it was efficacious.
Conclusion: PMEL17 is an attractive target for ADC therapy in melanoma.
Significance: Intracellular transmembrane proteins that transit the cell surface represent a new class of targets for ADCs.
Keywords: Antibodies, Anticancer Drug, Cancer Therapy, Melanogenesis, Melanoma, Membrane Trafficking
Abstract
Melanocytes uniquely express specialized genes required for pigment formation, some of which are maintained following their transformation to melanoma. Here we exploit this property to selectively target melanoma with an antibody drug conjugate (ADC) specific to PMEL17, the product of the SILV pigment-forming gene. We describe new PMEL17 antibodies that detect the endogenous protein. These antibodies help define the secretory fate of PMEL17 and demonstrate its utility as an ADC target. Although newly synthesized PMEL17 is ultimately routed to the melanosome, we find substantial amounts accessible to our antibodies at the cell surface that undergo internalization and routing to a LAMP1-enriched, lysosome-related organelle. Accordingly, an ADC reactive with PMEL17 exhibits target-dependent tumor cell killing in vitro and in vivo.
Introduction
One of the major challenges in oncology drug development is that of deriving sufficient specificity to limit off-target effects, which frequently compromise the safety of a therapeutic in unanticipated ways. The specificity naturally inherent to antibodies overcomes this challenge, limiting the effects of the therapeutic only to cells expressing the target. Nevertheless, toxicities still occur, particularly when the target exhibits widespread normal tissue distribution, as exemplified by the xerodermatitis frequently associated with anti-EGF receptor therapy (1). Therefore, the ideal antibody target would be essential to cancer cell viability, yet be absent or functionally irrelevant in normal tissues.
These rather stringent criteria can, in part, be circumvented if the contribution of the target to cancer cell viability is disregarded, leaving only the requirement of highly restricted normal tissue distribution. Targets of this nature are amenable to antibody drug conjugate (ADC)2 therapy, wherein a highly potent cytotoxic agent is directed to the tumor by appending it to an antibody (2). The drug is released upon internalization of the ADC and kills the cell through a generalized mechanism not reliant upon antibody target function, nor any specific genetic attributes of the tumor cell. Moreover, if the cytotoxic mechanism requires cell proliferation, normal non-dividing cells that express the target will be affected to a lesser extent than proliferating cancer cells. The ADC approach is also facilitated by the recent availability of gene expression data from a wide variety of cancer and normal tissues that enable the rapid assessment of prospective targets (3).
In considering potential targets for ADC therapy, we reasoned that gene products that support functions or structures unique to highly specialized cells would, by definition, display restricted normal tissue distribution. Accordingly, the synthesis of melanin pigments is largely restricted to melanocytes and the postmitotic pigment epithelium of the eye (4, 5). Melanin pigments are produced in melanosomes, a specialized lysosome-related organelle wherein the pigment is deposited on fibrils composed of proteolytic fragments derived from the PMEL17 protein. PMEL17 is an integral membrane protein that undergoes export from the endoplasmic reticulum to the Golgi apparatus where it is glycosylated and, ultimately, trafficked to the melanosome (6). The specific route by which mature PMEL17 makes it way to the melanosome has been a subject of debate. However, it is apparent that some fraction of the protein is presented transiently at the cell surface prior to its entry into stage I melanosomes (7–10). Thus, internalization and routing of cell-surface PMEL17 could make it amenable to targeting with an antibody drug conjugate.
ADCs share the common feature of targeting internalizing cell surface proteins with an antibody covalently linked to a highly potent cytotoxic compound (2). In principal, this enables higher local exposure of the tumor to the drug than that permissible by systemic delivery of the free drug. Thus, ADCs are prodrugs that release their cell killing potential upon internalization and subsequent digestion in the protease-enriched vesicles. Recent advances in this technology have resulted in some very encouraging objective clinical responses, and numerous ADCs are now in various stages of development (2, 11, 12).
Encouraged by the success of ADCs in the cancer clinic, we sought to develop antibodies suitable for this approach in the treatment of melanoma. Accordingly, we evaluated the pigment-forming protein PMEL17 as a potential target because of its highly restricted expression pattern in normal tissues. Several antibodies to PMEL17 were generated and characterized, and one was selected for conjugation via a peptide linker to the potent antimitotic monomethylauristatin E. These antibodies help elucidate the secretory fate of PMEL17 and show that it is a surprisingly efficient target for ADC therapy.
EXPERIMENTAL PROCEDURES
Cell Culture
526mel, 888mel, 928mel, and 1300mel were a generous gift from Paul Robbins (Center for Cancer Research, Tumor Immunology Section). All others were either from the ATCC or NCI-60 (National Cancer Institute), and all were grown in appropriate media at 37 °C with 5% CO2. Normal human melanocytes were obtained from Invitrogen and grown in Invitrogen-recommended media.
Plasmid Constructs and Transfections
The full-length PMEL17 clone was initially obtained from Invitrogen Life Technology and subsequently PCR-amplified for subcloning into the pRKtkneo vector with an N-term gD tag. All the constructs for epitope mapping were carried out by PCR amplifications of each fragment and subcloning into either the pRKtkneo vector with the N-term gD tag or into the pRKtkneo vector with the N-term gD tag and C-term glycophosphatidylinositol anchor. Transfections were performed using FuGENE 6 (Roche), and an empty vector was used as control.
Development of Anti-PMEL17 Antibodies
Two separate groups of Balb/C mice (Charles River Laboratories, Hollister, CA) were hyperimmunized with either purified human PMEL17 extracellular domain in Ribi adjuvant (Group 1, Genentech) or with plasmid DNA encoding full-length PMEL17 (Group 2, Genentech) via hydrodynamic tail vein injection using a modified version of a protocol described previously (13–15). Following a final boost with protein (Group1) or PC3 cells overexpressing PMEL17 (Group 2) 3 days prior to fusion, B cells from mice demonstrating strong specific binding to PC3 cells by FACS from both groups were electrofused (BTX ECM 2001, Harvard Apparatus) at a 1:1 ratio with X63-Ag8.653 mouse myeloma cells (ATCC), plated at 100,000 cells/well in culture medium containing 1× azaserine and hypoxanthine (Sigma-Aldrich, St. Louis, MO) and incubated at 37 °C, 7% CO2. After 10–14 days, the supernatants were harvested and screened by ELISA and FACS. Positive clones demonstrating strong PMEL17-specific binding by FACS were then expanded and subcloned by limiting dilution. Following two rounds of subcloning and screening, the final clones were cultured in bioreactors (Integra Biosciences, Chur, Switzerland), and supernatants were purified by protein A affinity chromatography as described previously (16).
Indirect Fluorescence Microscopy and Direct Fluorescence Confocal Microscopy
Melanoma cells were seeded in Lab-Tek II cell culture-treated 4-well chamber slides (Nalge Nunc International) and incubated with PMEL17 mAb in the presence of the lysosomal protease inhibitors leupeptin and pepstatin A (Sigma-Aldrich) at 50 nm and 5 nm, respectively, at 2 μg/ml for either 2 h or 20 h in a 37 °C incubator with 5% CO2. Cells were then washed with PBS and fixed in 4% paraformaldehyde (Polysciences, Inc.) for 5 min at room temperature and permeabilized with 0.05% saponin (Sigma-Aldrich) in PBS with 0.5% BSA for 5 min at 37 °C. Cells were then incubated for 1 h with 2 μg/ml rabbit polyclonal anti-LAMPI (Sigma-Aldrich) followed by one hour incubation of Cy3-labeled anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) and Alexa Fluor 488-labeled anti-rabbit IgG (Invitrogen). PMEL17 antibodies were also directly labeled with either Alexa Fluoro 555 or Alexa Fluoro 488 (Invitrogen) and stained cells after they were fixed and permeabilized as above. A Leica SP5 confocal microscope (Leica Microsystems) was used for imaging.
Dual Color Confocal Live Imaging and Flow Cytometry
928mel cells were lifted from culture dishes with 5 mm EDTA and incubated with Alexa Fluor 488-derivitized 17A9 antibody and/or Alex Fluor 555-derivitized 77E6 antibody for 1 h on ice. As controls, cells were incubated with Alexa Fluor 488 and Alexa Fluor 555-derivitized normal mouse IgG. All subsequent washing and incubation was carried out at 4 °C. After incubation, cells were used for live imaging with a Leica SP5 confocal microscope (Leica Microsystems) and the rest for flow cytometry with FACSCalibur (BD Biosciences).
Immunohistochemistry
Immunohistochemistry was performed on 4-μm-thick formalin-fixed, paraffin-embedded tissue sections mounted on glass slides. All immunohistochemistry steps were carried out on the Ventana Discovery XT (Ventana Medical Systems, Tucson, AZ) autostainer. Pretreatment was done with cell conditioner 1, standard time. Primary antibody PMEL17 clone 31D1.6.7 was used at a concentration of 10 μg/ml and was incubated on slides for 1 h at 37 °C. Ventana Mouse OmniMap (Ventana Medical Systems) was used as the detection system. Ventana DAB and hematoxylin II were used for chromogenic detection and counterstain.
Inhibition of in Vitro Cell Proliferation
Proliferation in the presence of armed antibodies was assessed using cells plated at 2000/well in 50 μl of normal growth medium in 96-well clear-bottom plates (PerkinElmer Life Sciences). Twenty-four hours later, an additional 50 μl of culture medium with serial dilutions of armed antibodies was added to triplicate wells. Three or 5 days later, cell numbers were determined using CellTiter-GloII (Promega Corp.) and with an EnVision 2101 multilabel reader (PerkinElmer).
In Vivo Antitumor Efficacy
All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, “Guide for the Care and Use of Laboratory Animals.”
Antibodies were conjugated with monomethylauristatin E (MMAE) as described previously (17). For efficacy studies with antibody drug conjugates, female CRL Nu/Nu mice from Charles River Laboratories were inoculated subcutaneously in the dorsal right flank with 5 million SK23 cells in Hanks' balanced salt solution with Matrigel. When tumor volumes reached ∼200 mm3 (day 0), animals were randomized into groups of 10 each and administered a single IV injection of antibody conjugated to MMAE through the valine citrulline linker (17). MMAE conjugated to anti-GP120 antibody was used as a control. Tumor volumes were measured twice per week until study end. Tumor volumes were determined using digital calipers (Fred V. Fowler Company, Inc.) using the formula (L × W × W)/2. Tumor growth inhibition (%TGI) was calculated as the percentage of the area under the fitted curve (AUC) for the respective dose group per day in relation to the vehicle, so that %TGI = 100 × [1–(AUCtreatment/day)/(AUCvehicle/day)]. Curve fitting was applied to Log2 transformed individual tumor volume data using a linear mixed-effects model using the R package nlme, version 3.1-96 in R v2.10.1.
Microarray Gene Expression Analysis
For the analysis of PMEL17 mRNA expression in multiple human tumor and normal biopsy samples (Fig. 1A), the Affymetrix data were obtained from Gene Logic, Inc. (Gaithersburg, MD). The analysis shown is for probe set ID 209848_s_at, performed using the HGU133 Plus v2 GeneChip on 3879 normal human tissue samples, 1605 human cancer tissue samples (1291 primary and 314 metastatic), and 3872 human non-cancer disease tissue samples. Microarray data were normalized using the Affymetrix MAS (Microarray Analysis Suite) version 5.0 software, with sample expression values scaled to a trimmed mean of 500. Analysis of cultured cell lines was performed in a similar fashion, and a complete list of the cell lines and the corresponding average difference values are presented in supplemental Table 1.
FIGURE 1.

Expression of PMEL17 mRNA. Measurements were carried out on the Affymetrix U133P chip and are expressed as scaled average difference. A, relative expression of PMEL17 in human tissues. Dots above and below the horizontal lines represent a specimen from the indicated normal or neoplastic tissue, respectively. B, relative expression of PMEL17 mRNA in cultured human cancer cell lines. BC, breast cancer; CRC, colorectal cancer; GB, glioblastoma; NSCLC, non-small cell lung cancer; SC, small cell lung cancer; NHL, Non-Hodgkin's lymphoma; MEL, melanoma; MM, multiple myeloma; OVCA, ovarian cancer; PANC, pancreatic cancer; PROS, prostate cancer. Only 5% of the 487 different cell lines analyzed are identified in the figure. For a complete list, see supplemental Table 1.
RESULTS
Melanocytes synthesize melanin pigments and harbor a variety of highly specialized gene products specifically suited for this function (18). Some of these genes remain active following the neoplastic transformation of melanocytes and, thereby, represent highly specific markers for melanoma (19, 20). Such markers could provide a specific entry point for antibody drug conjugate therapy, provided they are accessible at the cell surface and are internalized and degraded subsequent to ADC binding. Although most of the pigment-forming gene products reside in intracellular vesicles, ample evidence indicates that the type I transmembrane protein PMEL17 is at least transiently present at the cell surface (7–10). Highly restricted normal tissue expression is a key feature for ADC targets. To determine whether PMEL17 would make a suitable target for ADC therapy, we assessed the distribution of its mRNA transcript across a very large panel of normal and neoplastic human tissues derived from a wide variety of organs. High-level expression of PMEL17 mRNA was strikingly restricted to neoplasms derived from skin, and all of these were classified as melanoma (Fig. 1A). A small number of benign kidney tumors that were also strongly positive were classified as epitheloid angiomyolipomas, whereas a handful of positive lymphomas were identified as cutaneous T-cell lymphomas. We also examined mRNA expression in a panel of 487 human cancer cell lines derived from various tissues and found expression only in those classified as melanoma (Fig. 1B and supplemental Table 1).
The highly restricted pattern of expression prompted us to generate antibodies to PMEL17 to be evaluated for use in ADC therapy. Several monoclonal antibodies were obtained by immunization of Balb-C mice with purified recombinant PMEL17 protein. One antibody, designated 17A9, reacted strongly by FACS with live melanoma cells, normal human melanocytes, and a PC3 cell line stably expressing PMEL17 cDNA (Fig. 2). The PC3 parental cell line was not reactive with 17A9 (not shown). We also immunized mice with cDNA coding for PMEL17 and obtained an additional FACS+ monoclonal antibody designated 77E6. Although 77E6 reacted with live cell lines expressing PMEL17, it did so inconsistently and more weakly than 17A9 (Fig. 2A). The heterogeneous staining of melanoma cells with 77E6 was more apparent when the antibodies were directly labeled with Alexa Fluor. Costaining of mel928 cells with 17A9-Alexa Fluor 488 and 77E6-Alexa Fluor 555 revealed uniform reactivity with 17A9, whereas populations of high and low reactivity were observed with 77E6 (Fig. 2B)
FIGURE 2.
Binding of PMEL17 antibodies to intact cells. A, the melanoma cell lines SK-MEL-23, 1300mel, SK-MEL-5, and UACC257, normal melanocytes, and the prostate cancer cell line PC3 stably expressing PMEL17 were subjected to fluorescence-activated flow cytometry using antibody 17A9 (red line), 77E6 (blue line), and secondary antibody conjugated with phycoerythrin or the conjugated secondary antibody only (black line). B, fluorescence-activated cell sorting of mel928 cells stained with antibodies 17A9 and 77E6 directly labeled with Alexa Fluor 488 (vertical axis) and Alexa Fluor 555 (horizontal axis), respectively.
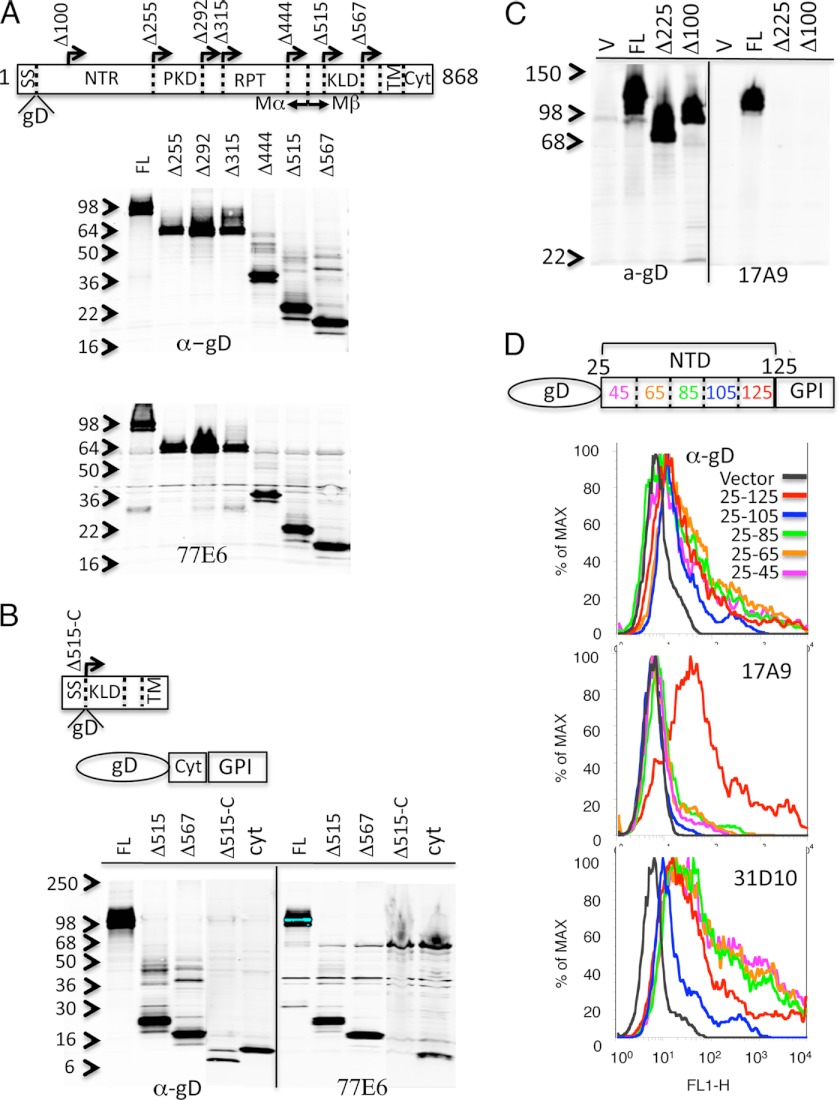
To gain insight into the differential reactivity observed with 77E6 and 17A9, the antibodies were reacted with various fragments of PMEL17 to localize their binding sties. A signal sequence and a gD epitope tag were fused to the N-terminal sequence of a series of deletion mutants that were analyzed by immunoblotting for reactivity with 77E6 (Fig. 3A). Surprisingly, the antibody recognized all deletion constructs, including Δ567, which contained only a small, undefined domain, the transmembrane domain and the cytoplasmic region. The C-terminal region of PMEL17 was further analyzed by generating a Δ515 fragment lacking the cytoplasmic region (Δ515-C) and another fragment containing only the cytoplasmic domain (Cyt) (Fig. 3B). The reactivity with these two constructs demonstrates that 77E6 reacts with the sequence present in the cytoplasmic domain of PMEL17.
FIGURE 3.
Localization of antibody binding sites on PMEL17. A, the PMEL17 deletion series represented in the schematic were expressed in mammalian cells, and the lysates were analyzed by immunoblotting with anti-gD and antibody 77E6. A signal sequence (SS) and the gD epitope were fused in-frame to each construct, followed by the PMEL17 sequence initiated at the indicated amino acid position (arrows). B, the Δ515 construct lacking the cytoplasmic domain (Δ515-C) and the cytoplasmic domain fused to a C-terminal GPI anchor (Cyt) were analyzed as in A. C, the indicated constructs were expressed and analyzed by immunoblotting with antibody 17A9. D, amino acid residues 25–125, 24–104, 25–85, 25–65, and 25–45 from the PMEL717 N-terminal domain (NTD) were fused to the gD epitope at the N terminus and GPI anchor at the C terminus. Constructs were expressed in 293 cells subjected to fluorescence-activated cell sorting with anti-gD (top panel), 17A9 (center panel), and 31D10 (bottom panel).
A similar assessment with 17A9 demonstrated a loss of immunoblotting reactivity upon deletion of the N-terminal region, Δ225, as well as with an additional construct, Δ100, lacking only the first 100 N-terminal amino acids (Fig. 3C). We further assessed this 100 amino segment with five additional constructs containing progressive deletions of 20 amino acids, each from the C terminus. A GPI anchor was fused to the C terminus to accommodate plasma membrane binding, and a gD epitope tag was fused to the N terminus to assess expression. Upon transient transfection into 293 cells, expression of all constructs generated FACS+ signals, as evidenced by anti-gD binding (Fig. 3D). Reactivity with 17A9 was lost upon deletion of amino acids 80–100 of this 100-amino acid N-terminal PMEL17 fragment. One of our additional monoclonal antibodies, 31D1, reacted with all of the constructs, providing further support for their expression at the cell surface.
Although the binding of 17A9 to N-terminal sequence of PMEL17 is consistent with the expected orientation of this protein in the cell membrane, we were surprised to find that the FACS+ 77E6 antibody reacted with the cytoplasmic domain. PMEL17 is known to undergo rather complex processing during its routing to the melanosome, some of which involves proteolytic cleavages ultimately resulting in deposition of limited RPT domain-containing fragments into fibrillar structures within the mature organelle (21–26). This processing involves cleavage at Arg-469 by a proprotein convertase, resulting in an N-terminal Mα and a C-terminal Mβ fragment that remain tethered by a disulfide bond. The Mβ fragment is cleaved further by a metalloproteinase at Gln-583 and by γ-secretase in the transmembrane region. Thus, regions of PMEL17 containing the cytoplasmic and N-terminal epitopes are dissociated during this processing.
To assess the subcellular distribution of these PMEL17 epitopes in fixed permeabilized cells, we directly labeled 77E6 with Cy3 Fluorophor and the antibody 14C10 with Alexa Fluor 488. Our monoclonal antibody 14C10 reacts with the same deletion fragments (presented in Fig. 3D) as 17A9 but is more suitable for immunofluorescent analysis of fixed, permeabilized cells. The results show that a large pool of PMEL17 containing both the cytoplasmic and the N-terminal epitopes is present in a perinuclear structure, likely the endoplasmic reticulum (Fig. 4 A, yellow). However, in extending toward the cell periphery, a downward gradient of staining intensity by 77E6 (Fig. 4A, red) is evident, followed by an upward gradient of 14C10 staining (Fig. 4A, green) that includes reactivity at the cell membrane. These results suggest that the cytoplasmic epitope recognized by 77E6 is removed during trafficking of PMEL17 to the plasma membrane whereas that the N-terminal epitope progresses to the cell surface.
FIGURE 4.
Immunfluorescent localization of PMEL17 in melanoma cells. A, fixed and permeabilized mel928 cells were reacted with antibodies 14C10 and 77E6 that were directly labeled with Alexa Fluor 488 (green) and Alexa Fluor 555 (red), respectively. Two examples are presented for the independent channels and the merged channel images. B, fluorescent activated cell sorting of mel928 cells stained with antibodies 17A9 and 77E6 directly labeled with Alexa Fluor 488 (light green) and Alexa Fluor 555 (red), respectively. Cells were also reacted with both antibodies simultaneously (blue), normal mouse IgG labeled with Alexa Fluor 488 (dark green), or Alexa Fluor 555 (fuchsia) or no antibody (black). Cytometry was gated for detection of Alexa Fluor 488 (left panel) or Alexa Fluor 555 (right panel). C, dual color confocal live imaging of mel928 cells reacted antibodies 17A9 and 77E6 that were directly labeled with Alexa Fluor 488 (green) and Alexa Fluor 555 (red), respectively.
Consistent with the release of cytoplasmic structure during secretory processing, we found that PMEL17 recovered from conditioned media also failed to react with 77E6 (supplemental Fig. 1). When excised from non-reducing SDS-gels, this secreted PMEL17 generated peptide sequences derived from both Mα and Mβ, but not from the cytoplasmic region, as determined by mass spectrometry (supplemental Fig. 2). Upon reduction with 2-mercaptoethanol, the secreted PMEL17 exhibited enhanced mobility on SDS-gels and Mα, but not Mβ, sequences were generated from this excised band (supplemental Fig. 2). These results suggest that the secreted PMEL17 contains Mα and Mβ regions tethered by a disulfide but lacks a cytoplasmic sequence.
The predominance of the N-terminal epitope at the cell surface relative to the cytoplasmic epitope is consistent with superior FACS signals observed with 17A9 over 77E6. Nevertheless, some portion of the cytoplasmic region must be present at the cell surface to account for the FACS signals observed with 77E6. To address this, we reacted live cells with the 77E6 and 17A9 antibodies that were directly labeled with Alexa Fluors 555 and 488, respectively. To demonstrate that the antibodies did not compete with each other for binding to live cells, we incubated cells with either of the labeled antibodies alone or both together and performed a FACS analysis gated for the individual fluorophors. The binding of the individual antibodies was unaffected by the presence of the other antibody (Fig. 4B). Visualization of live-labeled cells revealed uniform cell surface staining with 17A9, consistent with ample N-terminal epitopes accessible at the plasma membrane (Fig. 4C). In contrast, 77E6 staining was more isolated and patchy and largely non-overlapping with 17A9 reactivity. Although 17A9 stained all cells uniformly and consistently, 77E6 staining was variable from cell to cell, and the overall degree of staining varied across preparations. Subsequent experiments demonstrated that suspension of cells greatly enhanced the presence of 77E6 staining at the cell surface, indicating that culture conditions likely accounted for this variability (supplemental Fig. 3). Overall, the uniform and intense reactivity of 17A9 on melanoma cells led us to select this antibody as a candidate for drug conjugation. The antibody 31D1 also reacted uniformly with live cells by flow cytometry, but more weakly than 17A9, and was therefore not selected, as we have found this to correlate with cell killing by the conjugated antibody. Finally, antibody 14C10 appeared very similar to 17A9 and thus did not offer any advantage over it.
One of the key attributes of an ADC target is the ability to internalize the antibody into a degradative compartment in the cell. Accordingly, we incubated live melanoma cells with 17A9, and following incubation for 2 h, we fixed, permeabilized, and costained the cells for the lysosomal-associated membrane protein LAMP1. Considerable overlap of 17A9 and LAMP1 staining was apparent within the cells (Fig. 5A). Rapid uptake of 17A9 into additional PMEL17+ melanoma cell lines was observed consistently (supplemental Fig. 4). These results predict that a 17A9 ADC should effectively internalize, undergo degradation, and release drug to kill melanoma cells. We therefore conjugated the potent cytotoxin MMAE to 17A9 via a peptide linker susceptible to cleavage by cathepsins (17). Titration of a panel of PMEL17+ melanoma cells with the 17A9 ADC resulted in effective cell killing with IC50s ranging from 10–100 ng/ml of ADC (Fig. 5B). The prostate cancer cell line PC3 stably expressing PMEL17 was killed effectively, but no killing of the vector control PC3 parental cell line was noted out to 10 μg/ml of ADC.
FIGURE 5.
Antibody internalization and cell killing. A, live 1300mel melanoma cells were incubated with mouse 17A9 for 2 h, fixed, permeabilized, and then reacted with rabbit-anti LAMP1 followed by anti-rabbit-Alexa Fluor 488 (LAMP1, green) and anti-mouse-Cy3 (17A9, red). B, the indicated cells were incubated with serial dilutions of the 17A9 ADC (ng/ml ADC), and 5 days later cell numbers were determined using CellTiter-GloII.
The variation in ADC IC50 values for the melanoma cell lines could reflect the variation in the levels of PMEL17 accessible to the ADC, although additional factors likely contribute. For example, the 1300mel cell line expresses significantly more cell surface PMEL17 than the SK-MEL-5 cell line, yet the ADC IC50 is slightly higher for 1300mel. Variation in response of the cell lines to the released MMAE drug product could be another contributing factor. The IC50 values for free MMAE across eight melanoma cell lines ranged from ∼0.1–0.5 nm (supplemental Fig. 5A). Although we did not observe a strict correlation between the response to free drug and the ADC, a severalfold difference in free drug sensitivity could account for the variation in sensitivity to ADC. Indeed, the SK-MEL-5 was more sensitive to free MMAE than 1300mel, which could thus compensate for having lower levels of cell surface PMEL17 than 1300mel (Fig. 5B and supplemental Fig. 5). Other factors, including the relative rate of antibody internalization, might also affect the response to the ADC. Accordingly, we compared the ability of four different melanoma cell lines to accumulate and internalize antibody over time. As expected, cells with higher levels of cell surface PMEL17 accumulated more antibody, but the percentage of that amount internalized appeared comparable, indicating little difference in the relative efficiency of uptake (data not shown).
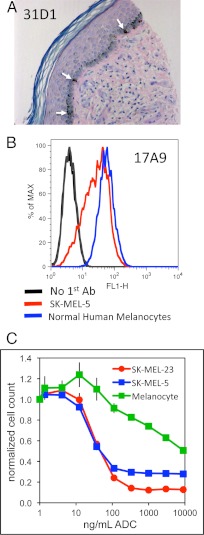
To assess the potential liability of targeting PMEL17 on normal melanocytes, we evaluated the level of expression of this target in normal human skin by immunohistochemistry. Staining with PMEL17 antibody 31D1 revealed intermittent reactivity along the basal layer of the dermis, consistent with the distribution of melanocytes in skin (Fig. 6A). Cultured normal human melanocytes were also FACS+ with antibody 17A9, and the intensity was comparable to that observed with melanoma cell line SK-MEL-5 (Fig. 6B). However, titration of the normal melanocytes with the 17A9 ADC resulted in cell killing with an IC50 nearly 100-fold higher than that observed with melanoma cell lines (Fig. 6C). This is consistent with the mechanism of action of the antimitotic drug MMAE and the reduced proliferative capacity of normal melanocytes relative to melanoma cells.
FIGURE 6.
Expression of PMEL17 on normal melanocytes. A, PMEL17 expression in a fixed paraffin-embedded section of normal human skin was stained with mouse anti-PMEL17 antibody 31D10 and hematoxylin and eosin. B, normal human melanocytes and SK-MEL-5 melanoma cells were reacted with chimeric 17A9 or no primary antibody followed by Alexa Fluor 488-labeled anti-human IgG and analyzed by flow cytometry. C, SK-MEL-5 and SK-MEL-23 melanoma cells or normal human melanocytes were incubated with serial dilutions of the 17A9 ADC, and 5 days later cell numbers were determined using CellTiter-GloII.
Finally we evaluated the expression of PMEL17 by immunohistochemistry across a panel of human melanoma tissue specimens using both 17A9 and 31D1. Staining was scored on an arbitrary scale ranging from zero for absent to 3+ for most intense. The full spectrum of staining was observed across the 58 human melanoma specimens, with the majority scoring positive with either antibody (Fig. 7A). Relative to 17A9, the staining with 31D1 was more highly skewed toward the upper end of the scale (Fig. 7A). These 58 specimens were obtained from 30 primary and 28 metastatic melanomas, with the majority of either of these subgroups scoring positive for PMEL17. We also sectioned fixed paraffin-embedded pellets of melanoma cell lines and stained them alongside the tissue specimens to gauge their staining intensity relative to actual melanoma (Fig. 7A).
FIGURE 7.
Relative expression of PMEL17 on melanoma cells and ADC-dependent anti-tumor activity. A panel of 58 fixed paraffin-embedded specimens of human melanoma and five sections obtained from pelleted paraffin-embedded melanoma cell lines were stained with anti-PMEL17 antibodies 17A9 or 31D10. Scores for relative staining intensity (0,1, 2, or 3) for the melanomas are listed (table, bottom panel), and representative images for each scoring level are presented next to images of the five stained melanoma cell lines. B, subcutaneous tumors were established in mice inoculated with SK-MEL-23 cells. When tumor volumes reached ∼200 mm3 (day 0), animals were given a single intravenous injection of PBS (vehicle), control ADC (anti-GP120vcMMAE), or anti-PMEL17 ADC (17A9vcMMAE) at either 2 or 6 mg/kg (MPK). Average tumor volumes with standard deviations were determined from 10 animals per group.
To evaluate in vivo anti-tumor activity of the 17A9 ADC, we selected the SK-MEL-23 melanoma cell line, which exhibited a staining intensity of 2+ to 3+ relative to the melanoma specimens. Subcutaneous tumor xenografts were established with SK-MEL-23 until the average volumes reached ∼200 mm3, at which point the animals were randomized into five groups of ten mice each. A single injection of the 17A9 ADC or the anti-GP120 control ADC was administered at a dose of 2 or 6 mg/kg, and tumor volumes were measured twice per week. Although the 2 mg/kg dose of 17A9 ADC, as well as both dose levels of the control ADC, had very little effect relative to the vehicle, the 6 mg/kg dose of 17A9 ADC retarded tumor growth for several weeks (Fig. 7B). These results demonstrate a robust and specific efficacy with an ADC targeting PMEL17 on a human melanoma tumor xenograft.
DISCUSSION
Enthusiasm for the use of antibody drug conjugates in cancer therapy has risen over the past few years. This is principally due to the advent of new approaches in linking drugs to antibodies, the utilization of more potent drugs, and new high throughput technologies enabling the identification of appropriate antibody targets. There are numerous ADCs currently in various phases of clinical testing, and one of them, SGN-35, was granted accelerated approval by the United States Food and Drug Administration (2, 12). Although the ability to specifically target cancer cells with increasingly potent drugs is appealing, there remain safety concerns associated with the unintended delivery of these highly toxic compounds to normal tissues. Some of this toxicity results from the normal clearance of the circulating antibody that, upon catabolism, generates the free active drug. Secondly, ADCs appear to undergo internalization, via a poorly understood endocytotic process, into normal cells lacking an antibody target. These toxicities resulting from antibody clearance and nonspecific ADC uptake are challenging impediments to dose escalation and will require further innovation of the platform ADC technologies to ameliorate.
An additional safety concern is more specific and relates to the choice of antibody target (27). The susceptibility of normal tissues to target-dependent toxicity will likely be a function of the mechanism of action of the drug. Antimitotic agents adversely affect regenerative tissue compartments such as bone marrow, intestine, and skin. For example, the targeting of the tubulin-disrupting agent maytansine to normal skin keratinocytes via conjugation to anti-CD44 resulted in severe epidermal toxicity (28). Thus target selection can be a critical factor in defining dose-limiting toxicities. Moreover, restricted target expression is less likely to impact antibody pharmocokinetics, which can be perturbed by dense target antigen sinks present in normal tissues (29). These characteristics led us to PMEL17 as a potential ADC target.
Cell surface expression and internalization to a degradative compartment are also key attributes of an ADC target. Thus, we were initially concerned about the description of PMEL17 as intracellular, where it undergoes glycolytic and proteolytic processing to ultimately form fibrillar sheets in the mature melanosome (7). However, there is ample evidence supporting cell surface accessibility of PMEL17, where its trafficking to the melanosome involves sorting of PMEL17 from the transgolgi network to the plasma membrane followed by endocytosis and sorting to stage I melanosomes (8, 22, 30–32). Our internalization studies with the PMEL17-specific 17A9 antibody confirmed rapid internalization and colocalization with the lysomomal marker LAMP1. Moreover, the 17A9 ADC readily killed melanoma cells, indicating that it was taken up and degraded to release the MMAE drug product.
In our initial characterization of monoclonal antibodies raised against PMEL17, the antibody designated 77E6, obtained by cDNA immunization, was FACS-positive on melanoma cells, yet the epitope mapped to a cytoplasmic or extralumenal sequence. Immunocytofluorescent analysis of live-labeled cells indeed confirmed the accessibility of 77E6 to cell surface PMEL17. However, unlike the staining observed with N-terminal-reactive 17A9, the staining with 77E6 was neither uniform nor consistent across cells. Moreover, staining with 17A9 and 77E6 appeared non-overlapping, suggesting that these epitopes reside in independent cell surface fragments derived from PMEL17. PMEL17 is known to undergo proteolytic processing by a furin-like proprotein convertase to an N-terminal-containing Mα fragment and a transmembrane-anchored Mβ fragment, which remain tethered to each other by a disulfide bond (21). The Mα fragment is released into the vesicle lumen, and further processing results in smaller fibrillogenic fragments that are localized to intralumenal vesicles in stage I melanosomes (7).
The release of Mα from the membrane could occur by disulfide reduction or, perhaps more likely, by proteolytic cleavage C-terminal to the Mβ cysteine that participates in the disulfide bond (6). In support of a proteolytic mechanism, a recent report described a juxtamembrane cleavage of PMEL17 at amino acid 583 by a metalloproteinase (24). The remaining membrane-associated fragment undergoes intramembrane cleavage by γ-secretase to release the cytoplasmic domain. These proteolytic events are consistent with the physical separation of the 17A9 and 77E6 epitopes that we have observed by immunostaining. Staining of fixed permeabilized cells with N-terminal reactive 14C10 and C-terminal reactive 77E6 further supports the separation of these two epitopes during PMEL17 processing. Abundant costaining with the two antibodies was evident in the perinuclear region where unprocessed PMEL17 is localized. However, at the plasma membrane, most of the 14C10 epitope is detected independent of 77E6 staining. By contrast, the majority of the 77E6 epitope is detected independently in the cytosol or secretory apparatus. These results are consistent with a model in which the excision of the cytoplasmic 77E6 epitope from PMEL17 largely occurs prior to the arrival of pMEL17 at the cell surface.
The lack of a cytoplasmic domain was also apparent in our analysis of secreted PMEL17. PMEL17 immunoprecipitated with 17A9 from media conditioned by mel928 cells was not reactive with 77E6 by immunoblotting. Moreover, mass spectrometric analysis of this material recovered from denaturing gels under non-reducing conditions revealed peptides derived from various regions of Mα and Mβ but no cytoplasmic domain peptides. Under reducing conditions, this immunoprecipitate exhibited increased mobility and contained peptides derived from Mα but none from Mβ. These results corroborate those of Hoashi et al. (23), who reported the presence of both Mα and Mβ fragments in secreted PMEL17. Our results also indicate that most of the plasma membrane-associated and secreted forms of PMEL17 lack the cytoplasmic domain epitope recognized by 77E6. Nevertheless, a fragment of PMEL17 containing this epitope is presented at the cell surface in low levels where it does not overlap with the N-terminal 17A9 epitope. This membrane-bound C-terminal fragment might be a transient product reported to result from metalloproteinase cleavage near the juxtamembrane region of PMEL17 (24).
The characterization of our PMEL17 antibodies led us to implement 17A9 as a candidate for drug conjugation. Mechanistic studies on antibody drug conjugates have revealed that the release of active drug occurs in the context of antibody degradation. For example, the use of non-cleavable linkers results in the generation of a drug product that remains associated with the linker and the amino acid residue to which the drug was originally appended (33). The coupling of drug release to antibody degradation suggests that the 17A9 ADC associated with cell-surface PMEL17 is likely degraded following its uptake into melanoma cells. Accordingly, we find considerable overlap in the staining patterns of internalized 17A9 and the late endosome/lysosome marker LAMP1 in melanoma cells. However, the route by which internalized 17A9 is trafficked to these LAMP1-enriched vesicles is unclear. Following endocytosis, PMEL17 enters early-stage melanosomes, which are independent of the lysosomal compartments enriched in LAMP1 (31). Therefore, the internalized17A9 could be associated with a minor fraction of PMEL17 that progresses through melanosomes to classical lysosomes. Alternatively, bound 17A9 could interfere with normal PMEL17 trafficking, resulting in its accumulation in lysosomes. Indeed, amino acid deletions or substitutions near the NTR-PKD boundary (amino acids 190–208), which resides near the 17A9 binding site (amino acids 120–124), result in aberrant accumulation of PMEL17 in LAMP1-enriched vesicles (30).
Antibody drug conjugates have recently emerged as a highly effective therapy for certain malignancies (2). So far, the targets entertained for this approach have either been anchored to or span the plasma membrane, thereby presenting an extracellular structure for antibody recognition. The effective killing of melanoma cells in vitro and in vivo with an ADC recognizing PMEL17 realizes a novel class of target-molecules destined for intracellular compartments that transit the plasma membrane en route to this location.
Supplementary Material

This article contains supplemental Figs. 1–5 and Table 1.
- ADC
- antibody drug conjugate
- MMAE
- monomethylauristatin E.
REFERENCES
- 1. Garrett C. R., Eng C. (2011) Cetuximab in the treatment of patients with colorectal cancer. Expert Opin. Biol. Ther. 11, 937–949 [DOI] [PubMed] [Google Scholar]
- 2. Alley S. C., Okeley N. M., Senter P. D. (2010) Antibody-drug conjugates. Targeted drug delivery for cancer. Curr. Opin. Chem. Biol. 14, 529–537 [DOI] [PubMed] [Google Scholar]
- 3. Wilhite S. E., Barrett T. (2012) Strategies to explore functional genomics data sets in NCBI's GEO database. Methods Mol. Biol. 802, 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schallreuter K. U., Kothari S., Chavan B., Spencer J. D. (2008) Regulation of melanogenesis. Controversies and new concepts. Exp. Dermatol. 17, 395–404 [DOI] [PubMed] [Google Scholar]
- 5. Yamaguchi Y., Hearing V. J. (2009) Physiological factors that regulate skin pigmentation. Biofactors 35, 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Theos A. C., Truschel S. T., Raposo G., Marks M. S. (2005) The Silver locus product Pmel17/gp100/Silv/ME20. Controversial in name and in function. Pigment Cell Res. 18, 322–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raposo G., Marks M. S., Cutler D. F. (2007) Lysosome-related organelles. Driving post-Golgi compartments into specialisation. Curr. Opin. Cell Biol. 19, 394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Theos A. C., Berson J. F., Theos S. C., Herman K. E., Harper D. C., Tenza D., Sviderskaya E. V., Lamoreux M. L., Bennett D. C., Raposo G., Marks M. S. (2006) Dual loss of ER export and endocytic signals with altered melanosome morphology in the silver mutation of Pmel17. Mol. Biol. Cell 17, 3598–3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valencia J. C., Watabe H., Chi A., Rouzaud F., Chen K. G., Vieira W. D., Takahashi K., Yamaguchi Y., Berens W., Nagashima K., Shabanowitz J., Hunt D. F., Appella E., Hearing V. J. (2006) Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2. Evidence for the polarized nature of melanocytes. J. Cell Sci. 119, 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yasumoto K., Watabe H., Valencia J. C., Kushimoto T., Kobayashi T., Appella E., Hearing V. J. (2004) Epitope mapping of the melanosomal matrix protein gp100 (PMEL17). Rapid processing in the endoplasmic reticulum and glycosylation in the early Golgi compartment. J. Biol. Chem. 279, 28330–28338 [DOI] [PubMed] [Google Scholar]
- 11. Burris H. A., 3rd, Rugo H. S., Vukelja S. J., Vogel C. L., Borson R. A., Limentani S., Tan-Chiu E., Krop I. E., Michaelson R. A., Girish S., Amler L., Zheng M., Chu Y. W., Klencke B., O'Shaughnessy J. A. (2011) Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J. Clin. Oncol. 29, 398–405 [DOI] [PubMed] [Google Scholar]
- 12. Younes A., Yasothan U., Kirkpatrick P. (2012) Brentuximab vedotin. Nat. Rev. Drug Discov. 11, 19–20 [DOI] [PubMed] [Google Scholar]
- 13. Herweijer H., Wolff J. A. (2003) Progress and prospects. Naked DNA gene transfer and therapy. Gene Ther. 10, 453–458 [DOI] [PubMed] [Google Scholar]
- 14. Liu F., Song Y., Liu D. (1999) Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 6, 1258–1266 [DOI] [PubMed] [Google Scholar]
- 15. Zhang G., Budker V., Wolff J. A. (1999) High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 10, 1735–1737 [DOI] [PubMed] [Google Scholar]
- 16. Hongo J. A., Tsai S. P., Moffat B., Schroeder K. A., Jung C., Chuntharapai A., Lampe P. A., Johnson E. M., Jr., de Sauvage F. J., Armanini M., Phillips H., Devaux B. (2000) Characterization of novel neutralizing monoclonal antibodies specific to human neurturin. Hybridoma 19, 303–315 [DOI] [PubMed] [Google Scholar]
- 17. Doronina S. O., Toki B. E., Torgov M. Y., Mendelsohn B. A., Cerveny C. G., Chace D. F., DeBlanc R. L., Gearing R. P., Bovee T. D., Siegall C. B., Francisco J. A., Wahl A. F., Meyer D. L., Senter P. D. (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 21, 778–784 [DOI] [PubMed] [Google Scholar]
- 18. Raposo G., Marks M. S. (2007) Melanosomes. Dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 8, 786–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uong A., Zon L. I. (2010) Melanocytes in development and cancer. J. Cell Physiol. 222, 38–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vachtenheim J., Borovanský J. (2010) “Transcription physiology” of pigment formation in melanocytes. Central role of MITF. Exp. Dermatol. 19, 617–627 [DOI] [PubMed] [Google Scholar]
- 21. Berson J. F., Theos A. C., Harper D. C., Tenza D., Raposo G., Marks M. S. (2003) Proprotein convertase cleavage liberates a fibrillogenic fragment of a resident glycoprotein to initiate melanosome biogenesis. J. Cell Biol. 161, 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harper D. C., Theos A. C., Herman K. E., Tenza D., Raposo G., Marks M. S. (2008) Premelanosome amyloid-like fibrils are composed of only Golgi-processed forms of Pmel17 that have been proteolytically processed in endosomes. J. Biol. Chem. 283, 2307–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoashi T., Tamaki K., Hearing V. J. (2010) The secreted form of a melanocyte membrane-bound glycoprotein (Pmel17/gp100) is released by ectodomain shedding. FASEB J. 24, 916–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kummer M. P., Maruyama H., Huelsmann C., Baches S., Weggen S., Koo E. H. (2009) Formation of Pmel17 amyloid is regulated by juxtamembrane metalloproteinase cleavage, and the resulting C-terminal fragment is a substrate for γ-secretase. J. Biol. Chem. 284, 2296–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonhardt R. M., Vigneron N., Rahner C., Cresswell P. (2011) Proprotein convertases process Pmel17 during secretion. J. Biol. Chem. 286, 9321–9337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watt B., van Niel G., Fowler D. M., Hurbain I., Luk K. C., Stayrook S. E., Lemmon M. A., Raposo G., Shorter J., Kelly J. W., Marks M. S. (2009) N-terminal domains elicit formation of functional Pmel17 amyloid fibrils. J. Biol. Chem. 284, 35543–35555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polakis P. (2005) Arming antibodies for cancer therapy. Curr. Opin. Pharmacol. 5, 382–387 [DOI] [PubMed] [Google Scholar]
- 28. Tijink B. M., Buter J., de Bree R., Giaccone G., Lang M. S., Staab A., Leemans C. R., van Dongen G. A. (2006) A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin. Cancer Res. 12, 6064–6072 [DOI] [PubMed] [Google Scholar]
- 29. Goldenberg D. M., Sharkey R. M., Paganelli G., Barbet J., Chatal J. F. (2006) Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J. Clin. Oncol. 24, 823–834 [DOI] [PubMed] [Google Scholar]
- 30. Leonhardt R. M., Vigneron N., Rahner C., Van den Eynde B. J., Cresswell P. (2010) Endoplasmic reticulum export, subcellular distribution, and fibril formation by Pmel17 require an intact N-terminal domain junction. J. Biol. Chem. 285, 16166–16183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raposo G., Tenza D., Murphy D. M., Berson J. F., Marks M. S. (2001) Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 152, 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robila V., Ostankovitch M., Altrich-Vanlith M. L., Theos A. C., Drover S., Marks M. S., Restifo N., Engelhard V. H. (2008) MHC class II presentation of gp100 epitopes in melanoma cells requires the function of conventional endosomes and is influenced by melanosomes. J. Immunol. 181, 7843–7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chari R. V. (2008) Targeted cancer therapy. Conferring specificity to cytotoxic drugs. Acc. Chem. Res. 41, 98–107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.