Background: Integrin-mediated ECM adhesion is required for mammary epithelial proliferation, but the mechanism is not known.
Results: Gene deletion studies show that β1-integrin-null mammary epithelial cells retain β3-integrins and the ability to undergo two-dimensional migration, and Rac1 rescues their proliferation defect.
Conclusion: β1-Integrins uniquely control proliferation in mammary cells via Rac1, whereas β3-integrins support two-dimensional migration.
Significance: Specific β-integrin-containing adhesions determine different cell-fate responses.
Keywords: Cell Adhesion, Cell Cycle, Epithelial cell, Integrin, Mammary Gland
Abstract
Understanding how cell cycle is regulated in normal mammary epithelia is essential for deciphering defects of breast cancer and therefore for developing new therapies. Signals provided by both the extracellular matrix and growth factors are essential for epithelial cell proliferation. However, the mechanisms by which adhesion controls cell cycle in normal epithelia are poorly established. In this study, we describe the consequences of removing the β1-integrin gene from primary cultures of mammary epithelial cells in situ, using CreER. Upon β1-integrin gene deletion, the cells were unable to progress efficiently through S-phase, but were still able to undergo collective two-dimensional migration. These responses are explained by the presence of β3-integrin in β1-integrin-null cells, indicating that integrins containing different β-subunits exert differential control on mammary epithelial proliferation and migration. β1-Integrin deletion did not inhibit growth factor signaling to Erk or prevent the recruitment of core adhesome components to focal adhesions. Instead the S-phase arrest resulted from defective Rac activation and Erk translocation to the nucleus. Rac inhibition prevented Erk translocation and blocked proliferation. Activated Rac1 rescued the proliferation defect in β1-integrin-depleted cells, indicating that this GTPase is essential in propagating proliferative β1-integrin signals. These results show that β1-integrins promote cell cycle in mammary epithelial cells, whereas β3-integrins are involved in migration.
Introduction
Cell cycle progression in metazoan cells is tightly regulated by adhesion to the surrounding extracellular matrix (ECM),2 cell-cell adhesion, and soluble factors. The integrin family of adhesion receptors acts at a pivotal point in the control of the cell cycle by integrating the signaling pathways initiated by growth factors (GFs) with adhesion signaling (1). Integrins impart numerous controls at both early and late phases of the cell cycle, and they determine the axis of cell division (2–4).
Genetic evidence for a role for β1-integrin in proliferation comes from in vivo studies in cartilage, skin, and mammary gland (5–9). The link between integrins and proliferation has been studied in fibroblasts, endothelial, and carcinoma cells, but the mechanisms by which β-integrins support proliferation in normal epithelial cells are not well understood.
Many of the key conclusions regarding the role of integrins in cell cycle have been arrived at by comparing adherent cells with those placed in suspension, where integrins are not ligated to ECM and are therefore inactivated (10). This experimental strategy limits the amount of mechanistic information that can be obtained because it does not distinguish between cell cycle mechanisms associated with changes in cell shape, the actin cytoskeleton and cell-cell adhesion, with those directly regulated by integrins (11). Moreover, it does not identify which β-integrin subunits are involved in cell cycle regulation.
Here we have developed a novel genetic strategy to delete the β1-integrin gene in situ from primary cultures of mammary epithelial cells (MECs). This was achieved by the addition of a drug, 4-hydroxytamoxifen (4OHT), to MECs isolated from bi-transgenic Itgβ1fx/fx;CreERTM mice. This approach provides a robust method to study the cellular role of specific integrin subunits without perturbing the cells in any other way, such as by trypsinizing the cells or otherwise changing their microenvironment. It therefore has allowed us to ask directly how specific integrin subunits are involved in growth regulation.
We hypothesized that deleting β1-containing integrins in situ might cause the mammary epithelia to lose their adhesions and change their morphology and to alter their proliferation as a consequence. However, this was not the case. Instead we discovered that β1-containing integrins are uniquely required for mammary epithelial S-phase progression, but they are not necessary for the maintenance of cell adhesion, focal adhesion complexes (adhesomes), or cell shape or for collective two-dimensional migration.
EXPERIMENTAL PROCEDURES
Mouse Strains
The Itgβ1fx/fx and CreERTM mouse lines were crossed to produce the Itgβ1fx/fx;CreERTM mouse line (12, 13). The genotype of all breeding pairs and mice for MEC cultures was verified by PCR.
Primary Cell Culture and β1-Integrin Gene Deletion
MECs from 15.5- to 17.5-day pregnant Itgβ1fx/fx;CreERTM or wild type (WT) ICR mice were cultured on rat-tail collagen I-coated dishes or MatrigelTM (BD Biosciences) in the presence of 10% FCS, 5 μm insulin, and 5 ng/ml EGF (14). MECs were treated with 100 nm 4OHT at the time of plating to delete the β1-integrin gene. Fresh primary cells were used for each experiment. In each case, β1-integrin protein levels were verified by immunoblotting. In some studies, cells were treated with 1 μm Mek inhibitor U0126 for 24 h or 100 μm Rac inhibitor NSC23766 for 20 h before harvesting. For these experiments, controls were treated with the equivalent volume of DMSO.
Genomic DNA PCR
Genomic DNA was isolated from control and 4OHT-treated MECs at various time points following 4OHT addition and analyzed by PCR (12).
FSK7 Cells and β1-Integrin Knockdown
Low passage FSK7 mouse mammary epithelial cells were cultured as described (15). The shRNAmiR sequence for mouse β1-integrin was 5′-GGCTCTCAAACTATAAAGAAA-3′. To create pshβ1 (which expresses sh-β1-integrin-RNA and GFP), double-stranded oligonucleotides were cloned into the pLVTHM shRNA transfer vector (Tronolab), and a TTTTTT sequence was added downstream of the shRNAmiR sequence to stop the transcript of H1 promoter. To create the rescue vector pshβ1-Rac, high cycling L61-Rac1 fused to GFP was cloned downstream of the EF1α promoter in pVenus containing the β1-integrin-specific shRNAmiR. 105 cells/cm2 were transfected with a total of 1 μg of DNA in 12-well plates for 3 h using LipofectamineTM and PlusTM reagent (Invitrogen), cultured for 3 days, and then replated at 105 cells/cm2 on FN-precoated coverslips before fixing and staining.
Immunoblotting
Primary antibodies for immunoblotting (16) were: β1-integrin (BD Transduction Laboratories 553715 and 610467), mitochondrial Hsp70 (Thermo Scientific MA3-028), vinculin (Sigma V4505), talin (Santa Cruz Biotechnology sc-7534), Ilk (Chemicon AB3812), phospho-Fak (Tyr(P)-397) (Invitrogen 44-624), phospho-Fak (Tyr(P)-577) (Invitrogen 44-625), Fak (BD Biosciences 610088), phospho-paxillin (Tyr(P)-118) (BIOSOURCE 44-72), paxillin (BD Biosciences 610052), calnexin (Bioquote SPC-108A/B), β3-integrin (Cell Signaling 4702), phospho-Erk (Cell Signaling 9101), Erk (Santa Cruz Biotechnology sc-154), phospho-Elk-1(Santa Cruz Biotechnology sc-7979), Rac (Upstate Biotech Millipore 05-389), phospho-Pak1 (Cell Signaling 2605), and Cre recombinase (Chemicon mAb3120).
Proliferation and Immunostaining
MECs were treated with 10 μm EdU (8 h) and stained with EdU-Click reaction (Invitrogen Click-iTTM EdU kit C10083). Primary antibodies for immunostaining (17) were: β1-integrin (Chemicon MAB1997), β3-integrin (2C9.G2 (HMβ3-1); Biolegend 104311), and phospho-histone H3 (Millipore 06-570), and others were as for immunoblotting.
Real-time Reverse Transcription-Polymerase Chain Reaction (Quantitative PCR)
RNA was extracted from cultured cells using the PARISTM kit (Ambion AM1921). cDNA was synthesized using the High Capacity RNA-to-DNA synthesis kit (Applied Biosystems 4387406). Gene expression was measured using the TaqMan gene expression master mix (4369514) and StepOnePlus (Applied Biosystems). TaqMan gene expression assay primer probe sets for each gene were used. The Gene Assay IDs of the TaqMan gene expression assays supplied by Applied Biosystems were Mm01253233_m1 for β1-integrin, Mm00443972_m1 for β3-integrin, Mm01266844_m1 for β4-integrin, Mm00439825_m1 for β5-integrin, Mm00445326_m1 for β6-integrin, and Mm00442479_m1 for MAPK. The calibration samples were control untreated cells, and MAPK was used as an endogenous control.
FACS
106 single cells were fixed in suspension, blocked with fresh PBS, 1% BSA, and stained with Alexa Fluor 488-anti-mouse β3-integrin (Biolegend). Cells were washed three times, suspended in 100 μl of PBS, and analyzed with Beckman Coulter CYANADP. Excitation with 488-nm laser and 530–540-nm filter was used for Alexa Fluor 488.
Adhesion Assay
4 × 104 cells were seeded per well of 96-well plates precoated with collagen I and FN, with or without 10 μg/ml function blocking antibodies to β1-integrin (18) or β3-integrin (2C9.G2).
Isolation of Mammary Gland Acini from Matrigel and Migration Analysis
MECs cultured as acini on Matrigel, with or without 4OHT, were scraped off the dish into PBS, 5 mm EDTA and replated onto plates precoated with collagen-I. Cell emigration from the isolated acini was followed by live cell imaging (AS MDW, Leica) for 72 h. Cell tracks were generated, and point-to-point measurements were made using the ImageJ plugin, MTrackJ. The Chemotaxis tool was used for the generation of chemotaxis plots. In some experiments, cell cycle was prevented by prior treatment with 10 μm mitomycin C for 30 min.
Endogenous Rac Activity
Cells were lysed in Nonidet P-40 lysis buffer and centrifuged at 17,500 × g (15 min, 4 °C). 25 μg of GST-Pak Pak-binding domain (PBD) coupled to glutathione-agarose beads (Calbiochem) was used to precipitate GTP-bound Rac from lysates (40 min, 4 °C). Active Rac was detected by immunoblotting with an anti-Rac antibody and quantified using Odyssey (LI-COR Biosciences).
Statistics
Each figure shows data from a minimum of three independent experiments. Statistical significance was carried out using a paired Student's t test or analysis of variance.
RESULTS
β1-containing Integrins Are Required for S-phase Progression in Mammary Epithelia
To determine the role for β1-integrins in MEC proliferation, a system was developed whereby the β1-integrin gene could be removed from primary cell cultures in situ, without other changes that might occur after isolating or selecting null cells and replating them onto culture dishes. In MECs from Itgβ1fx/fx;CreERTM mice, the addition of 100 nm 4OHT to the cell culture medium deleted the β1-integrin gene within 24 h (Fig. 1a). Loss of the β1-integrin protein was confirmed immunologically (Fig. 1, b and c).
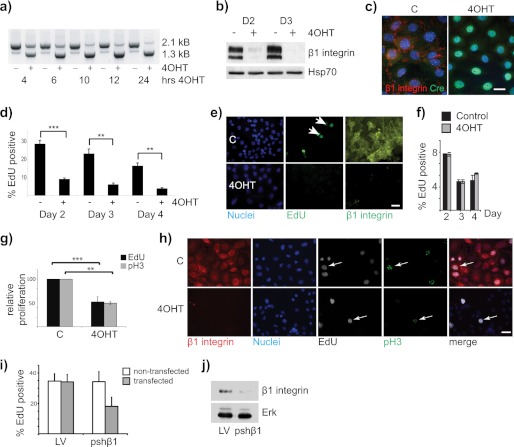
FIGURE 1.
β1-Integrin-null MECs display a proliferation block. a, genomic DNA was isolated from control and 4OHT-treated primary MECs over a time course of 24 h. PCR analysis was carried out to show the Cre-mediated recombination on genomic DNA and deletion of the β1-integrin gene. The 2.1-kb product is the full-length floxed allele, and the 1.3-kb product is the recombined allele. b, control (C) and β1-integrin-null (4OHT-treated at time of isolation) primary β1fx/fx;CreERTM MECs were fixed and stained for β1-integrin (red) and Cre-recombinase (green) to show loss of β1-integrin and nuclear localization of Cre-recombinase. Bar: 20 μm. c, immunoblotting confirmed β1-integrin deletion in 4OHT-treated MECs. D2 and D3, day 2 and day 3. d and e, untreated control (C) and 4OHT-treated β1fx/fx;CreERTM MECs were incubated with EdU 2, 3, or 4 days after isolation, fixed, and stained using EdU-Click reaction buffer and β1-integrin antibody. d, proliferation was quantified by counting the percentage of EdU-positive nuclei when compared with total number of cells. ∼1000 cells were counted per condition. The error bars are ± S.E. **, p = 0.04; ***, p = 0.003. e, representative images of day 4 samples. Bar: 38 μm. f, control and 4OHT-treated MECs from CreERTM-only mice were analyzed for EdU incorporation 2, 3, and 4 days after isolation. g and h, cells as in d were labeled with EdU 2 days after isolation and stained for EdU, β1-integrin, and phospho-histone H3. g, the graph shows the average of two independent experiments. **, p = 0.04; ***, p = 0.003. h, representative images. Arrows show phospho-histone H3 (pH3) staining in the EdU-labeled cells. Note that the sensitivity of the phospho-histone H3 stain was less than EdU, but the relative reduction in proliferation after integrin deletion was the same. Bar: 38 μm. i and j, FSK-7 cells were transfected with pshβ1 or control pLVTHM (LV) and replated for proliferation analysis with EdU. i, percentage of EdU incorporation in the transfected (GFP-positive) and nontransfected (GFP-negative) cells within the same dishes. Note that proliferation is only suppressed in the cells transfected with pshβ1. j, parallel culture with the transfected cells sorted by FACS and lysates immunoblotted to show β1-integrin knockdown.
The proliferation of primary MECs from Itgβ1fx/fx;CreERTM mice was assessed using EdU, which is incorporated into the DNA during S-phase (19). In 4OHT-treated cells, β1-integrin was deleted, and there was a significant decrease in the number of EdU-positive nuclei in β1-integrin-null cells when compared with controls (Fig. 1, d and e). The inhibition of S-phase progression following integrin deletion was evident up to 4 days of culture, after which the control primary cells lost their competence to proliferate (20). MECs isolated from CreERTM-only mice showed no difference in proliferation between control and 4OHT-treated MECs, indicating that the cell cycle defect was not due to Cre or 4OHT (Fig. 1f). Phospho-histone H3 staining showed a similar reduction in cell cycle to EdU staining (Fig. 1, g and h). To confirm the role for β1-integrin in MEC proliferation, we depleted it in FSK-7 MECs by expressing β1-integrin shRNAmiR together with a GFP marker. As with the previous results, there was a reduction in proliferation, assessed by EdU (Fig. 1, i and j). These results demonstrate that β1-containing integrins are required for progression of MECs through the G1/S-phase of the cell cycle, and they are consistent with our previous in vivo study (7).
Loss of β1-Integrin Does Not Affect MEC Shape, Cytoskeletal Organization, Adhesome Integrity, or Migration
Following β1-integrin gene deletion in situ, primary MECs remained adherent on the culture dishes. Moreover, the cells retained a similar morphology to the nondeleted control cells, and they assembled normal microfilament and microtubule networks (Fig. 2, a and b). We therefore reasoned that β1-null MECs expressed a compensatory integrin.
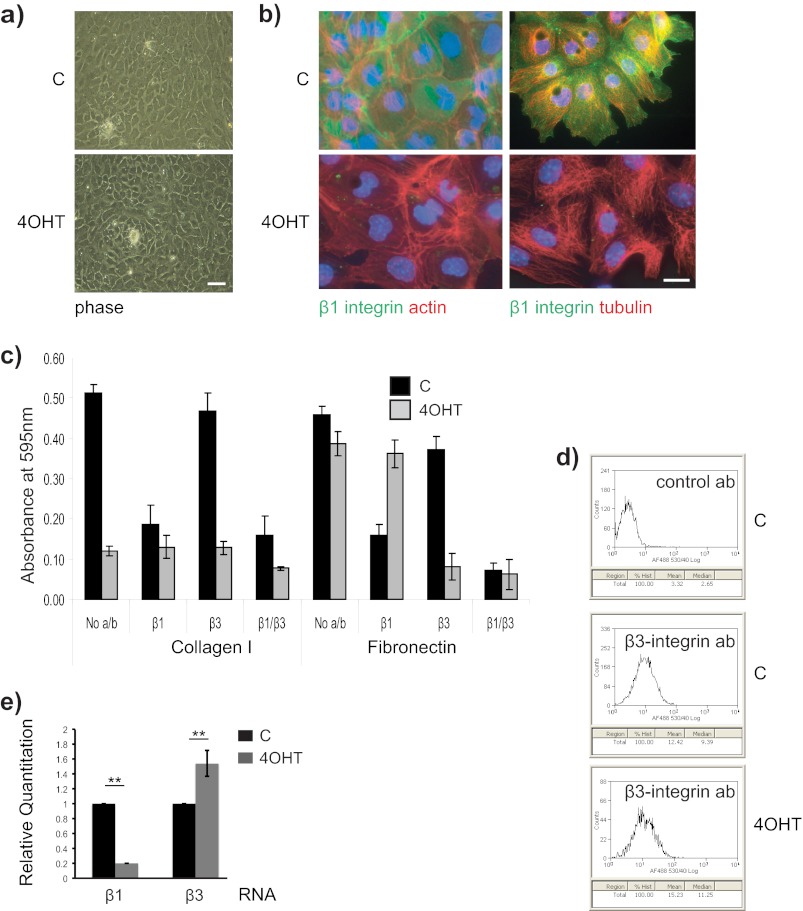
FIGURE 2.
β1-Integrin-null MECs contain functional β3-containing integrins. a, β1fx/fx;CreERTM MECs treated with 4OHT or without (C) were cultured on collagen-I coated coverslips, and the cell morphology was observed by phase microscopy after 3 days. Bar: 50 μm. b, similar cultures were stained for β1-integrin (green), and actin or tubulin (red). Bar: 20 μm. c, untreated control (C) and 4OHT-treated β1fx/fx;CreERTM MECs were used for an adhesion assay in serum-free medium on collagen I or FN, in the presence of β1-integrin and β3-integrin function blocking antibodies (10 μg/ml). The error bars represent S.D. of triplicate samples within a representative experiment (n = 3). No a/b, no antibodies, d, single cell suspensions of untreated control (C) and β1-integrin-deleted (4OHT) MECs were labeled with control or Alexa Fluor 488-conjugated anti-β3-integrin antibodies and analyzed by FACS. β3-Integrin was expressed on the surface of MECs, and its levels were similar following β1-integrin gene deletion. control ab, control antibody. e, β1-integrin and β3-integrin RNA levels were compared in control and 4OHT-treated β1fx/fx;CreERTM MECs 3 days after isolation from mice, by quantitative PCR. **, p < 0.01.
To identify compensatory integrins in β1-null MECs, we conducted adhesion assays in the presence of anti-integrin function-blocking antibodies (Fig. 2c). We compared adhesion to collagen I and FN because serum contains FN, which provides an additional ECM protein that MECs normally adhere to on collagen-coated culture dishes. Control cells adhered equally well to collagen-I and FN, and the adhesion was largely β1-integrin-dependent. In contrast, β1-null cells had poor adhesion to collagen-I, but adhered to FN in a β3-dependent manner. Thus, in the absence of β1-integrin, MECs are able to adhere to ECM proteins via β3-integrins.
To confirm β3-integrin expression, we carried out FACS analysis, which revealed cell surface β3-integrin on control MECs (Fig. 2d). In the β1-null cells, quantitative PCR showed an increase in β3-integrin RNA expression (Fig. 2e), but no changes were seen in the levels of other β-integrin subunits (not shown). β3-Integrin was therefore present in MECs regardless of β1-integrin, although we did not detect increased cell surface β3-integrin by FACS (Fig. 2d).
Immunostaining was used to examine the adhesomes of control and β1-integrin-null cells. No differences were revealed in the major components of the adhesomes of β1-null MECs, including talin, vinculin, or Fak (Fig. 3a). Furthermore, the adhesomes were capable of signaling because key proteins such as paxillin and Fak remained phosphorylated after integrin deletion (Fig. 3b); in controls, there was no change in the integrin and phospho-Fak levels after 4OHT treatment of CreERTM-only cells (Fig. 3c). Phospho-paxillin was also visible in the adhesomes of β1-integrin-null MECs (Fig. 3d). Finally, β3-integrin adhesomes were prominent in the β1-integrin-null cells (Fig. 3e).
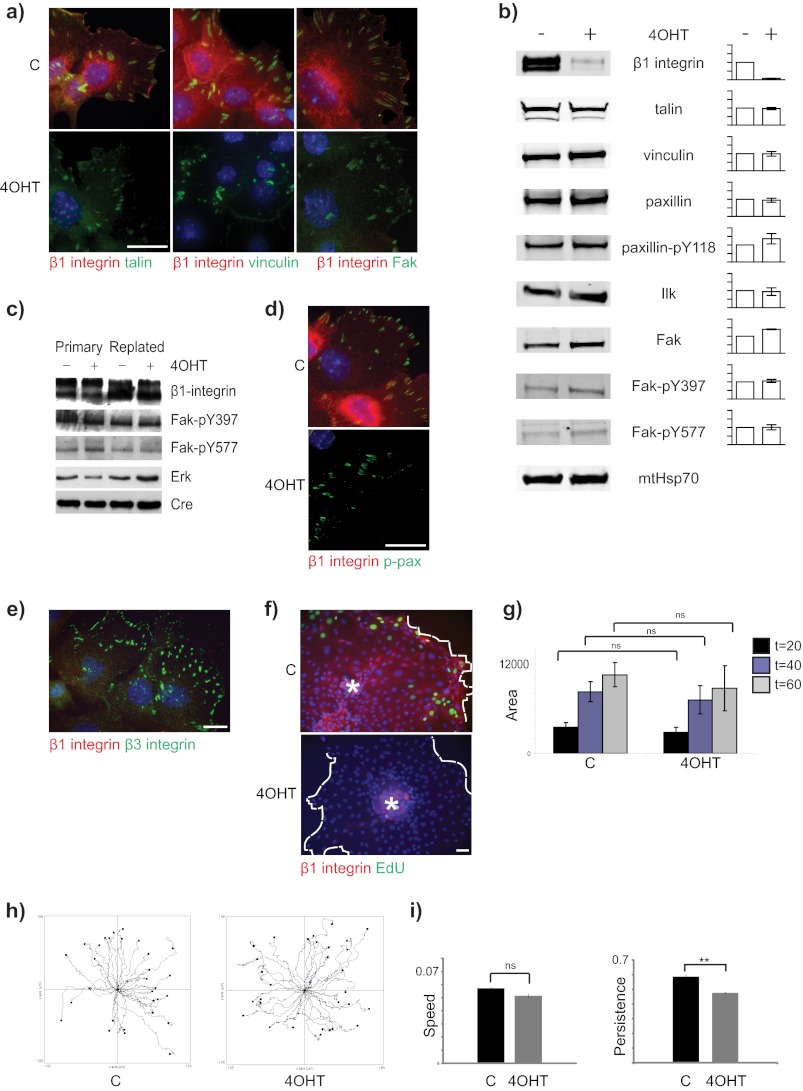
FIGURE 3.
β1-Integrin-null MECs contain functional adhesomes and show collective two-dimensional cell migration. a, β1fx/fx;CreERTM MECs treated with 4OHT or untreated controls (C) were cultured on collagen-I coated coverslips, fixed 3 days after isolation, and stained for β1-integrin (red) and talin, vinculin, or Fak (green). Bar: 20 μm. b, immunoblotting of lysates from untreated and β1-integrin-deleted (4OHT) MECs cultured for 3 days and probed for the indicated antigens. Mitochondrial Hsp70 (mtHsp70) was used a loading control. The intensity of the bands was quantified using the Odyssey system, and the level of signal in the 4OHT-treated samples is plotted relative to untreated. Error bars = S.E. c, immunoblotting of lysates from CreERTM-only MECs replated onto collagen I. d, immunofluorescence staining of paxillin-Tyr(P)-31 (p-pax) in control and 4OHT-treated MECs. Bar: 20 μm. e, β1-null MECs stained with β1- and β3-integrin antibodies. Bar: 20 μm. f, control (C) and β1-null (4OHT) acini were plated onto two-dimensional collagen I-coated plates. The acini were allowed to attach, and the cells migrated onto the culture surface. At the end of the experiment, the cells were stained for EdU and β1-integrin. Note the absence of EdU incorporation in the β1-null cells. Asterisks indicate location of acini from which the cells emigrated. White dotted line indicates migration front. Bar: 40 μm. g, acini were treated with 10 μm mitomycin C for 30 min and prior to cell migration onto the dishes. The area spread was calculated using ImageJ and was not significantly different (ns) in the controls (C) and β1-integrin-null (4OHT) MECs. h, MECs as in g were imaged by time-lapse cinematography, and the tracks of individual cells were followed using MTrackJ. i, the average speed (μm/min) and directional persistence of cell movements shown in h. **, p < 0.01.
To determine whether the removal of β1-integrin altered cell migration, multicellular MEC acini were cultured on MatrigelTM with and without 4OHT to delete the β1-integrin gene, and then either control or β1-integrin-null acini were isolated using EDTA and plated onto native collagen-I. The cells emigrated from the acini, and both the control and the β1-integrin-deleted cells collectively migrated to form cell sheets on the substratum (Fig. 3f). To rule out a role for proliferation in the migration response, we pretreated acini with mitomycin C and found that both the control and the β1-null cells migrated from the acini to a similar extent (Fig. 3g). Analysis of migration tracks using time-lapse microscopy revealed that the average speed of the control and β1-null MECs was not significantly different (Fig. 3h). The directional persistence (i.e. the ability of the cells to migrate in one direction) was slightly, but significantly, reduced in the β1-null MECs when compared with controls (Fig. 3i). Despite the requirement of β1-integrin for MEC proliferation, both control and β1-null MECs were able to undergo collective cell migration, indicating the presence of functional ECM interactions under each condition. These results demonstrate that β3-integrin assembles functional adhesomes in β1-integrin-deleted MECs, which remain competent to direct cytoskeleton formation and collective two-dimensional migration.
Rac1 Links β1-Integrins with Cell Cycle in MECs
The β1-null MECs were unable to undergo efficient cell proliferation, although they expressed β3-integrins. We reasoned that the differential ability of integrins to control the cell cycle machinery is reflected in altered signaling pathways downstream of β1- and β3-integrins.
One of the central pathways that regulates cell cycle progression is the GF receptor/MAP kinase signaling axis. In several cell types, this pathway is also under the control of ECM adhesion (1). The MAP kinase pathway was required for MEC cell cycle progression because treatment with the Mek inhibitor U0126 inhibited proliferation (Fig. 4a). However, there were no obvious differences in phospho-Erk in steady-state conditions in lysates of control and β1-null MECs during the 2–3 days of primary cell culture in which proliferation is at the highest levels (20) (Fig. 4b). In addition, Erk phosphorylation was similar in control and β1-null cells following an acute 30-min stimulation with serum (Fig. 4c). This indicates that β1-integrin regulation of the proliferation response does not occur at the level of growth factor receptor signaling to Erk.
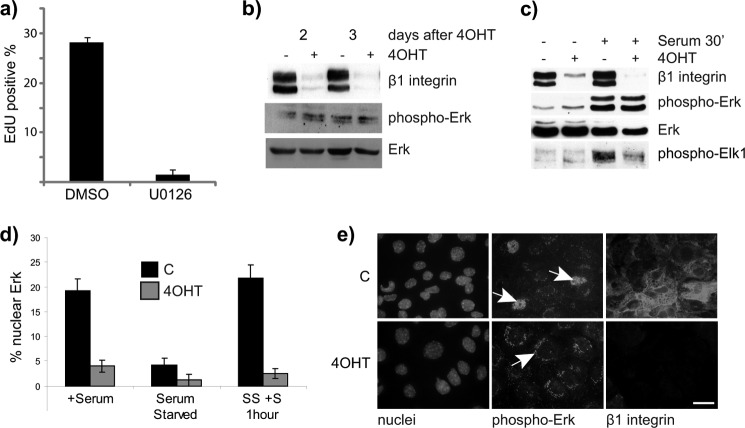
FIGURE 4.
β1-Integrin is required for nuclear translocation of pErk. a, WT MECs were isolated and cultured for 24 h and then treated with Mek inhibitor U0126 for 24 h before assessing the percentage of EdU-positive cells. The equivalent volume of DMSO was used as a control. b and c, cell lysates were harvested from untreated and 4OHT-treated β1fx/fx;CreERTM MECs that had been cultured in steady-state conditions with serum, 2 and 3 days after isolation from mice. b, they were analyzed by immunoblotting for β1-integrin, pErk, and total Erk. c, untreated and 4OHT-treated β1fx/fx;CreERTM MECs were serum-starved for 12 h and subsequently stimulated with full medium for 30 min before analyzing the protein levels of β1-integrin, pErk, total Erk, and pElk1. d and e, control (C) and β1-integrin-deleted (4OHT) MECs were cultured on collagen-coated coverslips, treated as in c, and immunostained for pErk. d, the percentage of nuclear Erk was quantified. Error bars = S.E. SS + S, serum-starved plus serum. e, representative images of cells in serum for 2 days, stained for β1-integrin (red) and pErk (green). The white arrow highlights the localization of pErk inside the nucleus of control cells (C) and outside the nucleus of β1-integrin-null (4OHT) cells. Scale bar: 30 μm.
Erks (Erk1/2) reside primarily in the cytoplasm, and upon phosphorylation and activation, Erk can translocate to the nucleus. Nuclear translocation of Erk is required for cell cycle entry due to the Erk-dependent phosphorylation of target transcription factors such as Elk-1 (21). We therefore examined the intracellular localization of phospho-Erk. Control MECs contained nuclear phospho-Erk, which, in contrast, was reduced in the β1-null cells (Fig. 4, d and e). This result indicates that β3-integrin adhesions are unable to support the final stages of the MAP kinase pathway involving the translocation of Erk into the nucleus. To confirm this, we examined Elk1 phosphorylation and found that it was decreased in β1-null MECs when compared with controls (Fig. 4c).
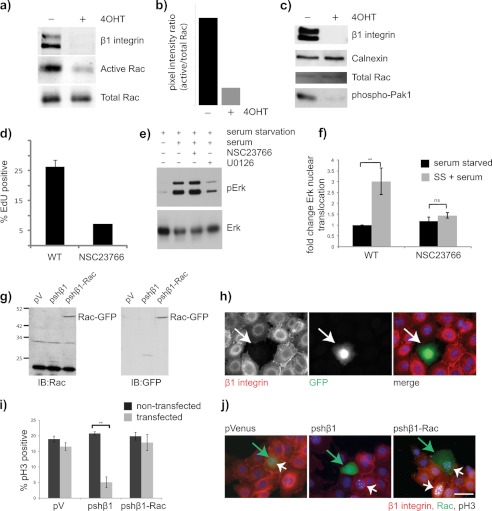
The GTPase Rac can also regulate proliferation by interacting with many different intracellular pathways (22). In the context of cell cycle, Rac and Pak directly influence the MAP kinase phosphorylation cascade (23). To examine whether there were any differences in Rac signaling between control and β1-null cells, a Rac activity assay was carried out. Control MECs contained high levels of active Rac, which was significantly decreased in β1-null cells (Fig. 5, a and b). Consistent with these results, we also observed a reduction in the phosphorylation of the downstream Rac effector kinase Pak1 (p21-activated kinase) in β1-null cells (Fig. 5c).
FIGURE 5.
Rac1 links β1-integrins with proliferation in MECs. a and b, control and 4OHT-treated β1fx/fx;CreERTM MECs were analyzed for Rac activity. Levels of β1-integrin and total Rac were assessed in the same lysates to confirm β1-integrin knockdown and correct loading. a, immunoblots. b, band quantification using the LI-COR Odyssey system. c, lysates of day 2 untreated (−) and β1-integrin-null (+4OHT) MECs were assessed by immunoblotting for β1-integrin, total Rac, and phospho-Pak1. Calnexin was used as a loading control. d, WT MECs were cultured for 24 h and then treated for 20 h with Rac inhibitor NSC23766 before assessing the percentage of EdU-positive cells. The equivalent volume of DMSO was used as a control. Error bars = S.E. e, WT MECs were cultured for 2 days, serum-starved for 12 h, treated with the Mek (24 h) or Rac (20 h) inhibitor, and then treated with serum for 1 h. Lysates were immunoblotted for pErk and total Erk. f, WT MECs were cultured with Rac inhibitor as in e, immunostained for pErk, and assessed for the presence of nuclear pErk. SS + serum, serum-starved plus serum. g and h, FSK7 cells were transfected with the empty pVenus vector (pV), pshβ1, or pshβ1-Rac and then replated onto glass coverslips for 24 h. g, lysates showing expression of Rac-GFP. IB, immunoblot. h, immunostain to show simultaneous loss of β1-integrin and expression of GFP in the transfected cells. i and j, cells as in g were immunostained for phospho-histone H3 (pH3). i, graph showing the percentage of phospho-histone H3-positive cells in nontransfected and transfected cells. **, p = 0.02. j, representative images. Green arrows indicate GFP-positive cells. White arrowheads point to cells in S-phase. Note that in the shβ1 cultures, the transfected cells (green) are phospho-histone H3-negative, whereas the neighboring untransfected cells (red) are phospho-histone H3-positive. In contrast, shβ1-Rac transfected cells were both β1-negative and phospho-histone H3-positive. Bar: 20 μm.
To determine whether Rac linked β1-containing-integrins and proliferation, WT MECs were treated with the Rac inhibitor NSC23766. The rate of proliferation was decreased in Rac-inhibited cells (Fig. 5d). Moreover, although Erk phosphorylation did not require Rac activity (Fig. 5e), the nuclear translocation of phospho-Erk was Rac-dependent (Fig. 5f). To confirm the role for Rac in linking β1-integrin with cell cycle, a rapid-recycling form of Rac1 was expressed in MECs at the same time as depleting β1-integrin (Fig. 5, g and h). The results revealed that Rac1 rescued the proliferation defect in β1-integrin-depleted MECs (Fig. 5, i and j).
These results demonstrate that integrins containing different β-subunits differentially regulate Rac1 and that Rac1 has a role in proliferation control of MECs. Moreover, specific β-integrin subunits are necessary for GFs to promote the translocation of pErk into the nucleus and thereby stimulate S-phase.
DISCUSSION
This study shows that integrins containing different β-subunits exhibit a striking specificity in the phenotypic responses they elicit. By using CreERTM to remove an integrin subunit in situ, we discovered that β1-containing integrins are uniquely required for S-phase progression in MECs. β3-containing integrins do not have this capacity. Thus, although β1- and β3-integrins assemble similar adhesomes, only β1-integrins signal efficiently to cell cycle and they do so via Rac1. In contrast, β3-integrins cannot license proliferation, but they can support collective cell migration. Epithelial cell fate is therefore dependent on the signaling pathways that emanate from specific β-containing integrin mediated adhesions.
In Situ Integrin Gene Deletion
Genetic manipulation is a powerful tool for analyzing how proteins work, but its use can be cumbersome in mammalian models. We have now taken advantage of the CreERTM methodology to delete integrin genes in situ (13). A simple treatment with 4OHT can delete both alleles of a floxed gene efficiently and rapidly in primary cells carrying the CreERTM transgene. Notably, we find that integrin-containing adhesomes are turned over rapidly in an epithelial monolayer in situ so that within 48 h, the β1-containing complexes disappear. This provides a robust method to study the cellular role of specific integrin subunits, without perturbing the cells in any other way, such as by trypsinizing the cells or otherwise changing their microenvironment.
β1- but Not β3-containing Integrins Are Required for Cell Cycle in MECs
A striking consequence of β1-integrin gene deletion in MECs is their inability to proliferate efficiently. Although proliferation defects have been described for in vivo β1-integrin deletion studies in epithelia, little is currently known about the mechanisms involved (6, 8, 9, 24). We previously identified a proliferation block following deletion of the β1-integrin gene in vivo, but the signals linking integrin to the cell cycle were not identified (7). One explanation was that the integrin loss could alter MEC shape, thereby preventing S-phase progression (17, 25). However, in the current study, deleting β1-integrin in spread cells in situ had no effect on cell shape. This indicates that mammary epithelial proliferation is controlled through a signaling mechanism that necessitates the β1-integrin subunit itself.
In some cell types and cancer cells, cell cycle progression depends on a close collaboration between integrins and receptor tyrosine kinases at the level of receptor interactions (4). However, MECs require integrins to propagate GF signaling downstream of the GF receptor because Erk phosphorylation (and Akt signaling, data not shown) is similar in control and β1-null cells. Our results show that β1-integrin signals feed into the GF signaling pathway at the level of Erk nuclear translocation. A previous comparison between adherent and suspension fibroblasts showed that adhesion regulates Erk translocation and the transcription of genes required for S-phase, but the integrins involved were not identified (26). Our study reveals that specifically β1-integrins, but not β3-integrins, enable Erk nuclear translocation, and moreover, this occurs independently of the alterations in cell shape, adhesome signaling, and cytoskeleton integrity that result from placing cells in suspension. Our results also show that integrin specificity for cell cycle signaling is determined by cell type. For example in fibroblasts, a β1-integrin COOH-terminal tail mutant perturbed Erk nuclear translocation and cell cycle, but those cells proliferated normally on a β3-integrin ligand (27).
Erk lacks a nuclear localization sequence, and it is not fully understood how Erk is transported across nuclear membranes. One possibility is that Erk translocation occurs in an energy-independent process via direct binding to nucleoporins (28). Another is that Erk-interacting proteins such as Mkp-7 may dictate its localization (29, 30).
β1- versus β3-Integrin Proximal Signals for Cell Cycle
A variety of mechanisms could explain the difference in the ability of β1- versus β3-integrin adhesions to support MEC proliferation. For example, the adhesomes assembled by β1-integrins may have different components to β3-containing adhesomes, which are required for a distal signal that is essential for cell cycle. One possibility is that the α-integrin subunits recruit specific cell cycle proteins. For example, the collagen-binding integrins might engage a different set of proteins from those recruited by the FN receptor, αvβ3-integrin. Another possibility is that although the cytoplasmic domains of β1- and β3-integrins are similar, there are sufficient sequence differences to mobilize different sets of noncore adhesion complex proteins (31).
Because inhibiting Rac activity prevents both Erk nuclear translocation and cell cycle progression in MECs, and Rac1 rescues the proliferation defect in β1-integrin-null MECs, we propose that β1-integrins uniquely activate Rac1, which then communicates with the receptor tyrosine kinase (RTK)-Erk pathway by facilitating the nuclear translocation of Erk. In endothelial cells, the Fak/PI3K and Fyn/Sos pathways determine ECM-specific Rac activation (32). This may not be the case in all cells because so far our data have revealed that control and β1-null MECs show similar levels of Fak Tyr-397 and Tyr-577 phosphorylation and Akt activity. Integrin-specific links to Rac occur in other cell types. For example, in both CHO cells and GD25 fibroblasts, elevating the levels of β1-integrin, but not the β3-subunit, enhances Rac1 activity (33, 34).
β1- versus β3-containing Integrins in MEC Migration
By using the novel strategy of gene deletion with 4OHT, we discovered that β1-integrins are not required exclusively for breast epithelial migration. Integrins are critical in cell migration, lending traction and acting as mechanosensors (35). In fibroblasts, endocytosis of surface integrins and vesicle trafficking provide key mechanisms of migration control (36). Persistent migration or random movement of fibroblasts depends on different methods of endocytic recycling of αvβ3- and α5β1-integrins (37). Epithelia move as cellular sheets rather than individual cells with lamellipodia and filopodia, and the role of integrin trafficking for collective migration has not yet been established. Interestingly, our results show that β3-integrin-dependent migrations are less persistent in β1-integrin-null cells than those of controls, possibly because of its reduced ability to activate Rac, which is known to have a role in persistent migration (38).
β-Integrins and MEC Proliferation in Cancer
It is notable that β1-integrins are required for cell cycle in some mouse models for breast cancer, for example in MMTV-PyMT transgenics (39). However, in the ErbB2 cancer model, β1-integrins are dispensable for the formation of primary tumors (40). It will therefore be important to determine the degree to which breast cancer oncogenes overcome the restriction on Erk translocation and S-phase that occurs in MECs lacking the β1-integrin subunit. Interestingly, a separate β-integrin, the β4-subunit, is required for tumor formation in a Neu breast cancer model (41). This may be a cancer-specific response because the β4-integrin subunit is not needed for normal mammary gland development in vivo (42). Thus, the cell cycle role of β-integrins may differ in the normal versus cancer context.
Our observation that cell migration still occurs in cells that have lost β1-integrin may indicate redundancy for cell migration during tissue repair or through different ECM environments. However, where β1-integrins are either naturally reduced or artificially inhibited in breast cancers, an unwanted side effect might be the ability of β3-integrin subunits to promote migration or even metastases (43–45).
Acknowledgments
We thank Cord Brakebush for providing the L61-Rac1 construct. The Wellcome Trust Centre for Cell-Matrix Research is supported by core funding from the Wellcome Trust (Grant 088785/Z/09/Z).
This work was supported by the Breast Cancer Campaign (Grant 2006NovPhD12) and Wellcome Trust (Grant 081203/Z/06/Z).
- ECM
- extracellular matrix
- GF
- growth factor
- MEC
- mammary epithelial cell
- 4-OHT
- 4-hydroxytamoxifen
- Fak
- focal adhesion kinase
- EdU
- 5-ethynyl-2′-deoxyuridine
- DMSO
- dimethyl sulfoxide
- pErk
- phospho-Erk
- FN
- fibronectin.
REFERENCES
- 1. Streuli C. H., Akhtar N. (2009) Signal co-operation between integrins and other receptor systems. Biochem. J. 418, 491–506 [DOI] [PubMed] [Google Scholar]
- 2. Walker J. L., Assoian R. K. (2005) Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 24, 383–393 [DOI] [PubMed] [Google Scholar]
- 3. Taddei I., Deugnier M. A., Faraldo M. M., Petit V., Bouvard D., Medina D., Fässler R., Thiery J. P., Glukhova M. A. (2008) β1-Integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 10, 716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Streuli C. H. (2009) Integrins and cell-fate determination. J. Cell Sci. 122, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aszodi A., Hunziker E. B., Brakebusch C., Fässler R. (2003) β1-Integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 17, 2465–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raghavan S., Bauer C., Mundschau G., Li Q., Fuchs E. (2000) Conditional ablation of β1-integrin in skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 150, 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li N., Zhang Y., Naylor M. J., Schatzmann F., Maurer F., Wintermantel T., Schuetz G., Mueller U., Streuli C. H., Hynes N. E. (2005) β1-Integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 24, 1942–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López-Rovira T., Silva-Vargas V., Watt F. M. (2005) Different consequences of β1-integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation, and intercellular communication. J. Invest. Dermatol. 125, 1215–1227 [DOI] [PubMed] [Google Scholar]
- 9. Brakebusch C., Grose R., Quondamatteo F., Ramirez A., Jorcano J. L., Pirro A., Svensson M., Herken R., Sasaki T., Timpl R., Werner S., Fässler R. (2000) Skin and hair follicle integrity is crucially dependent on β1-integrin expression on keratinocytes. EMBO J. 19, 3990–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assoian R. K., Schwartz M. A. (2001) Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell cycle progression. Curr. Opin. Genet. Dev. 11, 48–53 [DOI] [PubMed] [Google Scholar]
- 11. Huang S., Chen C. S., Ingber D. E. (1998) Control of cyclin D1, p27Kip1, and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol. Biol. Cell 9, 3179–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Graus-Porta D., Blaess S., Senften M., Littlewood-Evans A., Damsky C., Huang Z., Orban P., Klein R., Schittny J. C., Müller U. (2001) β1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31, 367–379 [DOI] [PubMed] [Google Scholar]
- 13. Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 14. Pullan S., Wilson J., Metcalfe A., Edwards G. M., Goberdhan N., Tilly J., Hickman J. A., Dive C., Streuli C. H. (1996) Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J. Cell Sci. 109, 631–642 [DOI] [PubMed] [Google Scholar]
- 15. Wang P., Ballestrem C., Streuli C. H. (2011) The C terminus of talin links integrins to cell cycle progression. J. Cell Biol. 195, 499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akhtar N., Streuli C. H. (2006) Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J. Cell Biol. 173, 781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naylor M. J., Li N., Cheung J., Lowe E. T., Lambert E., Marlow R., Wang P., Schatzmann F., Wintermantel T., Schüetz G., Clarke A. R., Mueller U., Hynes N. E., Streuli C. H. (2005) Ablation of β1-integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 171, 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klinowska T. C., Soriano J. V., Edwards G. M., Oliver J. M., Valentijn A. J., Montesano R., Streuli C. H. (1999) Laminin and β1-integrins are crucial for normal mammary gland development in the mouse. Dev. Biol. 215, 13–32 [DOI] [PubMed] [Google Scholar]
- 19. Buck S. B., Bradford J., Gee K. R., Agnew B. J., Clarke S. T., Salic A. (2008) Detection of S-phase cell cycle progression using 5-ethynyl-2′-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2′-deoxyuridine antibodies. BioTechniques 44, 927–929 [DOI] [PubMed] [Google Scholar]
- 20. Jeanes A. I., Maya-Mendoza A., Streuli C. H. (2011) Cellular microenvironment influences the ability of mammary epithelia to undergo cell cycle. PLoS One 6, e18144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brunet A., Roux D., Lenormand P., Dowd S., Keyse S., Pouysségur J. (1999) Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18, 664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mack N. A., Whalley H. J., Castillo-Lluva S., Malliri A. (2011) The diverse roles of Rac signaling in tumorigenesis. Cell Cycle 10, 1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eblen S. T., Slack J. K., Weber M. J., Catling A. D. (2002) Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell. Biol. 22, 6023–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang X., Mernaugh G., Yang D. H., Gewin L., Srichai M. B., Harris R. C., Iturregui J. M., Nelson R. D., Kohan D. E., Abrahamson D., Fässler R., Yurchenco P., Pozzi A., Zent R. (2009) β1-Integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136, 3357–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen C. S., Mrksich M., Huang S., Whitesides G. M., Ingber D. E. (1997) Geometric control of cell life and death. Science 276, 1425–1428 [DOI] [PubMed] [Google Scholar]
- 26. Aplin A. E., Stewart S. A., Assoian R. K., Juliano R. L. (2001) Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 153, 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hirsch E., Barberis L., Brancaccio M., Azzolino O., Xu D., Kyriakis J. M., Silengo L., Giancotti F. G., Tarone G., Fässler R., Altruda F. (2002) Defective Rac-mediated proliferation and survival after targeted mutation of the β1-integrin cytodomain. J. Cell Biol. 157, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yazicioglu M. N., Goad D. L., Ranganathan A., Whitehurst A. W., Goldsmith E. J., Cobb M. H. (2007) Mutations in ERK2-binding sites affect nuclear entry. J. Biol. Chem. 282, 28759–28767 [DOI] [PubMed] [Google Scholar]
- 29. Lidke D. S., Huang F., Post J. N., Rieger B., Wilsbacher J., Thomas J. L., Pouysségur J., Jovin T. M., Lenormand P. (2010) ERK nuclear translocation is dimerization-independent but controlled by the rate of phosphorylation. J. Biol. Chem. 285, 3092–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masuda K., Katagiri C., Nomura M., Sato M., Kakumoto K., Akagi T., Kikuchi K., Tanuma N., Shima H. (2010) MKP-7, a JNK phosphatase, blocks ERK-dependent gene activation by anchoring phosphorylated ERK in the cytoplasm. Biochem. Biophys. Res. Commun. 393, 201–206 [DOI] [PubMed] [Google Scholar]
- 31. Legate K. R., Fässler R. (2009) Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J. Cell Sci. 122, 187–198 [DOI] [PubMed] [Google Scholar]
- 32. Mettouchi A., Klein S., Guo W., Lopez-Lago M., Lemichez E., Westwick J. K., Giancotti F. G. (2001) Integrin-specific activation of Rac controls progression through the G1 phase of the cell cycle. Mol. Cell 8, 115–127 [DOI] [PubMed] [Google Scholar]
- 33. Danen E. H., Sonneveld P., Brakebusch C., Fassler R., Sonnenberg A. (2002) The fibronectin-binding integrins α5β1 and αvβ3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 159, 1071–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miao H., Li S., Hu Y. L., Yuan S., Zhao Y., Chen B. P., Puzon-McLaughlin W., Tarui T., Shyy J. Y., Takada Y., Usami S., Chien S. (2002) Differential regulation of Rho GTPases by β1- and β3-integrins: the role of an extracellular domain of integrin in intracellular signaling. J. Cell Sci. 115, 2199–2206 [DOI] [PubMed] [Google Scholar]
- 35. Schwartz M. A. (2010) Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb. Perspect. Biol. 2, a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caswell P. T., Vadrevu S., Norman J. C. (2009) Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10, 843–853 [DOI] [PubMed] [Google Scholar]
- 37. White D. P., Caswell P. T., Norman J. C. (2007) αvβ3- and α51-integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J. Cell Biol. 177, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bass M. D., Roach K. A., Morgan M. R., Mostafavi-Pour Z., Schoen T., Muramatsu T., Mayer U., Ballestrem C., Spatz J. P., Humphries M. J. (2007) Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J. Cell Biol. 177, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White D. E., Kurpios N. A., Zuo D., Hassell J. A., Blaess S., Mueller U., Muller W. J. (2004) Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170 [DOI] [PubMed] [Google Scholar]
- 40. Huck L., Pontier S. M., Zuo D. M., Muller W. J. (2010) β1-Integrin is dispensable for the induction of ErbB2 mammary tumors but plays a critical role in the metastatic phase of tumor progression. Proc. Natl. Acad. Sci. U.S.A. 107, 15559–15564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guo W., Pylayeva Y., Pepe A., Yoshioka T., Muller W. J., Inghirami G., Giancotti F. G. (2006) β4-Integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, 489–502 [DOI] [PubMed] [Google Scholar]
- 42. Klinowska T. C., Alexander C. M., Georges-Labouesse E., Van der Neut R., Kreidberg J. A., Jones C. J., Sonnenberg A., Streuli C. H. (2001) Epithelial development and differentiation in the mammary gland is not dependent on α3- or α6-integrin subunits. Dev. Biol. 233, 449–467 [DOI] [PubMed] [Google Scholar]
- 43. Gui G. P., Wells C. A., Browne P. D., Yeomans P., Jordan S., Puddefoot J. R., Vinson G. P., Carpenter R. (1995) Integrin expression in primary breast cancer and its relation to axillary nodal status. Surgery 117, 102–108 [DOI] [PubMed] [Google Scholar]
- 44. Park C. C., Zhang H. J., Yao E. S., Park C. J., Bissell M. J. (2008) β1-Integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 68, 4398–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sloan E. K., Pouliot N., Stanley K. L., Chia J., Moseley J. M., Hards D. K., Anderson R. L. (2006) Tumor-specific expression of αvβ3-integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 8, R20. [DOI] [PMC free article] [PubMed] [Google Scholar]