Background: Phospholipids containing polyunsaturated hydrocarbon chains play important roles in various biological membranes.
Results: Fluorescence microscopic analysis with a chemically synthesized fluorescent probe showed localization of phospholipids containing an eicosapentaenyl group at the bacterial cell division site.
Conclusion: Phospholipids containing polyunsaturated hydrocarbon chains form a membrane microdomain at the cell center, probably to promote cell division.
Significance: Hydrocarbon chain-dependent microdomain formation was visualized in vivo.
Keywords: Bacteria, Cell Division, Eicosapentaenoic acid (EPA), Membrane Lipids, Phospholipid, Polyunsaturated Fatty Acids, Fluorescent Probe, Membrane Microdomain
Abstract
In this study, we found that phospholipids containing an eicosapentaenyl group form a novel membrane microdomain at the cell division site of a Gram-negative bacterium, Shewanella livingstonensis Ac10, using chemically synthesized fluorescent probes. The occurrence of membrane microdomains in eukaryotes and prokaryotes has been demonstrated with various imaging tools for phospholipids with different polar headgroups. However, few studies have focused on the hydrocarbon chain-dependent localization of membrane-resident phospholipids in vivo. We previously found that lack of eicosapentaenoic acid (EPA), a polyunsaturated fatty acid found at the sn-2 position of glycerophospholipids, causes a defect in cell division after DNA replication of S. livingstonensis Ac10. Here, we synthesized phospholipid probes labeled with a fluorescent 7-nitro-2,1,3-benzoxadiazol-4-yl (NBD) group to study the localization of EPA-containing phospholipids by fluorescence microscopy. A fluorescent probe in which EPA was bound to the glycerol backbone via an ester bond was found to be unsuitable for imaging because EPA was released from the probe by in vivo hydrolysis. To overcome this problem, we synthesized hydrolysis-resistant ether-type phospholipid probes. Using these probes, we found that the fluorescence localized between two nucleoids at the cell center during cell division when the cells were grown in the presence of the eicosapentaenyl group-containing probe (N-NBD-1-oleoyl-2-eicosapentaenyl-sn-glycero-3-phosphoethanolamine), whereas this localization was not observed with the oleyl group-containing control probe (N-NBD-1-oleoyl-2-oleyl-sn-glycero-3-phosphoethanolamine). Thus, phospholipids containing an eicosapentaenyl group are specifically enriched at the cell division site. Formation of a membrane microdomain enriched in EPA-containing phospholipids at the nucleoid occlusion site probably facilitates cell division.
Introduction
Biological membranes are composed of lipids and proteins and play essential roles in all living organisms in nutrient uptake, waste efflux, signal transduction, morphogenesis, etc. According to the fluid mosaic model proposed by Singer and Nicolson (1), it was previously assumed that lipids are homogeneously distributed in biological membranes. However, this view was considerably modified by the discovery of membrane microdomains, each of which is supposed to have specific physiological functions.
Various lipid molecules form membrane microdomains in eukaryotes and prokaryotes. Eukaryotic cells contain membrane microdomains known as lipid rafts, which are enriched in sphingolipids and sterols (2). Lipid rafts serve as platforms for the colocalization of proteins involved in signaling and transport. In prokaryotes, cardiolipin microdomains exist at the poles and septa in the rod-shaped cells of Escherichia coli, Bacillus subtilis, and Pseudomonas putida (3, 4, 32). Recently, the membrane of B. subtilis was reported to contain microdomains functionally similar to the lipid rafts of eukaryotic cells (5). These observations indicate the importance of dissecting the distribution of individual lipid molecules in biological membranes to better understand the mechanisms behind biological phenomena observed at the cell membrane.
The long-chain ω-3 polyunsaturated fatty acids eicosapentaenoic acid (EPA4; 5,8,11,14,17-all-cis-20:5) and docosahexaenoic acid (DHA, 4,7,10,13,16,19-all-cis-22:6) have been attracting a great deal of attention mainly because of their beneficial effects on human health (6–8). EPA and DHA occur in the membranes of various organisms, including mammals and bacteria, as acyl chains of phospholipids. EPA and DHA serve as precursors of various eicosanoids and autacoids (9). DHA interacts with membrane-associated G protein-coupled receptors to support their function (10–12). In EPA-producing bacteria, EPA plays a beneficial role in cell division (13, 14). However, the molecular mechanisms of EPA and DHA action are not well defined, in particular, their roles in coordinating the spatial organization of the phospholipids that contain them in biological membranes.
The Gram-negative bacterium Shewanella livingstonensis Ac10, which produces EPA as an sn-2-acyl chain of phosphatidylethanolamine (PE) and phosphatidylglycerol (PG) (13, 15), is an excellent model organism for studies on in vivo EPA function for the following reasons (13, 15–17). (i) The genes coding for EPA synthase are known, enabling the construction of an EPA-less mutant by disruption of these genes. (ii) The phenotype of the EPA-less mutant is clear; depletion of EPA causes growth retardation at low temperatures and produces filamentous cells because of a defect in cell division. (iii) The structure-function relationship of EPA can be studied by externally adding a series of EPA analogs to the culture medium. (iv) The whole genome sequence is available for this bacterium. (v) The methods for the genetic manipulation of this bacterium have been established. (vi) As a bacterium, it has a much simpler membrane system than eukaryotic cells.
To gain more insight into the function of the EPA-containing phospholipids in the cell membrane, we synthesized fluorescent molecular probes in this study to visualize the distribution of phospholipids containing polyunsaturated hydrocarbon chains. Advances have recently been made in the methods for labeling small molecules to visualize their subcellular localization. For phospholipids, methods have been reported for visualization of phosphatidylserine (18), PE (19), phosphatidylcholine (20, 21), phosphatidic acid (22), and sphingomyelin (21, 23). However, hydrocarbon chain-dependent localization of phospholipids cannot be visualized using the currently available molecular probes. To achieve this, we introduced a fluorescent group into the headgroup of phospholipids. A polyunsaturated hydrocarbon chain was bound to the phospholipids via an ether bond. (An ester bond did not work because it was hydrolyzed in S. livingstonensis Ac10, resulting in the release of the polyunsaturated hydrocarbon chain from the probe molecule.) Using this fluorescent probe, we found that phospholipids with an eicosapentaenyl group are enriched at the cell division site of S. livingstonensis Ac10. This is in accordance with the physiological function of EPA-containing phospholipids in the cell division of this bacterium. These results greatly contribute to understanding the in vivo function of polyunsaturated fatty acids and indicate the usefulness of the probe we synthesized.
EXPERIMENTAL PROCEDURES
Synthesis of Fluorescent Phospholipid Probes
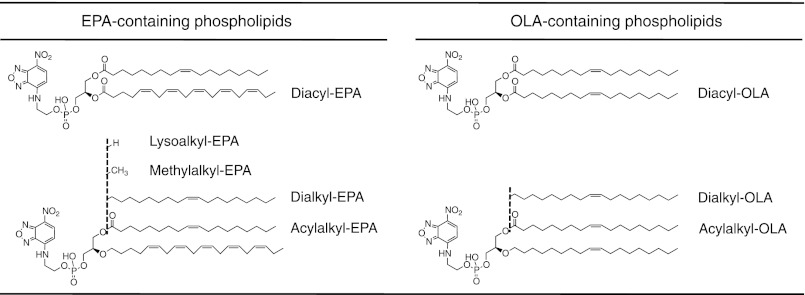
The 7-nitro-2,1,3-benzoxadiazol-4-yl (NBD)-labeled phospholipids shown in Fig. 1 were chemically synthesized as described under supplemental “Experimental Procedures.”
FIGURE 1.
NBD-labeled phospholipids synthesized in this study. Diacyl-EPA, diacyl-OLA, acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, and dialkyl-OLA were named after the binding mode between the glycerol moiety and the hydrocarbon chains, as well as the hydrocarbon chain species at the sn-2 position. Acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, dialkyl-OLA, methylalkyl-EPA, and lysoalkyl-EPA correspond to compounds 34, 33, 14, 21, 38, and 40, respectively, in supplemental Scheme S1.
Bacterial Strain and Growth Conditions
The EPA-less mutant of S. livingstonensis Ac10 used in this study was constructed by the disruption of orf5, one of the genes required for EPA synthesis (13). We previously found that this EPA-less mutant is longer than the wild-type strain because of retardation of cell division after DNA replication; this longer bacterial length facilitates microscopic analysis of the phospholipid subcellular localization, as fluorescence microscopic analysis of the much shorter wild-type strain cells is difficult. The cells were grown in LB medium containing 50 μg/ml rifampicin and 40 μg/ml kanamycin for 48 h at 18 °C. The seed culture was used to inoculate 5 ml of LB medium containing the various NBD-labeled phospholipids (0.13 mm) chemically synthesized in this study. The phospholipid solution in chloroform was dried in a sterilized tube with nitrogen gas, resolubilized in 80 μl of ethanol, and then hydrated with 5 ml of LB medium to a final concentration of 0.13 mm by vortexing. The cells were cultured at 6 °C for 250 h until they reached stationary phase.
Fluorescence Microscopic Analysis
The culture (100 μl each) was sampled at 150, 200, and 250 h, and the fluorescence images were obtained using an epifluorescence microscope. For nuclear labeling, the cells collected at 250 h were treated with Hoechst 33342 (final concentration of 25 μg/ml) and washed twice with 200 μl of buffer containing 0.75 m sucrose and 10 mm Tris-HCl (pH 7.5). The fluorescence images were also obtained after dithionite treatment of the cells to quench the NBD fluorescence in the outer leaflet of the outer membrane. Further details are described under supplemental “Experimental Procedures.”
Electrospray Ionization (ESI) MS Analysis of Phospholipids
After fluorescence microscopic analysis, lipids were extracted from the residual sample by the Bligh and Dyer method (24) and analyzed by ESI-MS. The details of the ESI-MS analysis are described under supplemental “Experimental Procedures.”
Dithionite Assay for Measuring Lipid Distribution in Inner (IM) and Outer (OM) Membranes
To determine the distribution of NBD-labeled phospholipids in the IM and OM of the bacterium, we employed a fluorescence-quenching assay using sodium dithionite. For this assay, the following three samples were prepared: non-treated, lysozyme-treated, and lysozyme-and Triton X-100-treated cells (supplemental Fig. S1A). In the non-treated samples, only NBD-labeled phospholipids at the outer leaflet of the OM (OMout) were accessible by dithionite, and their fluorescence was quenched. In the lysozyme-treated samples, the OM was disrupted, and both the inner leaflet of the OM (OMin) and the outer leaflet of the IM (IMout), in addition to the OMout, became accessible to dithionite for fluorescence quenching. In the lysozyme- and Triton X-100-treated samples, both the OM and IM were disrupted, and the inner leaflet of the IM (IMin), as well as the OMout, OMin, and IMout, became accessible to dithionite. Further details of the dithionite assay are described under supplemental “Experimental Procedures.”
RESULTS
Fate of Fluorescence-labeled Diacyl Phospholipids in Cell Membranes
To visualize EPA-containing phospholipids in S. livingstonensis Ac10, we first synthesized a fluorescence-labeled phospholipid, N-NBD-1-oleoyl-2-eicosapentaenoyl-sn-glycero-3-phosphoethanolamine (diacyl-EPA) (Fig. 1; see supplemental “Experimental Procedures” for the synthesis). EPA was introduced into the sn-2 position because a polyunsaturated fatty acid is primarily found at this position in phospholipids in S. livingstonensis Ac10 and other organisms (25). The fluorescent NBD group was introduced into the headgroup to avoid modification of the hydrocarbon chain, which was characterized in this study. As a control, we synthesized N-NBD-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (diacyl-oleic acid (OLA)), which contained the oleoyl group instead of the eicosapentaenoyl group at the sn-2 position (Fig. 1; see supplemental “Experimental Procedures” for the synthesis). In these phospholipids, OLA (9-cis-18:1) was introduced into the sn-1 position because OLA and OLA-containing phospholipids were shown to have neutral effects on the phenotype of the EPA-less mutant (13, 15).
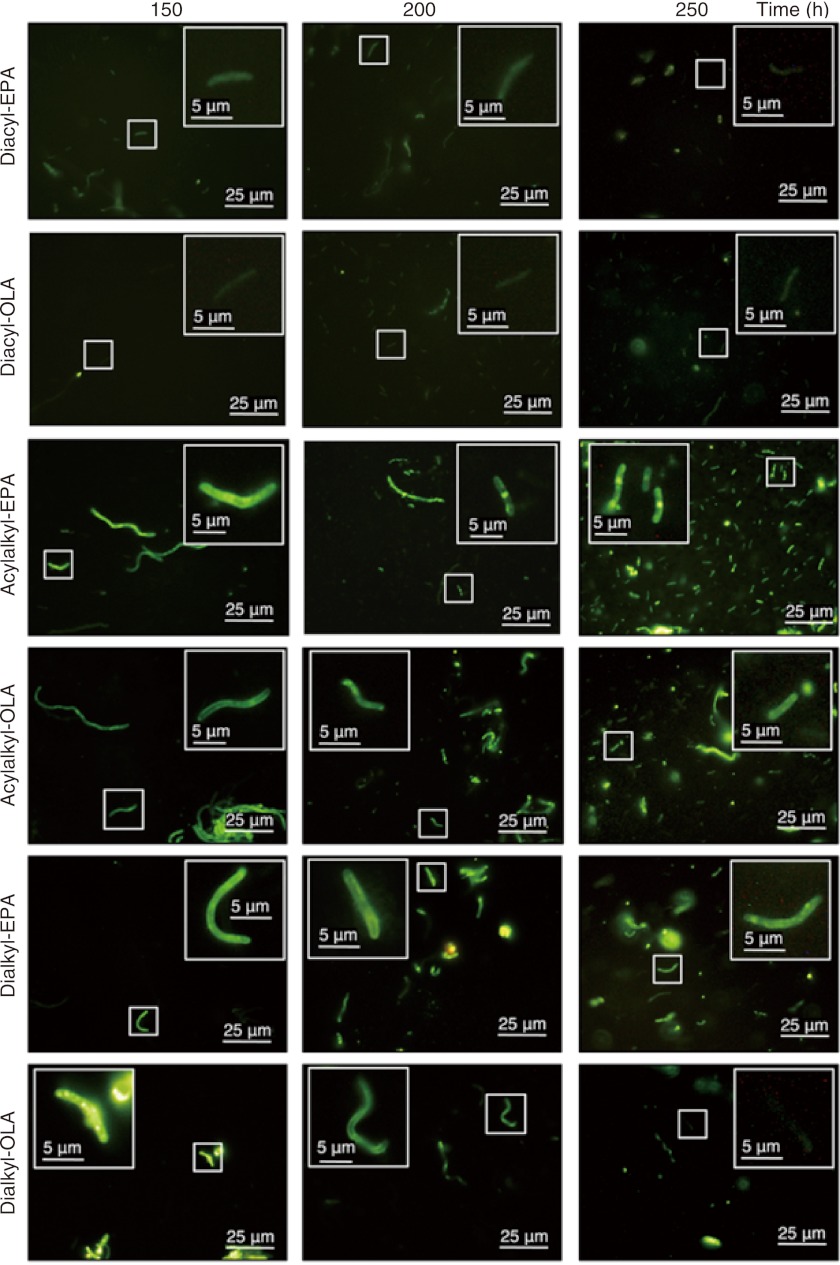
S. livingstonensis Ac10 was grown at 6 °C in LB medium supplemented with diacyl-EPA or diacyl-OLA, and the cellular phospholipids were analyzed by ESI-MS. We found that the amounts of diacyl-EPA and diacyl-OLA in cellular membranes gradually decreased during cultivation for 166 h, whereas PEs and PGs containing EPA or OLA gradually increased (supplemental Fig. S2). The results suggest that diacyl-EPA and diacyl-OLA are hydrolyzed to yield free fatty acids, which are then incorporated into cellular PEs and PGs. The OM phospholipase A likely catalyzed this hydrolysis (15, 26, 27). The NBD moiety of diacyl-EPA and diacyl-OLA was not introduced into cellular phospholipids as evaluated by ESI-MS analysis. In accordance with the ESI-MS data, fluorescence microscopic analysis revealed that the diacyl-EPA and diacyl-OLA fluorescence was hardly detected at 150 h and thereafter (Fig. 2). Thus, we learned that although EPA and OLA could be incorporated into PE and PG, they could not be tracked visually because the fluorescent NBD moiety was hydrolyzed and lost. Therefore, the NBD-labeled diacyl phospholipids were not suitable for continual visualization of EPA-containing phospholipids in vivo.
FIGURE 2.
Fluorescence microscopic images of S. livingstonensis Ac10 grown in presence of NBD-labeled phospholipids (diacyl-EPA, diacyl-OLA, acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, and dialkyl-OLA) for 150, 200, and 250 h.
Synthesis of Fluorescent Eicosapentaenyl Group-containing Phospholipids
For continual visualization of phospholipids in vivo, we synthesized NBD-containing hydrolysis-resistant PE derivatives to prevent the release of the hydrocarbon chain from the phospholipids. It was expected that replacement of the ester bond at the sn-2 position of phospholipids with an ether bond would suppress the hydrolytic release of the hydrocarbon chain from the glycerol backbone. Accordingly, we synthesized the following ether phospholipids to use in the in vivo imaging experiments: N-NBD-1-oleoyl-2-eicosapentaenyl-sn-glycero-3-phosphoethanolamine (acylalkyl-EPA), N-NBD-1-oleoyl-2-oleyl-sn-glycero-3-phosphoethanolamine (acylalkyl-OLA), N-NBD-1-oleyl-2-eicosapentaenyl-sn-glycero-3-phosphoethanolamine (dialkyl-EPA), and N-NBD-1,2-dioleyl-sn-glycero-3-phosphoethanolamine (dialkyl-OLA) (Fig. 1; see supplemental “Experimental Procedures” for the synthesis).
Cellular Uptake and Remodeling of Fluorescent Eicosapentaenyl Group-containing Phospholipids
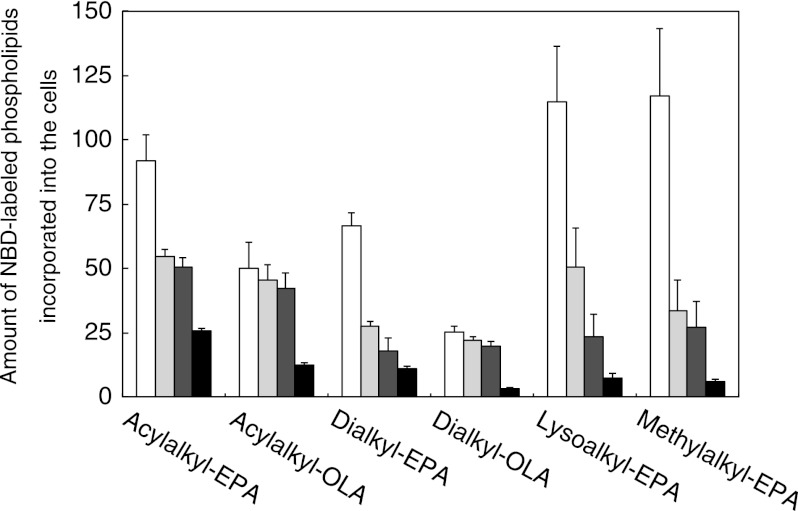
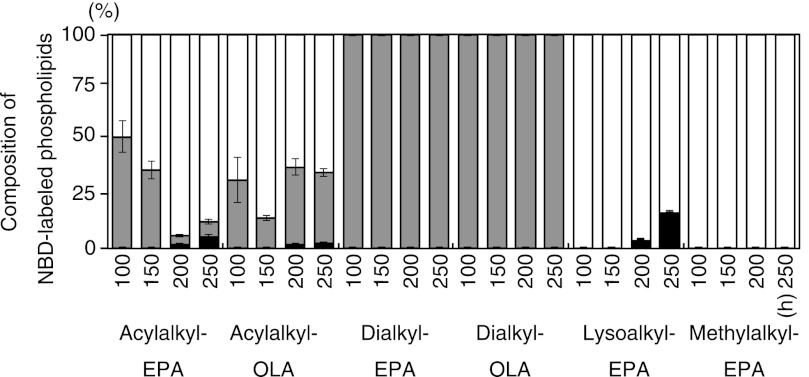
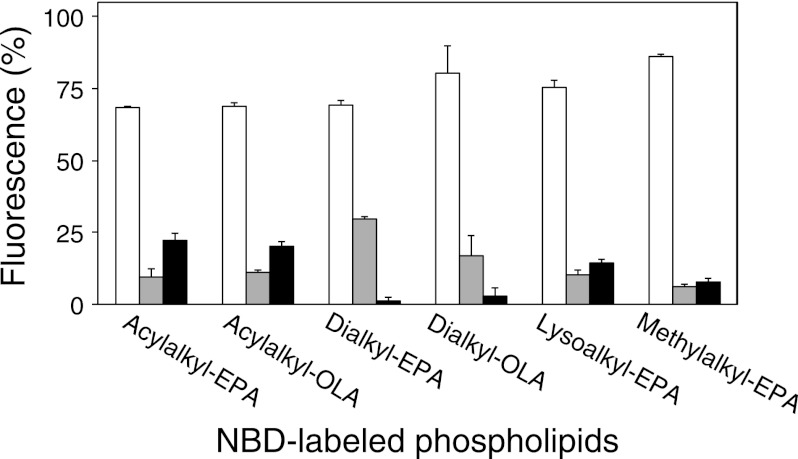
We tested whether these synthetic ether phospholipids are incorporated into the cell membrane of S. livingstonensis Ac10. The total lipids isolated from the cells grown in the presence of acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, or dialkyl-OLA were analyzed by ESI MS/MS (Figs. 3 and 4). NBD-labeled phospholipids were found in the cell membrane when any of the synthetic ether phospholipids were used (Fig. 3). We also found that acylalkyl-EPA and acylalkyl-OLA were hydrolyzed at the sn-1 (but not sn-2) position to yield N-NBD-2-eicosapentaenyl-sn-glycero-3-phosphoethanolamine (lysoalkyl-EPA) and N-NBD-2-oleyl-sn-glycero-3-phosphoethanolamine (lysoalkyl-OLA), respectively, during cultivation (Fig. 4, white bars). In addition to lysoalkyl-EPA and lysoalkyl-OLA, re-acylated NBD-labeled phospholipids containing two hydrocarbon chains were produced at 200 and 250 h (Fig. 4, black bars). The ratio of the total amount of the NBD-labeled phospholipids to the total amount of the unlabeled phospholipids in the cells grown in the presence of acylalkyl-EPA for 250 h was 9.8 ± 3.1%. The composition of the fatty acyl chains at the sn-1 position of the NBD-labeled phospholipids derived from acylalkyl-EPA is shown in Table 1. These phospholipids, constituting 1–5% of the total NBD-labeled phospholipids, were produced by the incorporation of a bacterial fatty acyl chain into the sn-1 position. In contrast to acylalkyl-EPA and acylalkyl-OLA, dialkyl-EPA and dialkyl-OLA were incorporated into the cell membrane without remodeling because the ether bonds at the sn-1 and sn-2 positions are both resistant to hydrolysis (Fig. 4).
FIGURE 3.
Amount of NBD-labeled phospholipids incorporated into S. livingstonensis Ac10 cells grown in presence of acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, dialkyl-OLA, lysoalkyl-EPA, and methylalkyl-EPA for 50 (white bars), 100 (light gray bars), 150 (dark gray bars), and 200 (black bars) h. The amount was calculated as described under “Experimental Procedures” and supplemental “Experimental Procedures.” The mean ± S.D. of three separate measurements is shown.
FIGURE 4.
Composition of NBD-labeled phospholipids in S. livingstonensis Ac10 cells. The cells were grown in the presence of acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, dialkyl-OLA, lysoalkyl-EPA, and methylalkyl-EPA for 100, 150, 200, and 250 h. The structures of NBD-labeled phospholipids extracted from the cells were analyzed by ESI-MS. Gray bars, externally added double-chain phospholipids; white bars, single-chain phospholipids derived from acylalkyl-EPA and acylalkyl-OLA and externally added lysoalkyl-EPA and methylalkyl-EPA; black bars, double-chain phospholipids produced in S. livingstonensis Ac10 by acylation at the sn-1 position of the single-chain phospholipids. The mean ± S.D. of three separate measurements is shown.
TABLE 1.
Composition of the sn-1 acyl chain of NBD-labeled phospholipids from cells cultivated with acylalkyl-EPA and lysoalkyl-EPA
| sn-1 Acyl chain | Relative amount |
|
|---|---|---|
| Acylalkyl-EPA | Lysoalkyl-EPA | |
| % | ||
| 13:0 | 4.0 ± 0.3 | 4.1 ± 0.6 |
| 13:1 | 1.2 ± 0.6 | 1.6 ± 0.6 |
| 14:0 | 4.0 ± 2.6 | 4.7 ± 2.6 |
| 14:1 | 1.6 ± 1.0 | 1.8 ± 1.0 |
| 15:0 | 19.0 ± 9.2 | 24.5 ± 12.3 |
| 15:1 | 0.9 ± 0.2 | 1.3 ± 0.6 |
| 16:0 | 0.7 ± 0.1 | 1.0 ± 0.2 |
| 16:1 | 29.6 ± 6.4 | 37.2 ± 11.4 |
| 17:0 | 6.6 ± 0.6 | 8.2 ± 0.7 |
| 17:1 | 3.3 ± 0.5 | 4.1 ± 0.2 |
| 18:0 | 2.1 ± 0.4 | 2.7 ± 0.6 |
| 18:1 | 27.0 ± 2.1 | 8.8 ± 1.1 |
| 20:5 | NDa | ND |
a ND, not determined.
Distribution of NBD-labeled Phospholipids in IM and OM
S. livingstonensis Ac10 is a Gram-negative bacterium possessing both the OM and IM. We measured the amount of NBD-labeled phospholipids distributed in these membranes by the dithionite quenching assay (see “Experimental Procedures” and supplemental “Experimental Procedures” for details). To identify the membrane-specific location of the NBD-labeled phospholipids, we prepared the following three samples from cells grown at 6 °C for 250 h: non-treated, lysozyme-treated, and lysozyme- and Triton X-100-treated cells (supplemental Fig. S1A). Using these samples, NBD-labeled phospholipids located in the OMout, in the OMin and IMout, and in the IMin can be measured. Note that the OMin and IMout cannot be distinguished by this assay.
We calculated the ratio of the NBD fluorescence at the OMout, OMin + IMout, and IMin in the cells grown in the presence of acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, or dialkyl-OLA for 250 h (Fig. 5). For dialkyl-EPA and dialkyl-OLA, the NBD fluorescence was found at the OMout and OMin + IMout, but almost no fluorescence was found at the IMin. In contrast, ∼25% of the fluorescence was found at the IMin when acylalkyl-EPA or acylalkyl-OLA was added to the cells.
FIGURE 5.
Distribution of NBD-labeled phospholipids in OMout (white bars), OMin + IMout (gray bars), and IMin (black bars). The cells were grown in the presence of the various NBD-labeled phospholipids indicated. The mean ± S.D. of three separate determinations is shown.
In these experiments, a large part of the NBD fluorescence was quenched even when non-treated cells were used. This was not due to quenching of NBD derivatives in the culture medium because the cells were washed well before dithionite treatment. The results rather indicate that a large part of the NBD-labeled phospholipids was localized in OMout. This uneven distribution between the different membrane surfaces is probably due to slow flip-flop of the lipids.
Fluorescence Microscopic Analysis of Eicosapentaenyl Group-containing Phospholipids
We cultivated S. livingstonensis Ac10 at 6 °C in LB medium containing the NBD-labeled ether phospholipids (acylalkyl-EPA, acylalkyl-OLA, dialkyl-EPA, and dialkyl-OLA) for fluorescence microscopic analysis. These cells showed more intense fluorescence than those grown in the presence of diacyl-EPA or diacyl-OLA, except for the cells grown in the presence of dialkyl-OLA for 250 h (Fig. 2). The disappearance of the fluorescence from the cells grown in the presence of dialkyl-OLA was probably due to degradation of the NBD-labeled phospholipids or their elimination from the cells (Fig. 3). We found that the fluorescence was localized at the center of the cells when they were grown in the presence of acylalkyl-EPA for 200 h or longer, whereas this localization was not observed in cells grown in the presence of the acylalkyl-OLA control (Figs. 2 and 6). Fluorescence localization was also not observed in the dialkyl-EPA and dialkyl-OLA experiment (Fig. 2).
FIGURE 6.
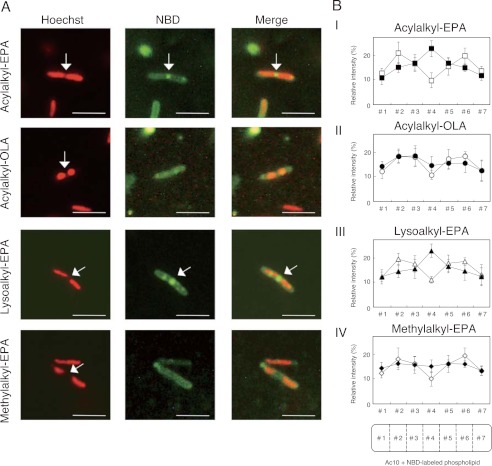
Fluorescence microscopic images of S. livingstonensis Ac10 grown in presence of NBD-labeled phospholipids. A, fluorescence microscopic images of the cells stained with Hoechst 33342 (false-colored red) (left panels) and NBD-labeled phospholipids (center panels) and merged images (right panels). The cells were grown at 6 °C for 250 h in the presence of the NBD-labeled phospholipids indicated on the left. Scale bars = 5 μm. B, quantification of subcellular localization of NBD-labeled phospholipids and Hoechst 33342. The cells were grown in the presence of acylalkyl-EPA (panel I), acylalkyl-OLA (panel II), lysoalkyl-EPA (panel III), and methylalkyl-EPA (panel IV) at 6 °C for 250 h. In each case, 15 cells during cell division were randomly chosen, and fluorescence intensities in seven equally sized sections of the cells (#1–#7) were determined using ImageJ software. Closed symbols, fluorescence of NBD-labeled phospholipids; open symbols, fluorescence of Hoechst 33342.
To further characterize the fluorescence localization, the cellular nucleoids were stained with Hoechst 33342 in cells grown with acylalkyl-EPA or acylalkyl-OLA for 250 h. Each of the 15 randomly chosen cells undergoing cell division was divided into seven equally sized sections, and the fluorescence intensities of NBD and Hoechst 33342 in each segment were measured (Fig. 6B). We found that the NBD-labeled ether phospholipids were localized at the nucleoid occlusion site at the center of the acylalkyl-EPA-treated cells undergoing cell division. This localization was not observed when acylalkyl-OLA was used. On the other hand, the same localization and cellular uptake and remodeling were observed when the sn-1-oleoyl group of acylalkyl-EPA was replaced with a palmitoleoyl group (data not shown). Thus, the fluorescence localization at the center of the cells was dependent on the eicosapentaenyl group of the phospholipid. The localization we observed in these experiments is in accordance with the physiological importance of EPA-containing phospholipids in cell division.
Imaging of Lysoalkyl-EPA and Its Analog, N-NBD-1-methyl-2-eicosapentaenyl-sn-glycero-3-phosphoethanolamine (Methylalkyl-EPA), in Cell Membranes
As shown in Figs. 2 and 6, we found that NBD-labeled phospholipids were localized at the center of the cells when acylalkyl-EPA was added to the cells. Under this condition, some of the acylalkyl-EPA added to the cells was converted into lysoalkyl-EPA, which was subsequently re-acylated to form phospholipids with two hydrocarbon chains (Fig. 4). Thus, the fluorescent phospholipids localized at the center of the cells could be the acylalkyl-EPA added to the cells, the converted lysoalkyl-EPA, or the re-acylated phospholipids. To distinguish between these and to examine whether lysoalkyl-EPA produced from acylalkyl-EPA was localized at the center of the cells, we synthesized a lysoalkyl-EPA analog, methylalkyl-EPA, for in vivo imaging (Fig. 1; see supplemental “Experimental Procedures” for the synthesis). Methylalkyl-EPA retains the hydrophilicity of lysoalkyl-EPA, which is considered to be important for the molecule to traverse through the periplasmic space to reach the IM. However, unlike lysoalkyl-EPA, it is not susceptible to acylation at the sn-1 position. We also synthesized lysoalkyl-EPA as a control (Fig. 1; see supplemental “Experimental Procedures” for the synthesis).
S. livingstonensis Ac10 was grown in the presence of lysoalkyl-EPA or methylalkyl-EPA, and the cellular phospholipids were analyzed by ESI-MS. The amounts of NBD-labeled phospholipids found incorporated into the cells were not significantly different between lysoalkyl-EPA and methylalkyl-EPA (Fig. 3). We also determined the amounts of NBD-labeled phospholipids distributed to the OM and IM by the dithionite quenching assay (Fig. 5). NBD-labeled phospholipids were found not only in the OMout and OMin + IMout, but also in the IMin, irrespective of whether lysoalkyl-EPA or methylalkyl-EPA was used. Thus, methylalkyl-EPA mimics lysoalkyl-EPA in these respects. On the other hand, methylalkyl-EPA is different from lysoalkyl-EPA in that methylalkyl-EPA was not acylated at the sn-1 position, as expected (Fig. 4). In the case of lysoalkyl-EPA, the sn-1 position was acylated, and the acyl chain composition at the sn-1 position was similar to that of the NBD-labeled phospholipids derived from acylalkyl-EPA (Table 1). Fluorescence microscopic analysis showed fluorescence localization at the center of the cells when lysoalkyl-EPA was added to the cells, whereas this localization was not observed when methylalkyl-EPA was used (Fig. 6). This finding suggested that the localization at the center of the cells was dependent on the acylation at the sn-1 position.
Fluorescence Microscopic Analysis of Cells Treated with Dithionite to Quench NBD Fluorescence in OMout
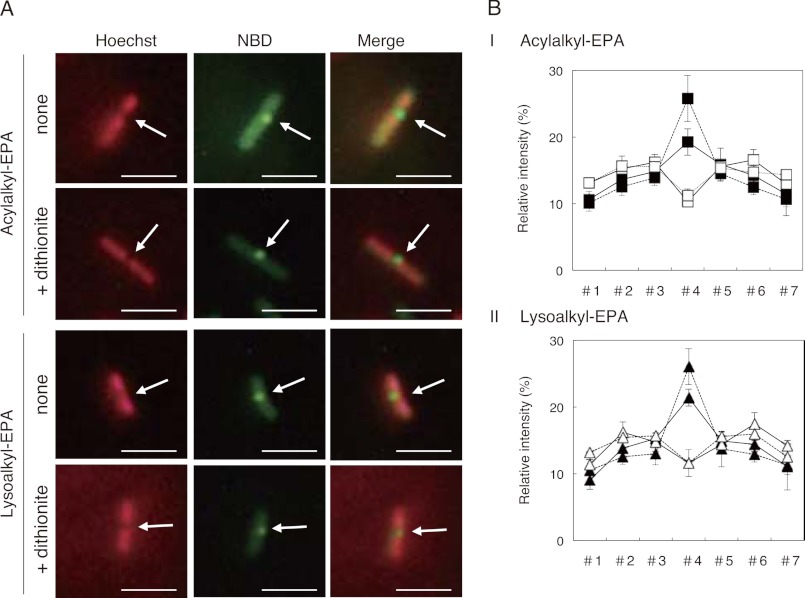
S. livingstonensis Ac10 was grown at 6 °C for 250 h in the presence of acylalkyl-EPA or lysoalkyl-EPA, and the NBD fluorescence in the OMout was quenched with sodium dithionite before fluorescence microscopic analysis as described under “Experimental Procedures” and supplemental “Experimental Procedures.” The relative fluorescence intensity at the cell division site was increased by the dithionite treatment of both acylalkyl-EPA-labeled and lysoalkyl-EPA-labeled cells (Fig. 7).
FIGURE 7.
Fluorescence microscopic images of dithionite-treated S. livingstonensis Ac10 grown in presence of NBD-labeled phospholipids. A, fluorescence microscopic images of the cells stained with Hoechst 33342 (false-colored red) (left panels) and NBD-labeled phospholipids (center panels) and merged images (right panels). The cells were grown at 6 °C for 250 h in the presence of acylalkyl-EPA or lysoalkyl-EPA as indicated on the left. For each of the acylalkyl-EPA-labeled and lysoalkyl-EPA-labeled cells, fluorescence images with (lower panels) and without (upper panels) dithionite treatment are shown. Scale bars = 5 μm. B, quantification of the subcellular localization of NBD-labeled phospholipids and Hoechst 33342. The cells were grown in the presence of acylalkyl-EPA (panel I) and lysoalkyl-EPA (panel II) at 6 °C for 250 h. In each case, 15 cells during cell division were randomly chosen, and fluorescence intensities in seven equally sized sections of the cells (#1–#7) were determined using ImageJ software. Closed symbols, fluorescence of NBD-labeled phospholipids; open symbols, fluorescence of Hoechst 33342. The dashed and solid lines indicate fluorescence intensities of the cells with and without dithionite treatment, respectively.
DISCUSSION
Identity of Fluorescent Phospholipid Localized at Center of Cells during Cell Division
We found that the NBD-labeled fluorescent phospholipids localized to the nucleoid occlusion site at the cell center during cell division when acylalkyl-EPA was added to S. livingstonensis Ac10 (Figs. 2 and 6). This localization was eicosapentaenyl group-dependent because it was not observed when the cells were grown in the presence of acylalkyl-OLA. Notably, when another phospholipid containing the eicosapentaenyl group was used, namely dialkyl-EPA, this localization was not observed (Fig. 2). This may be because of the difference in their subcellular distribution. The NBD-labeled phospholipids were detected in the IMin when acylalkyl-EPA was used, whereas the NBD-labeled phospholipids were not found in the IMin with dialkyl-EPA (Fig. 5). This is likely because the sn-1 position of dialkyl-EPA was not hydrolyzed, rendering it less capable of migrating into the IMin. Because the OMin and IMout cannot be distinguished by the dithionite quenching assay, it is possible that the NBD-labeled phospholipids did not even reach the IMout when dialkyl-EPA was used. Thus, the data indicate that the NBD-labeled phospholipids that localize at the nucleoid occlusion site may come from the IM. This view is supported by the fluorescence microscopic analysis of the cells treated with dithionite. Dithionite treatment of the cells to quench the NBD fluorescence at the OMout increased relative fluorescence intensities at the cell division site (Fig. 7).
When we used diacyl-EPA, the cells were not labeled efficiently. This was probably due to the catalytic action of the OM phospholipase A (15, 26, 27); this enzyme likely catalyzed the cleavage of both ester-bonded hydrocarbon chains in diacyl-EPA. Thus, the resulting fluorescent N-NBD-sn-glycero-3-phosphoethanolamine was likely released from the OM because of its high hydrophilicity and was not subsequently incorporated into any cellular membrane components, rendering it impossible for us to detect it by fluorescence methods.
Acylalkyl-EPA was converted into lysoalkyl-EPA (Fig. 4, white bars) and then re-acylated to form NBD-labeled phospholipids with two hydrocarbon chains, one of which was the eicosapentaenyl group and the other an acyl group produced by the bacterial cells (Fig. 4, black bars). Thus, the fluorescent phospholipids observed in our study that localized to the cell center could be acylalkyl-EPA, lysoalkyl-EPA, or re-acylated phospholipids. We found that the fluorescent phospholipids localized to the center of the cells when lysoalkyl-EPA (but not methylalkyl-EPA) was used (Fig. 6). Methylalkyl-EPA mimics lysoalkyl-EPA in its distribution in the OM and IM but differs from lysoalkyl-EPA in that it cannot be acylated at the sn-1 position. Thus, the results suggest that the fluorescence localization at the nucleoid occlusion site was not due to lysoalkyl-EPA itself but was due to re-acylated phospholipids. In Gram-negative bacteria, 2-acylglycerophosphoethanolamine acyltransferase, an enzyme that catalyzes the acylation of lysophospholipids at the sn-1 position, is localized to the IM (28, 29). Considering that the NBD-labeled phospholipids were found in the IM when lysoalkyl-EPA was added to the cells (Fig. 5), acylation of lysoalkyl-EPA at the sn-1 position may have been catalyzed by this enzyme once it reached the IM; this allowed it to travel to the nucleoid occlusion site in the center of the bacterial cells.
Dialkyl-EPA and dialkyl-OLA did not reach the IMin (Fig. 5), suggesting that phospholipids containing two hydrocarbon chains cannot traverse through the periplasmic space unless at least one of their hydrocarbon chains is cleaved off. The acyl group at the sn-1 position of acylalkyl-EPA and acylalkyl-OLA was probably hydrolyzed before they traversed through the periplasm to reach the IM. OM-resident phospholipase A most likely catalyzed this reaction to produce lysoalkyl-EPA and lysoalkyl-OLA, respectively (15, 26, 27). We suggest that lysoalkyl-EPA, produced by hydrolysis of acylalkyl-EPA, is re-acylated at its sn-1 position after reaching the IM, and the re-acylated NBD-labeled phospholipids are localized at the nucleoid occlusion site at the cell center during cell division.
Mechanisms and Significance of Formation of Membrane Microdomain Enriched in Polyunsaturated Hydrocarbon Chain-containing Phospholipids
We found that phospholipids containing an eicosapentaenyl group are enriched at the site of cell division. This is in accordance with our finding that EPA-containing phospholipids play an important functional role in the cell division in S. livingstonensis Ac10 (13). Although the molecular mechanism for the formation of the membrane domain enriched in polyunsaturated hydrocarbon chains remains unknown, we suggest the following two possibilities. The first is that the phospholipids containing the eicosapentaenyl group are enriched in a membrane region with a high curvature because of their physicochemical properties. We consider this possibility because the bending rigidity of bilayers rich in phospholipids with a polyunsaturated hydrocarbon chain is known to be low (30). The cellular membrane at the site of cell division has a high curvature, which may cause the enrichment of phospholipids with the eicosapentaenyl group in this region. The second possibility is that phospholipids containing an eicosapentaenyl group interact with proteins localized at the center of the cells during cell division. Considering that EPA-containing phospholipids promote cell division, there may be a direct or indirect interaction between these phospholipids and cell division-related proteins at the cell center. These interactions may cause enrichment of the eicosapentaenyl group-containing phospholipids at the site of cell division.
Polyunsaturated hydrocarbon chains have much greater diversity of energetically accessible conformational states than saturated hydrocarbon chains (31). This allows polyunsaturated hydrocarbon chains to solvate membrane proteins with rough surfaces with little energetic penalty. Thus, interaction with EPA-containing phospholipids may be beneficial for the stability and function of various membrane proteins, including those involved in cell division. The molecular interactions between EPA-containing phospholipids and membrane proteins involved in cell division are currently under investigation to better understand the role of EPA-containing phospholipids at the site of cell division. The occurrence of such membrane microdomains in other organisms and their specific physiological roles should also be examined in future studies. In this study, the visualization of the membrane microdomain enriched in phospholipids with a polyunsaturated hydrocarbon chain was made possible using a newly synthesized fluorescent probe with an uncleavable bond between the hydrocarbon chain and the glycerol backbone. This type of probe would be useful in examining whether membrane microdomains like the ones we observed in this study occur more generally among various organisms.
Supplementary Material
Acknowledgment
We thank Prof. Masaaki Okazaki (Hirosaki University) for helpful advice on chemical synthesis.
This work was supported in part by Grants-in-aid for Scientific Research (B) 20360372 and 22404021 from the Japan Society for the Promotion of Science, Grant-in-aid for Challenging Exploratory Research 22658028, and grants from the Institute for Fermentation (Osaka) and the Japan Foundation for Applied Enzymology (to T. K.); by Grant for Research for Promoting Technological Seeds 10-041 from the Japan Science and Technology Agency (to J. K.); and by Japan Society for the Promotion of Science research fellowships for young scientists (to S. S.).
- EPA
- eicosapentaenoic acid
- DHA
- docosahexaenoic acid
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- NBD
- 7-nitro-2,1,3-benzoxadiazol-4-yl
- ESI
- electrospray ionization
- IM
- inner membrane
- OM
- outer membrane
- OMout
- OM outer leaflet
- OMin
- OM inner leaflet
- IMout
- IM outer leaflet
- IMin
- IM inner leaflet
- OLA
- oleic acid.
REFERENCES
- 1. Singer S. J., Nicolson G. L. (1972) The fluid mosaic model of the structure of cell membranes. Science 175, 720–731 [DOI] [PubMed] [Google Scholar]
- 2. Lingwood D., Simons K. (2010) Lipid rafts as a membrane-organizing principle. Science 327, 46–50 [DOI] [PubMed] [Google Scholar]
- 3. Matsumoto K., Kusaka J., Nishibori A., Hara H. (2006) Lipid domains in bacterial membranes. Mol. Microbiol. 61, 1110–1117 [DOI] [PubMed] [Google Scholar]
- 4. Renner L. D., Weibel D. B. (2011) Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. U.S.A. 108, 6264–6269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. López D., Kolter R. (2010) Functional microdomains in bacterial membranes. Genes Dev. 24, 1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wall R., Ross R. P., Fitzgerald G. F., Stanton C. (2010) Fatty acids from fish: the anti-inflammatory potential of long-chain ω-3 fatty acids. Nutr. Rev. 68, 280–289 [DOI] [PubMed] [Google Scholar]
- 7. Watts G. F., Mori T. A. (2011) Recent advances in understanding the role and use of marine ω-3 polyunsaturated fatty acids in cardiovascular protection. Curr. Opin. Lipidol. 22, 70–71 [DOI] [PubMed] [Google Scholar]
- 8. Sato S. B., Sato S., Kawamoto J., Kurihara T. (2011) Differential roles of internal and terminal double bonds in docosahexaenoic acid: comparative study of cytotoxicity of polyunsaturated fatty acids to HT-29 human colorectal tumor cell line. Prostaglandins Leukot. Essent. Fatty Acids 84, 31–37 [DOI] [PubMed] [Google Scholar]
- 9. Schmitz G., Ecker J. (2008) The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47, 147–155 [DOI] [PubMed] [Google Scholar]
- 10. Feller S. E., Gawrisch K. (2005) Properties of docosahexaenoic acid-containing lipids and their influence on the function of rhodopsin. Curr. Opin. Struct. Biol. 15, 416–422 [DOI] [PubMed] [Google Scholar]
- 11. Carrillo-Tripp M., Feller S. E. (2005) Evidence for a mechanism by which ω-3 polyunsaturated lipids may affect membrane protein function. Biochemistry 44, 10164–10169 [DOI] [PubMed] [Google Scholar]
- 12. Grossfield A., Feller S. E., Pitman M. C. (2006) A role for direct interactions in the modulation of rhodopsin by ω-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. U.S.A. 103, 4888–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kawamoto J., Kurihara T., Yamamoto K., Nagayasu M., Tani Y., Mihara H., Hosokawa M., Baba T., Sato S. B., Esaki N. (2009) Eicosapentaenoic acid plays a beneficial role in membrane organization and cell division of a cold-adapted bacterium, Shewanella livingstonensis Ac10. J. Bacteriol. 191, 632–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawamoto J., Sato T., Nakasone K., Kato C., Mihara H., Esaki N., Kurihara T. (2011) Favorable effects of eicosapentaenoic acid on the late step of the cell division in a piezophilic bacterium, Shewanella violacea DSS12, at high hydrostatic pressures. Environ. Microbiol. 13, 2293–2298 [DOI] [PubMed] [Google Scholar]
- 15. Sato S., Kurihara T., Kawamoto J., Hosokawa M., Sato S. B., Esaki N. (2008) Cold adaptation of eicosapentaenoic acid-less mutant of Shewanella livingstonensis Ac10 involving uptake and remodeling of synthetic phospholipids containing various polyunsaturated fatty acids. Extremophiles 12, 753–761 [DOI] [PubMed] [Google Scholar]
- 16. Kawamoto J., Kurihara T., Kitagawa M., Kato I., Esaki N. (2007) Proteomic studies of an Antarctic cold-adapted bacterium, Shewanella livingstonensis Ac10, for global identification of cold-inducible proteins. Extremophiles 11, 819–826 [DOI] [PubMed] [Google Scholar]
- 17. Miyake R., Kawamoto J., Wei Y. L., Kitagawa M., Kato I., Kurihara T., Esaki N. (2007) Construction of a low temperature protein expression system using a cold-adapted bacterium, Shewanella sp. strain Ac10, as the host. Appl. Environ. Microbiol. 73, 4849–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reutelingsperger C. P., Dumont E., Thimister P. W., van Genderen H., Kenis H., van de Eijnde S., Heidendal G., Hofstra L. (2002) Visualization of cell death in vivo with the annexin A5 imaging protocol. J. Immunol. Methods 265, 123–132 [DOI] [PubMed] [Google Scholar]
- 19. Emoto K., Umeda M. (2000) An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J. Cell Biol. 149, 1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jao C. Y., Roth M., Welti R., Salic A. (2009) Metabolic labeling and direct imaging of choline phospholipids in vivo. Proc. Natl. Acad. Sci. U.S.A. 106, 15332–15337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuerschner L., Ejsing C. S., Ekroos K., Shevchenko A., Anderson K. I., Thiele C. (2005) Polyene lipids: a new tool to image lipids. Nat. Methods 2, 39–45 [DOI] [PubMed] [Google Scholar]
- 22. Neef A. B., Schultz C. (2009) Selective fluorescence labeling of lipids in living cells. Angew. Chem. Int. Ed. Engl. 48, 1498–1500 [DOI] [PubMed] [Google Scholar]
- 23. Yamaji A., Sekizawa Y., Emoto K., Sakuraba H., Inoue K., Kobayashi H., Umeda M. (1998) Lysenin, a novel sphingomyelin-specific binding protein. J. Biol. Chem. 273, 5300–5306 [DOI] [PubMed] [Google Scholar]
- 24. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 25. Shindou H., Hishikawa D., Harayama T., Yuki K., Shimizu T. (2009) Recent progress on acyl-CoA:lysophospholipid acyltransferase research. J. Lipid Res. 50, S46–S51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malinverni J. C., Silhavy T. J. (2009) An ABC transport system that maintains lipid asymmetry in the Gram-negative outer membrane. Proc. Natl. Acad. Sci. U.S.A. 106, 8009–8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dekker N. (2000) Outer membrane phospholipase A: known structure, unknown biological function. Mol. Microbiol. 35, 711–717 [DOI] [PubMed] [Google Scholar]
- 28. Harvat E. M., Zhang Y. M., Tran C. V., Zhang Z., Frank M. W., Rock C. O., Saier M. H., Jr. (2005) Lysophospholipid flipping across the Escherichia coli inner membrane catalyzed by a transporter (LplT) belonging to the major facilitator superfamily. J. Biol. Chem. 280, 12028–12034 [DOI] [PubMed] [Google Scholar]
- 29. Hsu L., Jackowski S., Rock C. O. (1991) Isolation and characterization of Escherichia coli K-12 mutants lacking both 2-acylglycerophosphoethanolamine acyltransferase and acyl-acyl carrier protein synthetase activity. J. Biol. Chem. 266, 13783–13788 [PubMed] [Google Scholar]
- 30. Rawicz W., Olbrich K. C., McIntosh T., Needham D., Evans E. (2000) Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79, 328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feller S. E. (2008) Acyl chain conformations in phospholipid bilayers: a comparative study of docosahexaenoic acid and saturated fatty acids. Chem. Phys. Lipids 153, 76–80 [DOI] [PubMed] [Google Scholar]
- 32. Mileykovskaya E., Dowhan W. (2009) Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim. Biophys. Acta 1788, 2084–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.