Background: Cytochrome c oxidase (COX), the final enzyme of the mitochondrial electron transport chain, requires several assembly factors for its proper function.
Results: ccdc56 knock-out flies showed developmental delay, lethality, and a dramatic decrease in the levels/activity of COX.
Conclusion: CCDC56 protein is necessary for COX function and for viability in flies.
Significance: Drosophila CCDC56 is a novel putative COX assembly factor conserved in humans.
Keywords: Cytochrome Oxidase, Drosophila, Drosophila Genetics, Gene Structure, Mitochondria, CCDC56, Complex IV, OXPHOS Function, Cytochrome c Oxidase
Abstract
In Drosophila melanogaster, the mitochondrial transcription factor B1 (d-mtTFB1) transcript contains in its 5′-untranslated region a conserved upstream open reading frame denoted as CG42630 in FlyBase. We demonstrate that CG42630 encodes a novel protein, the coiled coil domain-containing protein 56 (CCDC56), conserved in metazoans. We show that Drosophila CCDC56 protein localizes to mitochondria and contains 87 amino acids in flies and 106 in humans with the two proteins sharing 42% amino acid identity. We show by rapid amplification of cDNA ends and Northern blotting that Drosophila CCDC56 protein and mtTFB1 are encoded on a bona fide bicistronic transcript. We report the generation and characterization of two ccdc56 knock-out lines in Drosophila carrying the ccdc56D6 and ccdc56D11 alleles. Lack of the CCDC56 protein in flies induces a developmental delay and 100% lethality by arrest of larval development at the third instar. ccdc56 knock-out larvae show a significant decrease in the level of fully assembled cytochrome c oxidase (COX) and in its activity, suggesting a defect in complex assembly; the activity of the other oxidative phosphorylation complexes remained either unaffected or increased in the ccdc56 knock-out larvae. The lethal phenotype and the decrease in COX were partially rescued by reintroduction of a wild-type UAS-ccdc56 transgene. These results indicate an important role for CCDC56 in the oxidative phosphorylation system and in particular in COX function required for proper development in D. melanogaster. We propose CCDC56 as a candidate factor required for COX biogenesis/assembly.
Introduction
Cytochrome c oxidase (COX)5 or complex IV (EC 1.9.3.1) is the terminal enzyme of the electron transport chain, and it catalyzes electron transfer from reduced cytochrome c to molecular oxygen. Most cellular ATP is produced in mitochondria by the oxidative phosphorylation (OXPHOS) system comprising the electron transport chain complexes (plus two electron carriers, coenzyme Q, and cytochrome c) and the multimeric ATP synthase (complex V) (1). The energy released from the oxidation of carbohydrates and lipids is converted to reducing power (NADH + H+ and FADH2) in the mitochondrial matrix. The electron transport chain couples electron transfer from NADH and FADH2 to molecular oxygen with proton translocation from the matrix to the mitochondrial intermembrane space by complexes I, III, and IV. This proton translocation generates an electrochemical gradient that is used by complex V to generate ATP from ADP and inorganic phosphate.
Eukaryotic COX is a heteromeric enzyme of dual genetic origin (2, 3). The catalytic core of the enzyme is composed of three subunits encoded in the mitochondrial DNA (mtDNA): mt-CO1, mt-CO2, and mt-CO3. The structural subunits that surround the catalytic core are encoded by the nuclear genome (4). The nuclear genome-encoded subunits must be imported into the mitochondria, processed, and assembled together with the mtDNA-encoded subunits to form the holoenzyme. The nuclear genome-encoded subunits are necessary for the assembly/stability of the holoenzyme (5) and to regulate the catalytic activity of complex IV (6, 7).
More than 20 assembly factors required for correct COX function have been described in yeast; albeit the specific function of many of these factors remains elusive (for a review, see Ref. 8). Assembly factors are proteins involved in the biogenesis of the complex that are not present in the mature complex. They are involved in different biological processes, for example in the biogenesis and/or insertion of prosthetic groups (9–12), regulation of mt-CO1 translation (13), and stabilization of the mt-CO1 and mt-CO3 transcripts (14, 15).
Transcription of genes in bacteria and Archaea occurs in polycistronic messenger RNA, whereas in Eukaryota, the majority of genes are transcribed monocistronically (16). However, there are some exceptions in Eukaryota where genes are transcribed in polycistronic messages, and in general, these polycistronic genes tend to be involved in the same biological process as occurs in bacteria (17–20).
Mitochondrial gene expression is regulated by several nuclearly encoded proteins, including the mitochondrial transcription factor B1 (mtTFB1) (21). mtTFB1 is dual function protein that can activate mtDNA transcription in vitro (22) and act as an rRNA methyltransferase in vivo (23, 24). Previous work from our group in cultured Drosophila cells indicated a major role for mtTFB1 in mitochondrial translation (25). And more recently, Larsson and co-workers (26) have corroborated these data in mammals where they showed methylation of the 12 S rRNA mediated by mtTFB1 is required for assembly of the mitochondrial ribosome and therefore for mitochondrial translation. The mtTFB1 gene in Drosophila melanogaster was annotated as the protein-coding gene number CG7319 in the fly genome database (FlyBase). More recently, the FlyBase genome annotators have published changes affecting the annotation of the mtTFB1 gene that indicates the existence of an upstream open reading frame (uORF) in its 5′-untranslated region. The putative protein coding gene is annotated as CG42630 in the FlyBase database. Here we show that CG42630 is transcribed in a bicistronic RNA messenger with the mtTFB1 gene and is expressed in flies. BLAST analysis of the novel uORF indicated 42% amino acid identity with the human annotated coiled coil domain-containing protein 56 (CCDC56; NCBI accession number NP_001035521.1). Thus, we propose Drosophila CG42630 as the homolog of human CCDC56. Although the function of CCDC56 is unknown, it is highly conserved in higher eukaryotes. To study the function of the CCDC56 protein, we generated a D. melanogaster knock-out model by inducing genomic deletions by imprecise P element excision. Our results indicate that the CCDC56 homolog is a mitochondrial protein required for COX activity and assembly in D. melanogaster, suggesting a role as a COX assembly factor.
EXPERIMENTAL PROCEDURES
Drosophila Strains and Genetics
All fly crosses and stocks were grown at 25 °C on a standard Drosophila medium. ccdc56D6 and ccdc56D11 mutants were generated by inducing the transposition of the SUPor-P[kg07792] P element insertion using standard procedures (27). Deletion break points of alleles were determined by PCR followed by sequencing using specific primers (see Fig. 3, B and C). Sequences were assembled and analyzed using the Vector NTI software (Invitrogen). Transgenic lines for the pUASP-ccdc56, pUASP-mtTFB1, and pUASP-cDNAbi constructs were generated by the injection of Drosophila embryos (BestGene).
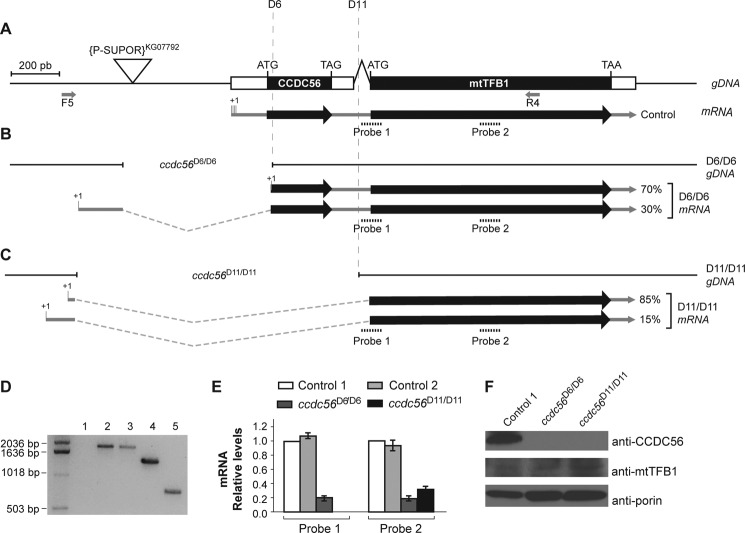
FIGURE 3.
Molecular characterization of the ccdc56D6 and ccdc56D11 alleles. A, genomic map of the ccdc56 and mtTFB1 genes showing the P element insertion (SUPor-P[kg07792]; triangle). Exons are indicated by boxes, coding regions are colored black for the CCDC56 and mtTFB1 proteins, and untranslated regions are represented in white. 5′-RACE from control (w1118) and mutant (ccdc56D6 and ccdc56D11) homozygous third instar larvae cDNAs identified the transcription start points, which are depicted as +1. The break points of the deletions generated in this work for the alleles ccdc56D6 and ccdc56D11 are shown in B and C, respectively. The ratios of the mRNA as determined by 5′-RACE in the clones analyzed are represented (ccdc56D6/D6, n = 8; ccdc56D11/D11, n = 10). PCR products amplified with F5 and R4 primers (shown in A) from genomic DNA of third instar larvae are shown in D. Lane 1, DNA from the stock containing the P element SUPor-P[kg07792] as a negative control; lane 2, DNA from w1118 control flies; lane 3, DNA from excised flies without any deletion used as an additional control; lane 4, DNA from excised flies of the ccdc56D6/D6 strain showing a 570-bp deletion; lane 5, DNA from excised flies of the ccdc56D11/D11 strain showing a 1168-bp deletion. E, transcript levels determined by qRT-PCR in third instar larvae of control and deleted homozygous lines after normalizing to w1118 flies using 18 S rRNA as an internal control. The two different TaqMan probes used are depicted in B. Control 1, w1118 flies; Control 2, excised flies without any deletion. Values are mean ± S.E. F, CCDC56 and mtTFB1 protein levels determined by immunoblotting of mitochondrial extracts (30 μg) of control and deleted homozygous larvae. Anti-voltage-dependent anion channel/porin antibody was used as a loading control.
Identification and Sequence Analysis of Bicistronic ccdc56-mtTFB1 cDNA and CCDC56
cDNAs from Drosophila control larvae (w1118 and Oregon R-C) and Schneider cells were prepared using the First Choice RLM-RACE cDNA amplification kit (Ambion). 5′-Rapid amplification of cDNAs ends (RACE) was performed using the following specific primers for Drosophila mtTFB1 cDNA (CG42631; formerly CG7319): R1, R2, and R3 (depicted in Fig. 1A). RACE products were cloned into the pCRII-TOPO vector (Invitrogen) and sequenced. Sequence analysis was performed using Vector NTI Advance 10 software (Invitrogen). Human CCDC56 (NCBI accession number NP_001035521.1) and CCDC56 homologs were identified by BLAST using the deduced amino acid sequence of the D. melanogaster CG2630 coding gene (28). Multiple sequence alignments of the predicted CCDC56 polypeptides were performed using the ClustalW 2.0.12 algorithm (29).
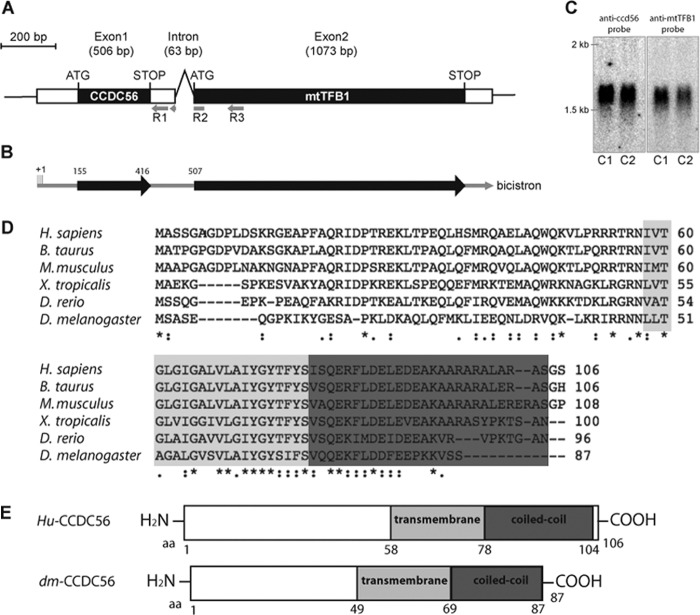
FIGURE 1.
The protein CCDC56, encoded in a bicistronic transcript together with mt-TFB1 in D. melanogaster, is conserved in metazoans. A, genomic map of CG42630/ccdc56 and mtTFB1. Exons are indicated by boxes, coding regions are colored black for the CCDC56 and mtTFB1 proteins, and untranslated regions are represented in white. B, bicistronic transcript determined by 5′-RACE from control flies (w1118 and OregonR-C) and cultured Schneider cell cDNAs. The transcription start point identified is depicted as +1. The primers R1, R2, and R3 used are represented in A. C, bicistronic ccdc56-mtTFB1 mRNA detected by Northern blot using 5 μg of RNA from w1118 (C1) and OregonR-C (C2) control larvae. The signal detected using a specific ccdc56 probe has the same migration as the signal detected when using a probe specific for mtTFB1. D, ClustalW alignment of Drosophila CCDC56 protein with CCDC56 sequences from other metazoan species. Accession numbers are as follows: fly (D. melanogaster), FlyBase annotation CG42630-PA; zebrafish (Danio rerio), UniProtKB accession number A8KB87; western clawed frog (Xenopus tropicalis), UniProtKB accession number A9UMl0-1; mouse (Mus musculus), NCBI accession number NP_080894.1; cow (Bos taurus), UniProtKB accession number Q3T0E3; human (Homo sapiens), NCBI accession number NP_001035521.1. Identical residues in all sequences (*), conserved substitutions (:), and semiconserved substitutions (.) are noted in the alignment. E, schematic diagram of the sequences of the human and D. melanogaster CCDC56 proteins showing the putative transmembrane and protein-protein interaction coiled coil domains. Hu, human; dm, D. melanogaster; aa, amino acids.
Northern Blotting
Five micrograms of total RNA from control flies were resolved on a 1.2% agarose gel and transferred to a Zeta-Probe GT membrane (Bio-Rad) following standard procedures. Invitrogen's 0.5–10-kb RNA ladder was used as a molecular size marker. A PCR fragment of 280 bp containing the complete ccdc56 ORF (261 bp) was used as a ccdc56-specific probe. This probe was amplified by PCR from the pUASP-ccdc56 construct using primers 9558F and 9559R (see below). The specific probe for the mtTFB1 coding sequence (322 bp) was obtained by PCR amplification using the primers F9 (5′-AGCACATCCCGGACACCTCA-3′) and R4 (5′-TTTAGGGGAATTAGCTTGACG-3′). Probes were radiolabeled with [P32]dCTP using the Amersham Biosciences Rediprime II Random Prime Labeling System (GE Healthcare) following the manufacturer's instructions.
Phenotyping Analysis
We carried out 2-h egg laying from the control w1118 stock and the stable mutant stocks ccdc56D6/TM6b-Tb and ccdc56D11/TM6B-Tb. To determine whether mutant larvae were developmentally arrested, developmentally delayed, or merely slow growing, their mouth hooks were examined daily under the microscope from the 5th day after egg laying (AEL). Fly vials were also photographed daily.
Bacterial Expression of Drosophila CCDC56 (d-CCDC56) and Generation of Anti-d-CCDC56 Antibody
To express d-CCDC56 in Escherichia coli, a PCR fragment encoding the d-CCDC56 open reading frame was cloned into the pRSET-B vector (Invitrogen) cut with NcoI and HindIII. The following primers were used: Fw, 5′-TTCCATGGCGGCGTCGGAGCAGGGACC-3′; and Rv, 5′-AGAAGCTTCTAGGAAGACACCTTCTTGGGCTC-3′ (bold nucleotides mean restriction enzyme sites). Polyclonal antibody was generated using standard procedures.
Constructs for the Generation of Transgenic Flies
The bicistronic ccdc56-mtTFB1 cDNA (1574 bp) was obtained from the cDNA clone LD40326 (GenBankTM accession number AY069635) and cloned into the BglII/XbaI restriction sites of the pUAST transformation vector to generate the pUAST-cDNAbi construct. To generate the pUASP-ccdc56 vector for transformation, a fragment containing exclusively the complete ccdc56 ORF (261 bp) was amplified by PCR using the primers 9558F (5′-TTTAGCAGCGTTTATAATGTCG-3′) and 9559R (5′-TAGGGATAACTAACGCGGACA-3′), subcloned into the pCAP vector (Roche Applied Science), and cloned into the NotI/XbaI restriction sites in the pUASP vector. To generate the pUASP-mtTFB1 construct, mtTFB1 ORF (990 bp) was obtained by digestion with KpnI/NotI of the pBluescript II KS+ vector (Stratagene) containing the mtTFB1 cDNA and cloned into the pUASP vector for transformation.
Drosophila CCDC56-FLAG Construct
D. melanogaster CCDC56 ORF was amplified by PCR from the pUASP-ccdc56 construct indicated above using the primers F (5′-TTGGTACCATGTCGGCGTCGGAGCAGGGACC-3′) and R (5′-TTGCGGCCGCCTACTTGTCGTCATCGTCTTTGTAGTCGGAAGACACCTTCTTGGGCTCC-3′) containing the FLAG epitope at the C terminus and the KpnI/NotI sites needed for cloning into the mammalian expression vector pcDNA3 (Invitrogen). Fidelity of the clones was confirmed by sequencing.
Transfection and Generation of CCDC56-FLAG-overexpressing Cell Lines
Human HeLa cells were grown in DMEM (Invitrogen) supplemented with 5% fetal bovine serum (Invitrogen). 1.5 × 105 HeLa cells were plated on coverslips and transfected with 2 μg of the pcDNA3-d-ccdc56-FLAG construct. Lipofectamine (Invitrogen) was used as a transfection reagent following the manufacturer's instructions.
Immunohistochemistry
To label the mitochondrial compartment, cells were incubated for 30 min with a 250 nm concentration of the mitochondrial dye MitoTracker Red (Invitrogen) 24 h after transfection, washed, and fixed for 15 min in 2% paraformaldehyde. Primary anti-FLAG antibody (1:1000; Stratagene) and secondary Alexa Fluor 488 anti-mouse antibody (1:200; Molecular Probes) were used. Images were collected using a confocal microscope (Leica).
Imaginal discs from third instar larvae of each genotype were dissected in PBS and fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. They were blocked in PBS, 1% bovine serum albumin, 0.3% Triton X-100 for 1 h; incubated with the primary antibody overnight at 4 °C (dilution, 1:50); washed; and incubated with the appropriate secondary antibody for 2 h at room temperature in the dark (dilution, 1:200). Finally, they were washed and mounted in Vectashield (Vector Laboratories). Primary antibodies used were rabbit anti-phosphohistone 3 (Sigma-Aldrich) and rabbit anti-caspase 3 (Cell Signaling Technology). Secondary antibodies were coupled to the fluorochrome Alexa Fluor 647 or Alexa Fluor 555 (Invitrogen). Preparations were visualized under a Leica TCS SP2 laser-scanning microscope.
Mitochondrial Enzyme Assays and Mitochondrial RNA (mtRNA) and mtDNA Quantification
For enzymatic activity measurements, mitochondrion-enriched homogenates were prepared from ∼30 third instar larvae ground in SETH buffer (250 mm sucrose, 2 mm EDTA, 100 units/liter heparin, 10 mm Tris-HCl, pH 7.4), fractionated by differential centrifugation, and sonicated (6 s at 4 °C). The activities of the respiratory chain complexes I, II, III, and IV and the mitochondrial mass marker citrate synthase were measured by spectrophotometric methods as described previously (30) and expressed in nanomoles of substrate catalyzed/minute/milligram of protein. For mtRNA quantification, RNA was extracted using TRIzol reagent (Invitrogen), and 1 μg from each genotype was converted into cDNA and amplified in a 7900 Fast Real Time PCR System (Applied Biosystems) using the TaqMan probes. For relative quantification of mtDNA, genomic DNA was isolated from third instar larvae and quantified using standard methods. Ten nanograms of each DNA were used as template. TaqMan probes for mt-ND5 and mt-CO1 were used.
Immunoblotting and Subcellular Fractionation
Thirty micrograms of each mitochondrial protein extract, obtained by differential centrifugation, were separated on 10 or 15% SDS-polyacrylamide gels and transferred to Immobilon P PVDF membranes (Millipore). Filters were preincubated for 1 h in 5% skim milk in TBS, 0.1% Tween 20 followed by an overnight incubation with the corresponding primary antibody. Monoclonal antibody against porin (1:2000; voltage-dependent anion channel) was obtained from Molecular Probes, polyclonal antibody against d-CCDC56 was generated as described above (1:50), and polyclonal antibody anti-mtTFB1 was previously generated by Dr. L. S. Kaguni (1:1000) (25). For subcellular fractionation, D. melanogaster embryos were homogenized in 250 mm sucrose, 10 mm TES, 1 mm EDTA, pH 7.4 with five strokes at 1000 rpm using a motor-driven Teflon pestle. Homogenates were then centrifuged at 900 × g for 10 min at 4 °C, and the supernatant was centrifuged again at 9000 × g for 10 min at 4 °C to obtain the mitochondrial fraction (pellet) and postmitochondrial supernatant. Fifty micrograms of each fraction were loaded onto 12% SDS-polyacrylamide gels, transferred to PVDF membranes, and probed with anti-porin (1:1000), anti-GAPDH (1:1000; Stressgene), and anti-d-CCDC56 (1:50) antibodies.
Blue Native Gel Analyses
Mitochondrial pellets isolated from larvae were resuspended in 1.5 m aminocaproic acid, 75 mm Bis-Tris, pH 7.0. Respiratory chain complexes were extracted with 2% lauryl maltoside for 20 min on ice and then centrifuged for 30 min at 4 °C. Blue native polyacrylamide gel electrophoresis (PAGE) and two-dimensional electrophoresis (two-dimensional SDS-PAGE) were performed as described (31), loading 40 μg of mitochondrial protein per lane. A 4–18% native acrylamide gradient gel was used for the first dimension, and 10% acrylamide gels were used for electrophoresis in the second dimension. Proteins were transferred overnight to a nitrocellulose membrane. Anti-mt-CO3 (Invitrogen) was used at a 1:200 dilution; polyclonal antibody against β-ATPase, generated in our laboratory (32), was used at 1:1000; and secondary goat-anti mouse antibody IgG-HRP (provided by Zymed Laboratories Inc.) was used at 1:2000.
Statistical Analysis
Statistical data analysis was performed with Prism 4.03 software (GraphPad Software). One-way analysis of variance (plus Bonferroni post-test) and Student's t test were used to determine statistical significance of the results, which was assumed at p < 0.05.
RESULTS
The D. melanogaster Gene CG42630 Is Expressed in a Bicistronic mRNA with mtTFB1 and Encodes a Novel Conserved Protein, CCDC56
A search in the Drosophila genome database (FlyBase) indicated the existence of a uORF in the 5′-UTR of the mtTFB1 transcript located in the first exon of the mRNA (Fig. 1A). This putative polypeptide is denoted as CG42630 in FlyBase, and the biological processes in which it is potentially involved are unknown. To confirm the existence of a bona fide bicistronic transcript of CG42630-mtTFB1, we isolated mRNA from Drosophila strains and from Drosophila SL2 Schneider cells and carried out RACE (Fig. 1B) using the strategy described under “Experimental Procedures.” We sequenced the cDNA clones obtained by 5′-RACE from different Drosophila strains (w1118, n = 8; Oregon R-C, n = 6) and from cultured Schneider cells (n = 6), and in all cases, sequence analyses detected a single transcript with a heterogeneous transcription start point located upstream from the CG42630 uORF (Fig. 1B). We excluded the presence of transcripts coding only for CG42630 by 3′-RACE (data not shown). These results suggest strongly that d-mtTFB1 and the putative gene, CG42630, are transcribed in the same mRNA and therefore are encoded in a bicistron. We tested the existence of this CG42630-mtTFB1 bicistronic mRNA by Northern blot in total RNA extracted from D. melanogaster flies (Fig. 1C) using two different probes: a 280-nucleotide probe specific for the CG42630 coding sequence and a 320-nucleotide probe specific for the mtTFB1 coding sequence (see “Experimental Procedures”). We detected with both probes a single signal of about the expected size of 1574 bp (Fig. 1A), confirming the existence of the CG42630-mtTFB1 bicistronic structure in D. melanogaster.
The putative CG42630 gene encodes a predicted polypeptide of 87 amino acids (FlyBase). The deduced amino acid sequence from Drosophila CG42630 was used to perform a BLAST analysis of the human genome (28), and a single protein of unknown function was identified: CCDC56. We found a 42% amino acid identity between D. melanogaster and its human homolog. CCDC56 has two putative domains: a single pass transmembrane domain (from amino acids 58 to 78 in humans and from amino acids 49 to 69 in D. melanogaster) and a protein-protein interaction coiled coil domain (from amino acids 79 to 104 in humans and from amino acids 70 to 87 in D. melanogaster) (Fig. 1E). We refer to the Drosophila CG42630 gene as ccdc56.
In contrast to the bicistronic structure detected in flies, human CCDC56 is encoded in a single transcript, and the gene is located on chromosome 17q.21.31 (Entrez Gene ID 28958). Human CCDC56 protein contains 106 amino acids (Swiss-Prot accession number Q9Y2R0). Orthologous CCDC56 proteins are present in metazoans, showing a high degree of conservation (Fig. 1D), but they are absent in yeast and plants. No mitochondrial signal peptide was detected in the amino acid sequences of the Drosophila and human CCDC56 proteins using the bioinformatics programs TargetP and iPSORT.
CCDC56 Is a Mitochondrial Protein
In prokaryotes, operons encode factors that are usually involved in the same metabolic pathway or biological process (33). Because mtTFB1 is an essential protein involved in mitochondrial translation, we first examined the possibility that CCDC56 is also located in mitochondria (25, 26). We cloned the Drosophila CCDC56 ORF tagged with the FLAG epitope (FLAG) at the C-terminal end (d-CCDC56-FLAG) and transiently transfected cultured HeLa cells (Fig. 2). Using anti-FLAG antibodies, we observed a clear colocalization with the mitochondrion-specific dye MitoTracker Red, indicating that the tagged CCDC56 version has a mitochondrial localization (Fig. 2C).
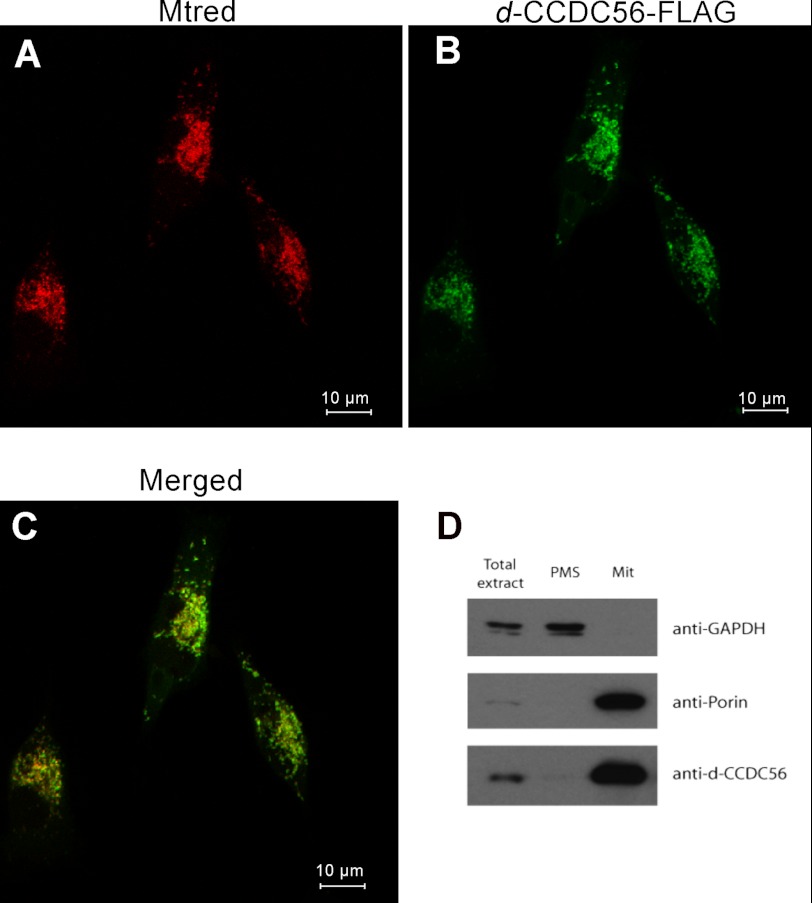
FIGURE 2.
CCDC56 protein localizes to mitochondria. Immunocytochemistry of HeLa cells transfected with recombinant d-CCDC56-FLAG is shown. A, MitoTracker (Mtred) staining is shown in red. B, the same cells immunostained for the FLAG epitope (green). The recombinant Drosophila protein colocalizes with the MitoTracker dye in the mitochondrial compartment (C). D, immunoblots of protein extracts (50 μg) from subcellular fractions of control embryos probed with anti-d-CCDC56, anti-porin, and anti-GAPDH antibodies. Total extracts, mitochondrial fraction (Mit), and postmitochondrial supernatant (PMS) are shown.
To detect the endogenous protein in the fly, we generated a polyclonal antibody against the Drosophila CCDC56 polypeptide. Immunoblot analysis of subcellular fractions of wild-type embryos demonstrated that endogenous d-CCDC56 is expressed and localized to the mitochondrial fraction (Fig. 2D). The protein shows an electrophoretic mobility that corresponds to a molecular mass of ∼10 kDa in accordance with the predicted size of the Drosophila CCD56 protein. Monoclonal anti-porin antibody was used as a mitochondrial marker.
Generation of ccdc56 Knock-out Alleles
To study the in vivo function of CCDC56, we generated transgenic flies harboring ccdc56 loss-of-function alleles. To generate deletions that specifically affect the ccdc56 gene, we mobilized the P{SUPor-P}mtTFB1[KG07792] transposon located in the proximal 5′-region of the gene using standard procedures (27). From ∼100 independent lines in which the P element had been removed, two lines carrying deletions that map specifically in the ccdc56 coding sequence without affecting the mtTFB1 coding sequence, ccdc56D6 and ccdc56D11, were selected (Fig. 3, A–C). A Drosophila line harboring an allele in which the P element was excised precisely without removing additional DNA sequence was used as a control (designated Control 2; Fig. 3, D and E). The control flies were obtained by the same process as those harboring the deletions. Homozygous ccdc56D6/D6 and ccdc56D11/D11 flies are not viable and die in the third larval instar stage, suggesting that ccdc56 is essential for development. ccdc56D6/D11 trans-heterozygotes showed the same lethal phenotype as homozygous ccdc56D6/D6 and ccdc56D11/D11 flies, indicating that these alleles did not complement each other, which is expected if the molecular lesion affects the same gene. To map precisely the deletion break points, we PCR-amplified and sequenced the genomic region flanking the P element (Fig. 3, A–C). The ccdc56D6 allele harbors a 570-bp-long deletion that includes the ATG initiation codon and the first 23 nucleotides of the ORF of CCDC56 (Fig. 3B). The ccdc56D11 allele shows a larger deletion of 1168 bp comprising the complete ccdc56 coding sequence and the first 26 nucleotides of the intron in the ccdc56-mtTFB1 mRNA (Fig. 3C).
Phenotype of ccdc56D6/D6 and ccdc56D11/D11 Drosophila Fly Lines
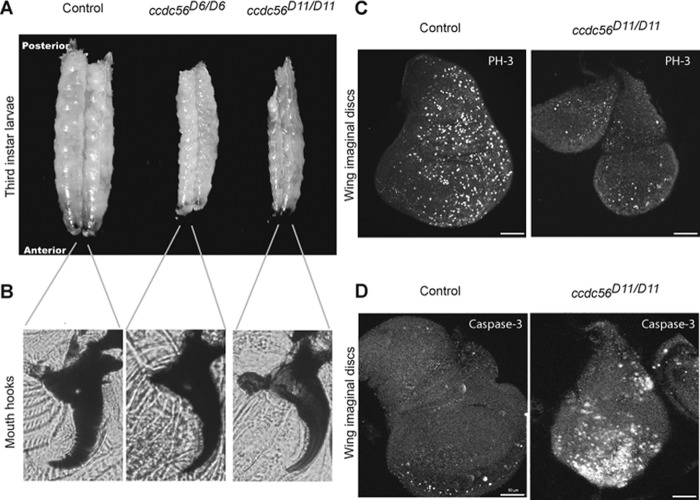
Heterozygous ccdc56D6/TM6B-Tb and ccdc56D11/TM6B-Tb flies are viable and fertile and can be distinguished easily by the genetic marker Tubby (Tb; generating small pupa size) that allows classifying the progeny. When raised at 25 °C, heterozygous mutant larvae, like control larvae, reached the third larval stage at 4–5 days AEL. However, ccdc56D6/D6 and ccdc56D11/D11 homozygotes took between 10 and 12 days to reach the third larval stage, monitored by analyzing the mouth hook morphology (Fig. 4B), indicating a developmental delay. Homozygous ccdc56D6/D6 and ccdc56D11/D11 third instar larvae were much smaller than controls, remained in this stage for over 20 days, and then died before pupariation (Fig. 4A). We used the ccdc56D11/D11 mutant to perform immunocytochemical analysis in wing imaginal discs, the proliferating larval epithelial cells that form the adult wings of the fly. Mutant imaginal discs were smaller than controls. Indeed, mutant wing discs showed a decreased number of mitotic cells as seen by a reduction in the number of cells that express phosphorylated histone 3, a mitotic cell marker (Fig. 4C). ccdc56D11/D11 mutants also showed increased levels of apoptosis in the wing discs as detected with an antibody that recognizes activated caspase 3, whereas cell death in control wing discs was minimal during most of the larval development (Fig. 4D). These results indicate that two of the processes that contribute to tissue growth, cell division and cell survival, are compromised in the ccdc56D11/D11 mutant.
FIGURE 4.
Lack of CCDC56 causes arrest at the third larval stage. A, size comparison of control (w1118) and homozygous mutant third instar larvae. Homozygous larvae for both alleles were smaller than control larvae in all cases tested. B, mouth hook morphology of control third instar larvae and mutant larvae 15 days AEL indicating third instar. C, wing imaginal discs from control (w1118) and homozygous ccdc56D11 third instar larvae were dissected and immunostained with anti-phosphohistone 3 (PH-3) antibody. Mutant wing discs showed lower cell proliferation levels as compared with controls. D, anti-caspase 3 activated antibody was used to detect apoptotic signals in wing imaginal discs. Increased levels of apoptosis were observed in homozygous ccdc56D11 flies as compared with control flies. Bar size, 50 μm.
The Phenotype of ccdc56D6/D6 and ccdc56D11/D11 Drosophila Lines Is Produced by the Absence of CCDC56
Because both deletions remove a large 5′-region upstream of the ccdc56/mtTFB1 genes that potentially contain critical promoter elements involved in regulating their transcription, we used RT-PCR to identify truncated transcripts encoding the mtTFB1 gene in the mutant lines. Interestingly, in both the homozygous D6 and D11 lines, the levels of truncated ccdc56/mtTFB1 transcript ranged between 20 and 30% as compared with the wild-type controls (Fig. 3E). Detailed analysis using 5′-RACE revealed the presence of a family of transcripts originating from the 5′-upstream region close to the break points (Fig. 3, B and C), indicating clearly that these regions still contain promoter activity. All the detected transcripts expressed in the mutant lines contained the complete d-mtTFB1 coding sequence (Fig. 3, B and C), but as expected, they lacked the complete ccdc56 coding sequence. Accordingly, no CCDC56 was detected by immunoblot analysis of mitochondrial extracts from ccdc56D6/D6 and ccdc56D11/D11 larvae, indicating that both are ccdc56-null alleles (Fig. 3F); in contrast, mtTFB1 is present in mitochondrial extracts prepared from the mutant larvae (Fig. 3F).
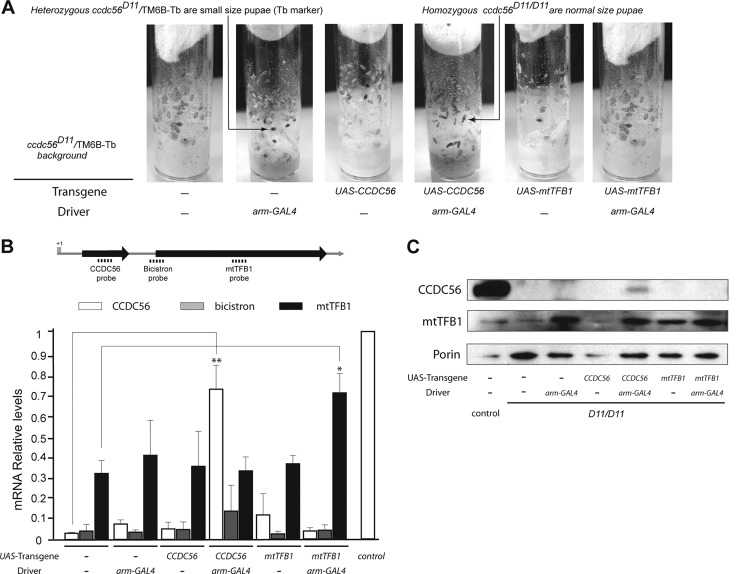
To demonstrate further that the lethal phenotype observed in the mutants isolated in our screen was due exclusively to the loss of CCDC56 function, we overexpressed independently ccdc56 or mtTFB1 in both D6 and D11 homozygous genetic backgrounds. We cloned the ccdc56 and mtTFB1 coding sequences in the pUASP vector and generated several UAS-ccdc56 and UAS-mtTFB1 transgenic lines (see “Experimental Procedures”). We selected two independent lines for each transgene that were viable upon homozygosis, and we generated stable stocks carrying these UAS constructs in homozygosis on chromosome II and the ccdc56D6 or ccdc56D11 allele balanced over the TM6B-Tb chromosome on chromosome III. To direct the expression of the transgenic UAS constructs, we used the ubiquitous arm-GAL4 driver in which expression of the transcription activator factor GAL4 is driven by the armadillo promoter (β-catenin homolog in mammals). We found that homozygous mutant larvae carrying one copy each of the UAS-ccdc56 and the arm-GAL4 transgenes developed beyond the third larval stage, reaching the pupal stage (Fig. 5A). Two independent transgenic lines expressing CCDC56 produced 62.8 ± 1.8 and 55.9 ± 4.8% of the homozygous mutant pupae progeny expected for a neutral allele (Table 1). However, the UAS-mtTFB1 construct directed by the same driver was not able to rescue lethality at the third larval stage for mutants carrying the D6 or D11 deletion allele (to simplify, only data are shown regarding the ccdc56D11 allele; Table 1 and Fig. 5A). The presence in a mutant background of a copy only of the arm-GAL4 driver or a copy only of the UAS-ccdc56 construct did not rescue mutant lethality. Accordingly, all pupae scored in the vials were heterozygous for the mutant allele (Table 1 and Fig. 5A).
FIGURE 5.
CCDC56 expression partially rescues the mutant lethality phenotype. A, flies homozygous for the ccdc56D11 allele reach only the pupal stage when they carry the UAS-ccdc56 and the arm-GAL4 transgenes on chromosome II. Larvae and pupae homozygous for the allele ccdc56D11 are of normal size. Larvae and pupae heterozygous for the deletion (genotype ccdc56D11/TM6B-Tb) carrying one copy of the Tb marker are smaller. B, qRT-PCR of CCDC56, bicistron, and mtTFB1 mRNA relative to 18 S rRNA from homozygous ccdc56D11 third instar larvae combined with the different UAS transgenes and with or without the ubiquitous arm-GAL4 driver. The three TaqMan probes used are depicted in the scheme. Data represent the mean ± S.E. of at least three independent determinations (*, p < 0.05; **, p < 0.01; Student's t test). C, immunoblot of mitochondrial extracts (30 μg) from homozygous ccdc56D11 third instar larvae of the genotypes indicated incubated with polyclonal anti-mtTFB1 and anti-CCDC56 antibodies and with monoclonal anti-voltage-dependent anion channel/porin antibody.
TABLE 1.
Rescue analysis of ccdc56D11/ccdc56D11 flies
| Genotype, chromosome II | No. of ccdc56D11/TM6B-Tb pupae scored | No. of ccdc56D11/ccdc56D11 pupae scored | Total no. of pupae scored | Mean (%) ccdc56D11/ccdc56D11 pupae scored/expecteda ±S.E. |
|---|---|---|---|---|
| +/+ | 1306 | 0 | 1306 | 0 |
| +/arm-GAL4 | 1527 | 0 | 1527 | 0 |
| UAS-ccdc56-1/+ | 1622 | 0 | 1622 | 0 |
| UAS-ccdc56-1/arm-GAL4 | 1823 | 487 | 2310 | 62.8 ± 1.8 |
| UAS-ccdc56-2/+ | 1786 | 0 | 1786 | 0 |
| UAS-ccdc56–2/arm-GAL4 | 1502 | 356 | 1858 | 55.9 ± 4.8 |
| UAS-mtTFB1-1/+ | 2039 | 0 | 2039 | 0 |
| UAS-mtTFB1-1/arm-GAL4 | 1984 | 0 | 1984 | 0 |
| UAS-mtTFB1-2/+ | 1629 | 0 | 1629 | 0 |
| UAS-mtTFB1-2/arm-GAL4 | 1626 | 0 | 1626 | 0 |
a Homozygous ccdc56D11/ccdc56D11 pupae scored/expected × 100 of at least three replicate experiments. Homozygous ccdc56D11/ccdc56D11 pupae expected are ⅓ of the total progeny of the cross. The Tb marker enables the progeny classes to be distinguished.
Quantification of transcript levels by qRT-PCR in homozygous third instar larvae of the different genotypes showed that the arm-GAL4 driver restores ccdc56 mRNA levels to 73.9 ± 0.1% of controls in a D11 mutant background (Fig. 5B, white bars). Despite this increase in mRNA levels, CCDC56 protein measured by immunoblot analysis was increased only to 15% of control levels (Fig. 5C). This result indicates that the induction of low levels of CCDC56 is sufficient to rescue the developmental arrest of the ccdc56D11/D11 mutant, permitting pupation of the larvae and most of the metamorphosis program of the flies (Fig. 5A). The fact that the rescue of lethality was not complete may be explained by the slight increase of the CCDC56 protein obtained under these conditions. Accordingly, by restoring CCDC56 to control levels (by inducing the expression of the bicistron), we obtained a stronger rescue of lethality: 96.77 ± 1.6% of the ccdc56D11/D11 mutant progeny reached the pupal stage, and 34.5 ± 4.5% reached the adult stage. As expected, the transcript levels assessed with the probe that recognizes the bicistronic mRNA were nearly absent in the D11 homozygous larvae for all transgene combinations as the D11 deletion (shown in Fig. 3C) includes part of the recognition sequence for this probe (Fig. 5B, gray bars, and Fig. 3E, Probe 1).
ccdc56 Knock-out Flies Show a Severe Isolated Cytochrome c Oxidase Deficiency
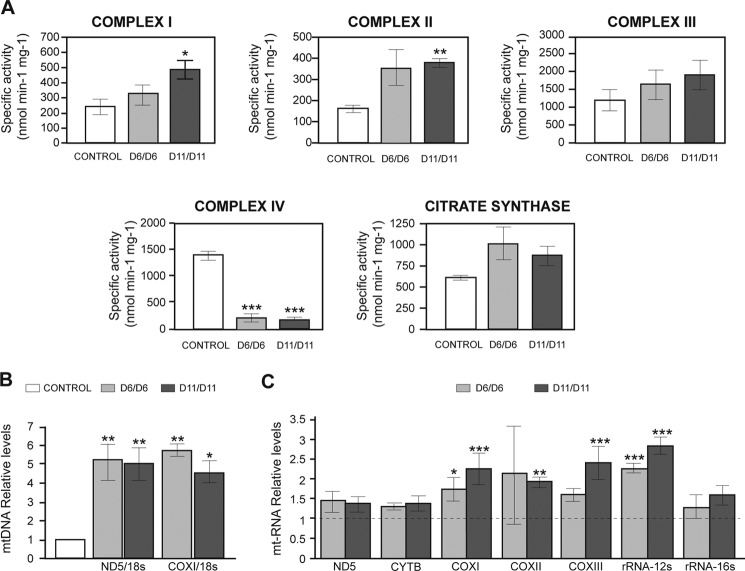
To study the effect of the absence of CCDC56 on mitochondrial function, we measured the OXPHOS enzyme activities in control and homozygous mutant third instar larvae. Both ccdc56D11/D11 and ccdc56D6/D6 mutant larvae showed a dramatic reduction in COX (complex IV) activity (Fig. 6A). The decrease in COX activity was almost complete when activities were normalized with respect to citrate synthase activity, an indicator of total mitochondrial mass (data not shown). This decrease in COX activity in ccdc56D11/D11 mutant larvae was confirmed by blue native PAGE (Fig. 7A). Interestingly, the enzyme activities of the remaining OXPHOS complexes were either unaffected or increased significantly (complexes I and II in ccdc56D11/D11 mutants; Fig. 6A), a result that may reflect a compensatory response to the dramatic reduction of COX activity in the mutants. Most interestingly, a huge increase (4–5-fold) in mtDNA levels was also observed in mutant mitochondria as compared with controls (Fig. 6B). In addition, a moderate increase was observed in the steady-state levels of mtRNA transcripts (Fig. 6C), including the small ribosomal RNA (rRNA-12 S) and the cytochrome c oxidase transcripts mt-CO1, mt-CO2, and mt-CO3 that are increased 2–2.5-fold (Fig. 6C). The increased mtRNA transcript levels in both mutants were confirmed by Northern blot analyses (data not shown).
FIGURE 6.
ccd56 knock-out flies exhibit an isolated complex IV enzyme deficiency. A, respiratory chain enzyme activities (complexes I, II, III, and IV) and citrate synthase activity were measured in mitochondrial extracts obtained from control and ccdc56 knock-out third instar larvae 15 days AEL. Both mutant alleles showed a severe decrease in complex IV enzyme activity. B, quantification of relative mtDNA levels by qRT-PCR using ND5 and COXI as mitochondrial gene probes and 18 S rRNA as a nuclear gene probe from control, ccdc56D6/D6, and ccdc56D11/D11 third instar larvae 15 days AEL. C, steady-state expression levels of representative genes from polycistronic transcripts from mitochondrial RNA were measured by qRT-PCR from ccdc56D6/D6 and ccdc56D11/D11 third instar larvae 15 days AEL and are shown relative to the levels found in control larvae after normalization to 18 S rRNA levels. CONTROL, genotype w1118; D6/D6, ccdc56D6/D6; D11/D11, ccdc56D11/D11. Data shown in A, B, and C represent the mean ± S.E. of at least three independent determinations (*, p < 0.05; **, p < 0.01; ***, p < 0.001; analysis of variance). CYTB, cytochrome b.
FIGURE 7.
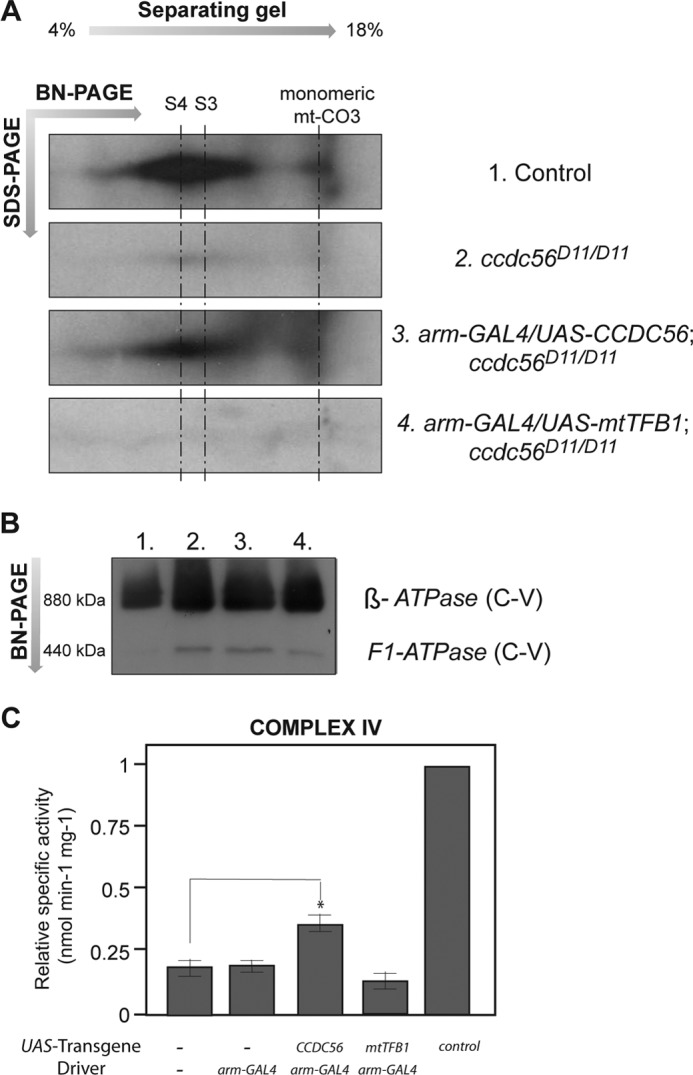
Loss of ccdc56 induces a complex IV assembly defect. A, two-dimensional blue native (BN)/SDS-PAGE analysis of mitochondrial extracts immunoblotted with an antibody against the mitochondrially encoded subunit mt-CO3 of the cytochrome c oxidase complex. Mitochondrion-enriched extracts were prepared from third instar larvae of the following genotypes: 1, control w1118; 2, mutant ccdc56D11/D11; 3, mutant ubiquitously expressing the UAS-ccdc56 transgene (arm-GAL4/UAS-ccdc56;ccdc56D11/D11); 4, mutant larvae expressing the UAS-mtTFB1 transgene under the same condition (arm-GAL4/UAS-mtTFB1;ccdc56D11/D11). The lack of fully assembled holo-COX exhibited by the ccdc56D11/D11 mutant is rescued partially by overexpression of the UAS-ccdc56 transgene. S4 is the fully assembled complex IV. The previously identified mt-CO3-containing COX subcomplex S3 is indicated. B, first dimension of duplicate blue native PAGE of mitochondrial extracts incubated with a polyclonal antibody against complex V (C-V) as a loading control for the two-dimensional blue native/SDS-PAGE shown in A. C, complex IV enzyme activity measured in mitochondrial extracts from homozygous ccdc56D11/D11 third instar larvae carrying the indicated constructs on chromosome II. Data were normalized to control larvae (w1118) and represent the mean S.E. (n = 3; *, p < 0.05; Student's t test).
The ubiquitous expression of the UAS-ccdc56 construct in a ccdc56D11/D11 genetic background induced a significant increase in COX enzyme activity (37.4 ± 0.043 versus 19.5 ± 0.040%; Fig. 7C). As expected, expression of the UAS-mtTFB1 construct under the same conditions had no effect, showing levels of COX activity similar to those of the mutants (14.3 ± 0.043%; Fig. 7C). These results suggest strongly that CCDC56 is required for cytochrome c oxidase function in D. melanogaster.
ccdc56 Knock-out Flies Show a Severe Reduction of Fully Assembled Complex IV
Because the only OXPHOS complex affected in ccdc56 knock-out flies is complex IV, we next explored by blue native PAGE analysis whether the assembly of this complex was affected in the mutants. Several antibodies against mammalian mitochondrial and nuclearly encoded COX subunits were tested against Drosophila protein extracts, but unfortunately, most showed little or no cross-reactivity (data not shown). To determine the levels of fully assembled complex IV in our mutant flies, we performed two-dimensional blue native PAGE followed by immunoblot analysis with an anti-mt-CO3 antibody that was able to recognize Drosophila mt-CO3. In ccdc56D11/D11 and ccdc56D6/D6 mitochondria, we observed a dramatic decrease in the levels of fully assembled holo-COX (indicated by complex S4; Fig. 7A). However, we did not detect the accumulation of any subcomplex or putative assembly intermediate in our mutants at least under the conditions tested (Fig. 7A). To demonstrate that the absence of CCDC56 function was responsible for the mutant complex IV assembly defect, we attempted to rescue this phenotype by inducing the ubiquitous expression of a UAS-ccdc56 transgene in a mutant background. Consistent with our hypothesis, we observed a recovery of fully assembled complex IV levels in mutants expressing UAS-ccdc56 (Fig. 7A, third panel) but not when we overexpressed mitochondrial translation factor B1 (Fig. 7A, fourth panel). This result indicates that CCDC56 is required for the proper assembly and/or stability of mitochondrial complex IV in D. melanogaster.
DISCUSSION
We have identified in D. melanogaster a novel mitochondrial protein, CCDC56, which is evolutionarily well conserved in metazoans. CCDC56 belongs to the coiled coil domain-containing family of proteins, and although its function is unknown, we found that loss of CCDC56 results in a severe isolated enzyme deficiency and assembly defect of mitochondrial cytochrome c oxidase. This indicates that CCDC56 plays a critical role in the biogenesis and activity of complex IV and is therefore essential for the function of the OXPHOS system.
In D. melanogaster, CCDC56 is annotated in a single transcription unit together with mitochondrial transcription factor B1 (FlyBase). BLAST analyses showed that this organization is also present in the other 11 Drosophila species for which sequence data are available (data not shown). Northern blot and RACE experiments demonstrated that ccdc56 and mtTFB1 are encoded on the same functional bicistronic transcript in D. melanogaster. Although the presence of operon-like structures is not frequent in eukaryotes, a comparative evolutionary study of 12 Drosophila genomes predicted the presence of 123 novel polycistronic transcripts (34). More recently, another comparative genomics approach using D. melanogaster and Anopheles gambiae transcript annotations and dipteran expressed sequence tags was performed to identify transcripts with uORFs. Interestingly, in dipterans, conserved uORFs occur preferentially in transcripts encoding mitochondrial proteins and methyltransferases (35). The uORF usually encodes proteins smaller than those encoded in the main ORF. Some examples in Drosophila are the translocase of the inner membrane 10, Tim 10 (FlyBase annotation CG9878), and the translocase of the inner membrane 9b, Tim 9b (CG17767). It is possible that at least some of these uORFs are vestiges of ancient prokaryotic operons that originated in the mitochondrion and were transferred to the nuclear genome over time. Another possible explanation would be that these structural organizations favor a coordinated regulation of genes involved in similar biochemical pathways (16).
We further investigated whether this novel peptide is targeted to the mitochondrial compartment, like mtTFB1, by transfecting HeLa cells with a recombinant d-CCDC56 FLAG-tagged protein. We observed a clear mitochondrial localization of CCDC56, and in addition, we detected by immunoblotting the endogenous CCDC56 only in the mitochondrial fraction. Therefore and in agreement with the results obtained in the comparative genomics study, we describe here a new case of a bicistronic transcript in which both proteins have a mitochondrial function. To our knowledge, this is the first demonstration of a eukaryotic bicistron in which both proteins have a mitochondrial function that is demonstrated to be functional in vivo.
We explored the consequences of the lack of function of mitochondrial protein CCDC56 by generating two independent Drosophila knock-out lines by inducing the excision of a P element located in the promoter region of the ccdc56-mtTFB1 genes. Its imprecise excision removed part of the flanking DNA and therefore generated specific deletions. Transcripts detected by RACE in the ccdc56D6 knock-out line lack the first 23 nucleotides and therefore the translation start codon of ccdc56, whereas no ccdc56-containing transcripts were detected in the ccdc56D11 knock-out line. As expected, CCDC56 was not detected in mitochondrial extracts from any of the mutant lines, indicating a total absence of CCDC56 protein in homozygous ccdc56D6 and ccdc56D11 animals. Furthermore, we have demonstrated that both mutant lines retained mtTFB1-encoding transcripts and mtTFB1 protein although in lower levels as compared with controls. Finally, we used the UAS-GAL4 system (36) to promote independent expression of CCDC56 or mtTFB1 in a mutant background. Overexpression of CCDC56 but not mtTFB1 rescued all mutant phenotypes: developmental delay, larval lethality, decreased complex IV enzyme activity, and reduction in the levels of fully assembled complex IV. Previous results have shown that mtTFB1 is essential for mitochondrial translation and more specifically for the stability of the small subunit of the mitochondrial ribosomes (12 S rRNA) (25, 26). Thus, if the functionality of mtTFB1 were affected in these alleles, we would expect a broader mitochondrial phenotype, not simply an isolated complex IV deficiency. Biochemical measurements showed no decrease in the other respiratory chain complex activities in mutant animals or in complex V levels detected by blue native PAGE, which were even higher in the mutants. Taken together, our results indicate clearly that the phenotypes exhibited by the mutants were due exclusively to loss of CCDC56 function.
The main biochemical phenotype of the lack of CCDC56 is a severe isolated complex IV deficiency, suggesting a role for CCDC56 in complex IV activity and consequently in the proper function of the OXPHOS system. Interestingly, in ccdc56 knock-out animals, the OXPHOS defect elicits a compensatory response that causes a significant increase in mtDNA, in mitochondrial transcript levels, in OXPHOS activities in complexes I and II, and in levels of fully assembled complex V. The molecular nature of this retrograde signaling is presently unknown.
The OXPHOS defect caused by the complex IV deficiency may be responsible for the developmental delay and 100% lethality in the third larval instar observed in ccdc56 knock-out individuals. Mutant wing discs showed a reduced size, a decrease in cell proliferation, and an increase in apoptosis levels as compared with controls. Larval to adult metamorphosis, which occurs during the pupal phase, is a high energy-requiring process. Thus, defects in the OXPHOS system and consequently defects in ATP production may be critical for successful completion of this developmental phase. This is supported by the phenotype observed in several other Drosophila mitochondrial gene mutants. For example, knockdown of mtTFB2, which encodes the mitochondrial transcription factor B2, or mutations in mitochondrial single-stranded DNA-binding protein, both involved in mtDNA replication, also cause a developmental delay and a developmental arrest in the third larval instar (37, 38).
Complex IV assembly was studied initially by Nijtmans et al. (39), and subsequently by others (40) using blue native gel electrophoresis in human cell lines or tissues from patients with genetic defects in assembly factors (for reviews, see Refs. 41 and 42). These studies describe complex IV biogenesis as a sequential process involving four intermediates, S1 to S4. The process starts with the incorporation of the prosthetic groups into mt-CO1 to form the subassembly intermediate S1. The last intermediate, S4 or holo-COX, constitutes the monomeric form of COX that subsequently dimerizes to form the active complex (39). Fully assembled holo-COX levels were decreased greatly in homozygous ccdc56D11 knock-out larvae as assessed by two-dimensional blue native electrophoresis using an anti-mt-CO3 antibody. As expected, fully assembled complex IV levels were restored partially by the ubiquitous expression of a UAS-ccdc56 transgene in the mutant background. These results indicate that CCDC56 is essential for the formation or stabilization of complex IV. No subassembly intermediates were detected under these conditions. mt-CO3 is incorporated into the S3 intermediate as indicated by the absence of fully assembled holo-COX and the accumulation of S1 and S2 in cultured fibroblasts from patients lacking mt-CO3 (Ref. 43, and for reviews, see Refs. 42 and 44). Unfortunately, we tried several antibodies against other complex IV subunits with no success, so we cannot rule out the possible accumulation of other subassembly intermediates not containing mt-CO3 and thus a role for CCDC56 in the early steps of complex IV biogenesis.
Although the function of CCDC56 in flies remains unknown, because ccdc56 knock-out strains have extremely low levels of fully assembled complex IV, it is tempting to suggest that CCDC56 constitutes a new complex IV assembly factor. In this regard, CCDC56 might participate in various steps of complex IV biogenesis: synthesis of mitochondrial COX subunits, synthesis of COX cofactors, the stability of different COX subunits, or their assembly into a functional holoenzyme. We suggest that it may function as a translational activator similar to TACO1 (13) or as a membrane insertion factor like OXA1 (45). Future experiments are warranted to explore these possibilities. Notably, an increasing number of essential assembly factors for the biogenesis of a functional cytochrome c oxidase have been identified in Saccharomyces cerevisiae (4). We found by BLAST analysis that CCDC56 homologs are present in metazoans that show high conservation levels. However, no CCDC56 homolog was detected in yeast. The absence of a CCDC56 homolog in yeast might be due to low evolutionary conservation or could suggest the presence of novel levels of regulation of the complex IV assembly process in metazoans, thus highlighting the importance of using different model systems for the identification of the complete mitochondrial proteome.
In conclusion, we have identified CCDC56 as a novel mitochondrial protein essential for cytochrome c oxidase activity in D. melanogaster and hence OXPHOS function. The high degree of conservation between the human and Drosophila proteins suggests strongly that the biological function of CCDC56 has been preserved in metazoans, making CCDC56 a new candidate gene to study in human mitochondrial diseases involving isolated cytochrome c oxidase deficiency.
Acknowledgments
We thank Verónica Domingo for excellent technical assistance and Dr. Francisca Diaz for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM45295 (to L. S. K.). This work was also supported by grants from the Center for Biomedical Research on Rare Diseases (CIBERER), Instituto de Salud Carlos III Grants PI 07/0167 and PI 10/0703 (to R. G), Comunidad de Madrid Grant GEN-0269/2006 (to R. G.), Instituto de Salud Carlos III-Agencia Laín Entralgo Grant PI 08/0021 (to C. U.).
- COX
- cytochrome c oxidase
- CCDC56
- coiled coil domain-containing protein 56
- RACE
- rapid amplification of cDNA ends
- OXPHOS
- oxidative phosphorylation
- mt
- mitochondrial
- mtTFB1
- mitochondrial transcription factor B1
- uORF
- upstream open reading frame
- AEL
- after egg laying
- d-CCDC56
- Drosophila CCDC56
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Tb
- Tubby
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Wallace D. C., Fan W., Procaccio V. (2010) Mitochondrial energetics and therapeutics. Annu. Rev. Pathol. 5, 297–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1995) Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 Å. Science 269, 1069–1074 [DOI] [PubMed] [Google Scholar]
- 3. Szuplewski S., Terracol R. (2001) The cyclope gene of Drosophila encodes a cytochrome c oxidase subunit VIc homolog. Genetics 158, 1629–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fontanesi F., Soto I. C., Barrientos A. (2008) Cytochrome c oxidase biogenesis: new levels of regulation. IUBMB Life 60, 557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galati D., Srinivasan S., Raza H., Prabu S. K., Hardy M., Chandran K., Lopez M., Kalyanaraman B., Avadhani N. G. (2009) Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex: implications in mitochondrial dysfunction and ROS production. Biochem. J. 420, 439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold S., Kadenbach B. (1997) Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur. J. Biochem. 249, 350–354 [DOI] [PubMed] [Google Scholar]
- 7. Kadenbach B., Hüttemann M., Arnold S., Lee I., Bender E. (2000) Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic. Biol. Med. 29, 211–221 [DOI] [PubMed] [Google Scholar]
- 8. Barrientos A., Gouget K., Horn D., Soto I. C., Fontanesi F. (2009) Suppression mechanisms of COX assembly defects in yeast and human: insights into the COX assembly process. Biochim. Biophys. Acta 1793, 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Papadopoulou L. C., Sue C. M., Davidson M. M., Tanji K., Nishino I., Sadlock J. E., Krishna S., Walker W., Selby J., Glerum D. M., Coster R. V., Lyon G., Scalais E., Lebel R., Kaplan P., Shanske S., De Vivo D. C., Bonilla E., Hirano M., DiMauro S., Schon E. A. (1999) Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 23, 333–337 [DOI] [PubMed] [Google Scholar]
- 10. Valnot I., von Kleist-Retzow J. C., Barrientos A., Gorbatyuk M., Taanman J. W., Mehaye B., Rustin P., Tzagoloff A., Munnich A., Rötig A. (2000) A mutation in the human heme A:farnesyltransferase gene (COX10 ) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 9, 1245–1249 [DOI] [PubMed] [Google Scholar]
- 11. Antonicka H., Mattman A., Carlson C. G., Glerum D. M., Hoffbuhr K. C., Leary S. C., Kennaway N. G., Shoubridge E. A. (2003) Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 72, 101–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stiburek L., Vesela K., Hansikova H., Hulkova H., Zeman J. (2009) Loss of function of Sco1 and its interaction with cytochrome c oxidase. Am. J. Physiol. Cell Physiol. 296, C1218–C1226 [DOI] [PubMed] [Google Scholar]
- 13. Weraarpachai W., Antonicka H., Sasarman F., Seeger J., Schrank B., Kolesar J. E., Lochmüller H., Chevrette M., Kaufman B. A., Horvath R., Shoubridge E. A. (2009) Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat. Genet. 41, 833–837 [DOI] [PubMed] [Google Scholar]
- 14. Xu F., Addis J. B., Cameron J. M., Robinson B. H. (2012) LRPPRC mutation suppresses cytochrome oxidase activity by altering mitochondrial RNA transcript stability in a mouse model. Biochem. J. 441, 275–283 [DOI] [PubMed] [Google Scholar]
- 15. Xu F., Morin C., Mitchell G., Ackerley C., Robinson B. H. (2004) The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA. Biochem. J. 382, 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blumenthal T. (2004) Operons in eukaryotes. Brief. Funct. Genomic. Proteomic. 3, 199–211 [DOI] [PubMed] [Google Scholar]
- 17. Andrews J., Smith M., Merakovsky J., Coulson M., Hannan F., Kelly L. E. (1996) The stoned locus of Drosophila melanogaster produces a dicistronic transcript and encodes two distinct polypeptides. Genetics 143, 1699–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brogna S., Ashburner M. (1997) The Adh-related gene of Drosophila melanogaster is expressed as a functional dicistronic messenger RNA: multigenic transcription in higher organisms. EMBO J. 16, 2023–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Betrán E., Ashburner M. (2000) Duplication, dicistronic transcription, and subsequent evolution of the alcohol dehydrogenase and alcohol dehydrogenase-related genes in Drosophila. Mol. Biol. Evol. 17, 1344–1352 [DOI] [PubMed] [Google Scholar]
- 20. Estes P. S., Jackson T. C., Stimson D. T., Sanyal S., Kelly L. E., Ramaswami M. (2003) Functional dissection of a eukaryotic dicistronic gene: transgenic stonedB, but not stonedA, restores normal synaptic properties to Drosophila stoned mutants. Genetics 165, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scarpulla R. C. (2008) Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 88, 611–638 [DOI] [PubMed] [Google Scholar]
- 22. Falkenberg M., Gaspari M., Rantanen A., Trifunovic A., Larsson N. G., Gustafsson C. M. (2002) Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat. Genet. 31, 289–294 [DOI] [PubMed] [Google Scholar]
- 23. Seidel-Rogol B. L., McCulloch V., Shadel G. S. (2003) Human mitochondrial transcription factor B1 methylates ribosomal RNA at a conserved stem-loop. Nat. Genet. 33, 23–24 [DOI] [PubMed] [Google Scholar]
- 24. Cotney J., Shadel G. S. (2006) Evidence for an early gene duplication event in the evolution of the mitochondrial transcription factor B family and maintenance of rRNA methyltransferase activity in human mtTFB1 and mtTFB2. J. Mol. Evol. 63, 707–717 [DOI] [PubMed] [Google Scholar]
- 25. Matsushima Y., Adán C., Garesse R., Kaguni L. S. (2005) Drosophila mitochondrial transcription factor B1 modulates mitochondrial translation but not transcription or DNA copy number in Schneider cells. J. Biol. Chem. 280, 16815–16820 [DOI] [PubMed] [Google Scholar]
- 26. Metodiev M. D., Lesko N., Park C. B., Cámara Y., Shi Y., Wibom R., Hultenby K., Gustafsson C. M., Larsson N. G. (2009) Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab. 9, 386–397 [DOI] [PubMed] [Google Scholar]
- 27. Engels W. R., Johnson-Schlitz D. M., Eggleston W. B., Sved J. (1990) High-frequency P element loss in Drosophila is homolog dependent. Cell 62, 515–525 [DOI] [PubMed] [Google Scholar]
- 28. Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson J. D., Higgins D. G., Gibson T. J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pérez-Carreras M., Del Hoyo P., Martín M. A., Rubio J. C., Martín A., Castellano G., Colina F., Arenas J., Solis-Herruzo J. A. (2003) Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38, 999–1007 [DOI] [PubMed] [Google Scholar]
- 31. Calvaruso M. A., Smeitink J., Nijtmans L. (2008) Electrophoresis techniques to investigate defects in oxidative phosphorylation. Methods 46, 281–287 [DOI] [PubMed] [Google Scholar]
- 32. Peña P., Garesse R. (1993) The β subunit of the Drosophila melanogaster ATP synthase: cDNA cloning, amino acid analysis and identification of the protein in adult flies. Biochem. Biophys. Res. Commun. 195, 785–791 [DOI] [PubMed] [Google Scholar]
- 33. Overbeek R., Fonstein M., D'Souza M., Pusch G. D., Maltsev N. (1999) The use of gene clusters to infer functional coupling. Proc. Natl. Acad. Sci. U.S.A. 96, 2896–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stark A., Lin M. F., Kheradpour P., Pedersen J. S., Parts L., Carlson J. W., Crosby M. A., Rasmussen M. D., Roy S., Deoras A. N., Ruby J. G., Brennecke J., Harvard FlyBase curators, Berkeley Drosophila Genome Project, Hodges E., Hinrichs A. S., Caspi A., Paten B., Park S. W., Han M. V., Maeder M. L., Polansky B. J., Robson B. E., Aerts S., van Helden J., Hassan B., Gilbert D. G., Eastman D. A., Rice M., Weir M., Hahn M. W., Park Y., Dewey C. N., Pachter L., Kent W. J., Haussler D., Lai E. C., Bartel D. P., Hannon G. J., Kaufman T. C., Eisen M. B., Clark A. G., Smith D., Celniker S. E., Gelbart W. M., Kellis M. (2007) Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures. Nature 450, 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hayden C. A., Bosco G. (2008) Comparative genomic analysis of novel conserved peptide upstream open reading frames in Drosophila melanogaster and other dipteran species. BMC Genomics 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brand A. H., Perrimon N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- 37. Adán C., Matsushima Y., Hernández-Sierra R., Marco-Ferreres R., Fernández-Moreno M. A., González-Vioque E., Calleja M., Aragón J. J., Kaguni L. S., Garesse R. (2008) Mitochondrial transcription factor B2 is essential for metabolic function in Drosophila melanogaster development. J. Biol. Chem. 283, 12333–12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maier D., Farr C. L., Poeck B., Alahari A., Vogel M., Fischer S., Kaguni L. S., Schneuwly S. (2001) Mitochondrial single-stranded DNA-binding protein is required for mitochondrial DNA replication and development in Drosophila melanogaster. Mol. Biol. Cell 12, 821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nijtmans L. G., Taanman J. W., Muijsers A. O., Speijer D., Van den Bogert C. (1998) Assembly of cytochrome-c oxidase in cultured human cells. Eur. J. Biochem. 254, 389–394 [DOI] [PubMed] [Google Scholar]
- 40. Fornuskova D., Stiburek L., Wenchich L., Vinsova K., Hansikova H., Zeman J. (2010) Novel insights into the assembly and function of human nuclear-encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b. Biochem. J. 428, 363–374 [DOI] [PubMed] [Google Scholar]
- 41. Diaz F. (2010) Cytochrome c oxidase deficiency: patients and animal models. Biochim. Biophys. Acta 1802, 100–110 [DOI] [PubMed] [Google Scholar]
- 42. Fernández-Vizarra E., Tiranti V., Zeviani M. (2009) Assembly of the oxidative phosphorylation system in humans: what we have learned by studying its defects. Biochim. Biophys. Acta 1793, 200–211 [DOI] [PubMed] [Google Scholar]
- 43. Tiranti V., Corona P., Greco M., Taanman J. W., Carrara F., Lamantea E., Nijtmans L., Uziel G., Zeviani M. (2000) A novel frameshift mutation of the mtDNA COIII gene leads to impaired assembly of cytochrome c oxidase in a patient affected by Leigh-like syndrome. Hum. Mol. Genet. 9, 2733–2742 [DOI] [PubMed] [Google Scholar]
- 44. Fontanesi F., Soto I. C., Horn D., Barrientos A. (2006) Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am. J. Physiol. Cell Physiol. 291, C1129–C1147 [DOI] [PubMed] [Google Scholar]
- 45. Bonnefoy N., Fiumera H. L., Dujardin G., Fox T. D. (2009) Roles of Oxa1-related inner-membrane translocases in assembly of respiratory chain complexes. Biochim. Biophys. Acta 1793, 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]